Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2574
Revised: July 2, 2024
Accepted: July 5, 2024
Published online: August 27, 2024
Processing time: 92 Days and 2.6 Hours
Study on influencing factors of gastric retention before endoscopic retrograde cholangiopancreatography (ERCP) background: With the wide application of ERCP, the risk of preoperative gastric retention affects the smooth progress of the operation. The study found that female, biliary and pancreatic malignant tumor, digestive tract obstruction and other factors are closely related to gastric retention, so the establishment of predictive model is very important to reduce the risk of operation.
To analyze the factors influencing preoperative gastric retention in ERCP and establish a predictive model.
A retrospective analysis was conducted on 190 patients admitted to our hospital for ERCP preparation between January 2020 and February 2024. Patient baseline clinical data were collected using an electronic medical record system. Patients were randomly matched in a 1:4 ratio with data from 190 patients during the same period to establish a validation group (n = 38) and a modeling group (n = 152). Patients in the modeling group were divided into the gastric retention group (n = 52) and non-gastric retention group (n = 100) based on whether gastric retention occurred preoperatively. General data of patients in the validation group and modeling group were compared. Univariate and multivariate logistic regression analyses were performed to identify factors influencing preoperative gastric retention in ERCP patients. A predictive model for preoperative gastric retention in ERCP patients was constructed, and calibration curves were used for validation. The receiver operating characteristic (ROC) curve was analyzed to evaluate the predictive value of the model.
We found no statistically significant difference in general data between the validation group and modeling group
Gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction are factors influencing preoperative gastric retention in ERCP patients. A predictive model established based on these factors has high predictive value.
Core Tip: The innovation of the study of gastric retention before endoscopic retrograde cholangiopancreatography lies in the accurate prediction of gastric retention by GCSI scale. It is found that female, biliary and pancreatic malignant tumor and digestive tract obstruction are independent risk factors. The important arguments are: Establishing a highly accurate prediction model, identifying high-risk patients in advance, and optimizing preoperative preparation.
- Citation: Jia Y, Wu HJ, Li T, Liu JB, Fang L, Liu ZM. Establishment of predictive models and determinants of preoperative gastric retention in endoscopic retrograde cholangiopancreatography. World J Gastrointest Surg 2024; 16(8): 2574-2582
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2574.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2574
Endoscopic retrograde cholangiopancreatography (ERCP) is a diagnostic method used to examine the pancreas and biliary system. It involves inserting a duodenoscope into the descending part of the duodenum to locate the major duodenal papilla. A catheter is then inserted through the biopsy channel of the duodenoscope into the opening of the papilla, and contrast medium is injected for X-ray imaging to clearly display the morphology and position of the pancreatic and bile ducts, as well as any existing lesions[1]. ERCP is primarily employed for the diagnosis of biliary and pancreatic diseases such as biliary tract stones, tumors, obstructive jaundice, inflammatory strictures, chronic pancreatitis, pancreatic cancer, and lesions in the ampulla of Vater[2]. Prior to the examination, patients are required to fast and undergo tests such as complete blood count, coagulation function, and liver and kidney function tests to ensure the accuracy of the results[3]. After the examination, patients are advised to drink plenty of water to facilitate the excretion of the contrast medium. Preoperative gastric retention before ERCP is a particular concern[4]. Gastric retention refers to delayed gastric emptying, which may lead to restricted visibility and difficulties in operation during the procedure, and may even increase the risk of surgical complications[5]. Therefore, understanding the factors influencing preoperative gastric retention is beneficial for physicians to conduct comprehensive preoperative assessments and formulate rational surgical plans, ensuring the safety and efficacy of the procedure. For patients undergoing ERCP, assessing gastric emptying to determine the presence of gastric retention is crucial. If gastric retention is identified, then the procedure may need to be postponed until the symptoms of gastric retention improve. Thus, understanding the influencing factors of preoperative gastric retention not only facilitates comprehensive preoperative assessments and rational surgical planning but also ensures the safety and efficacy of the procedure. The establishment of predictive models also contributes to the efficient utilization of medical resources. By predicting the risk of preoperative gastric retention in patients, hospitals can reasonably arrange surgical timing and medical resources, avoiding unnecessary waste and delays. Additionally, for high-risk patients, hospitals can make adequate preparations and response measures in advance to ensure smooth surgical procedures. In this study, we retrospectively analyzed 190 patients who underwent preparation for ERCP in our hospital from January 2020 to December 2023. We aimed to analyze the influencing factors of preoperative gastric retention in ERCP and establish a predictive model, providing clinical reference.
A retrospective analysis was conducted on 190 patients who underwent ERCP preparation at the Chengdu Shangjinnanfu Hospital, West China Hospital of Sichuan University from January 2020 and February 2024. Inclusion criteria were as follows: (1) All patients met the surgical indications for ERCP; (2) Patients aged > 18 years; and (3) Patients with stable vital signs. Exclusion criteria were as follows: (1) Patients with severe cardiac or pulmonary dysfunction; (2) Patients with mental disorders; and (3) Patients with other surgical contraindications. This study was approved by the Medical Ethics Committee of West China Hospital, Sichuan University (No. 2022-1674), and patient informed consent was exempted.
Patient baseline clinical data were collected using an electronic medical record system. Patients were randomly matched in a 1:4 ratio with data from 190 patients during the same period to establish a validation group (n = 38) and a modeling group (n = 152). Patients in the modeling group were divided into the gastric retention group (n = 52) and non-gastric retention group (n = 100) based on whether gastric retention occurred preoperatively. The criteria for gastric retention were as follows: (1) Repeated episodes of nausea and vomiting with vomitus containing undigested food and a sour odor, without bile content; (2) Abdominal pain characterized by dull, colicky, or burning sensations, typically relieved after vomiting; and (3) Residual gastric contents observed during endoscopic visualization. Any of the above criteria constituted gastric retention.
(1) General data between the validation group and modeling group were compared; (2) Factors influencing preoperative gastric retention in ERCP patients in the modeling group were examined via univariate analysis; (3) Factors influencing preoperative gastric retention in ERCP patients were determined via multivariate logistic regression analysis; (4) A predictive model for preoperative gastric retention in ERCP patients was constructed, with calibration curve validation; and (5) Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive value of the model for preoperative gastric retention in ERCP patients, including the area under the curve (AUC), sensitivity, and specificity.
Experimental data were analyzed using SPSS 27.0. Normally distributed continuous data were expressed as mean ± SD and compared using independent sample t-tests. Count data were expressed as numbers or rates and compared using χ2 tests or Fisher’s exact test. Univariate and binary logistic regression analyses were used to analyze factors influencing preoperative gastric retention in ERCP patients. The ROC curve was used to evaluate the predictive value of the model for preoperative gastric retention in ERCP patients, and statistical significance was set at P < 0.05.
We found no statistically significant difference in general data between the validation and modeling groups (P > 0.05), as shown in Table 1.
| General information | The validation groups (n = 38) | The modeling groups (n = 152) | t/χ2 | P value | |
| Age (years) | 60.83 ± 12.16 | 61.52 ± 11.85 | 0.319 | 0.750 | |
| Gender | Male | 16 | 92 | 4.205 | 0.122 |
| Female | 22 | 60 | |||
| BMI (kg/m2) | 21.16 ± 2.46 | 21.37 ± 2.11 | 0.530 | 0.597 | |
| Primary disease | Malignant biliary and pancreatic tumors | 20 | 77 | 0.047 | 0.977 |
| Other biliary diseases | 18 | 75 | |||
| Hypertension | Yes | 8 | 35 | 0.068 | 0.967 |
| No | 30 | 117 | |||
| Diabetes | Yes | 7 | 30 | 0.034 | 0.983 |
| No | 31 | 122 | |||
| Jaundice | Yes | 12 | 62 | 1.085 | 0.581 |
| No | 26 | 90 | |||
| Opioid use | Yes | 13 | 55 | 0.051 | 0.975 |
| No | 25 | 97 | |||
| Gastrointestinal obstruction | Yes | 15 | 58 | 0.022 | 0.989 |
| No | 23 | 94 |
We found no statistically significant difference in age, body mass index (BMI), hypertension, and diabetes between the two groups (P > 0.05). However, we noted statistically significant differences in gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction (P < 0.05), as shown in Table 2.
| General information | The gastric retention group (n = 52) | Non-gastric retention group (n = 100) | t/χ2 | P value | |
| Age (years) | 60.55 ± 10.16 | 60.50 ± 10.45 | 0.028 | 0.978 | |
| Gender | Male | 17 | 75 | 25.631 | < 0.001 |
| Female | 35 | 25 | |||
| BMI (kg/m2) | 21.39 ± 2.22 | 21.35 ± 2.14 | 0.108 | 0.914 | |
| Primary disease | Malignant biliary and pancreatic tumors | 39 | 38 | 18.741 | < 0.001 |
| Other biliary diseases | 13 | 62 | |||
| Hypertension | Yes | 15 | 20 | 1.510 | 0.470 |
| No | 37 | 80 | |||
| Diabetes | Yes | 8 | 22 | 0.945 | 0.623 |
| No | 44 | 78 | |||
| Jaundice | Yes | 32 | 30 | 14.090 | 0.001 |
| No | 20 | 70 | |||
| Opioid use | Yes | 28 | 27 | 10.680 | 0.005 |
| No | 24 | 73 | |||
| Gastrointestinal obstruction | Yes | 27 | 31 | 6.347 | 0.042 |
| No | 25 | 69 |
Using gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction as independent variables and preoperative gastric retention occurrence as the dependent variable (occurring = 1 and not occurring = 0), multivariate logistic regression analysis showed that gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction were independent factors influencing preoperative gastric retention in ERCP patients (P < 0.05; Table 3).
| Risk factors | β | SE | Ward | OR | 95%CI | P value |
| Gender | 0.883 | 0.361 | 5.988 | 2.419 | 1.192-4.908 | < 0.001 |
| Primary disease | 1.199 | 0.385 | 9.695 | 3.316 | 1.559-7.052 | < 0.001 |
| Jaundice | 1.072 | 0.372 | 8.303 | 2.921 | 1.409-6.056 | < 0.001 |
| Opioid use | 1.152 | 0.401 | 8.251 | 3.164 | 1.442-6.943 | < 0.001 |
| Gastrointestinal obstruction | 1.242 | 0.412 | 9.085 | 3.462 | 1.544-7.763 | < 0.001 |
On the basis of the results of logistic regression analysis, gender, primary disease, jaundice, opioid use, and gastroin
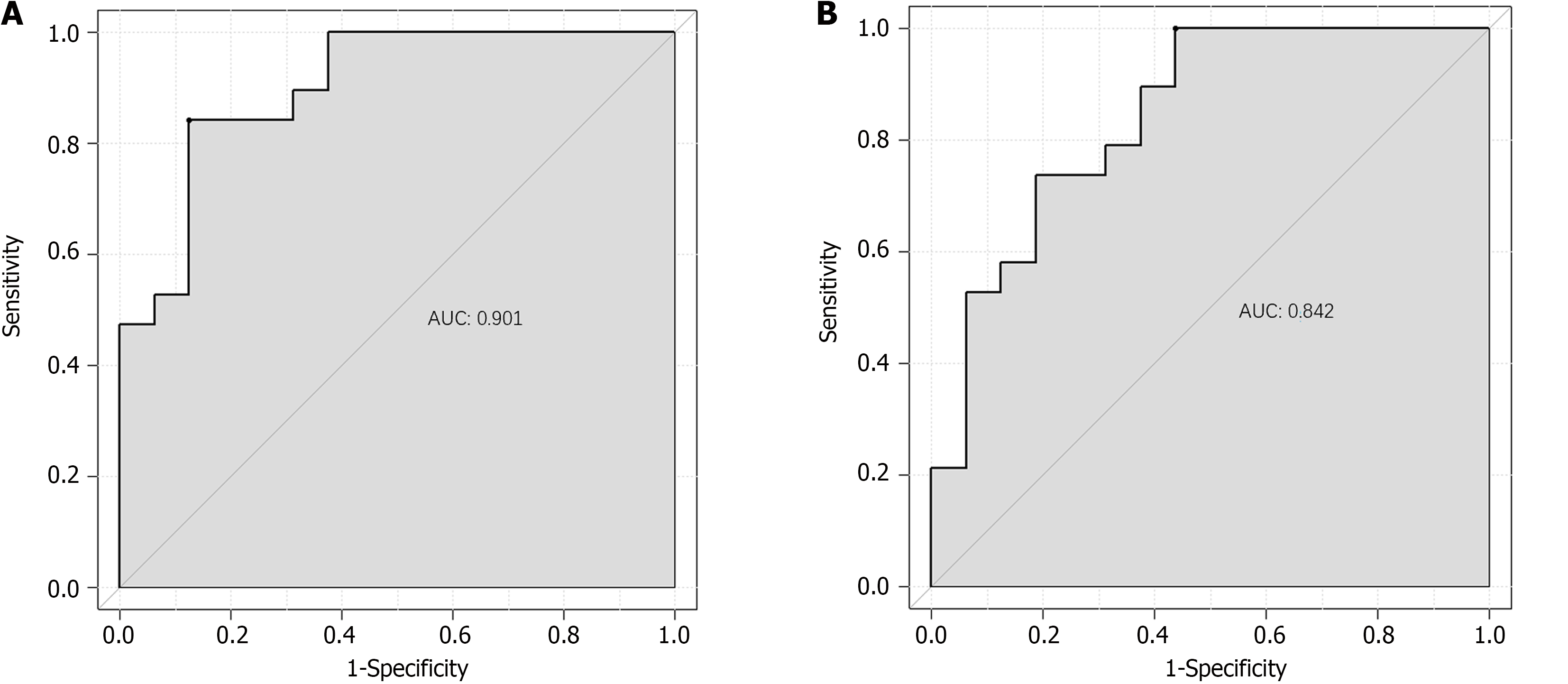
The ROC analysis results showed that the AUC of the model for predicting preoperative gastric retention in ERCP patients in the training set was 0.901, with a standard error of 0.023 (95%CI: 0.8264-0.9567); the optimal cutoff value was 0.71, with a sensitivity of 87.5 and specificity of 84.2 (Figure 3A). For the validation set, the AUC of the model was 0.842, with a standard error of 0.013 (95%CI: 0.8061-0.9216); the optimal cutoff value was 0.56, with a sensitivity of 56.2 and specificity of 100.0 (Figure 3B).
Preoperative gastric retention is a relatively common clinical issue in ERCP. The occurrence of gastric retention may vary due to individual differences, underlying diseases, medication use, and inadequate preoperative preparation. In ERCP, the presence of gastric retention in patients may increase the difficulty and risk of ERCP procedures, affecting patient psychological state and cooperation during surgery. Furthermore, gastric retention may influence postoperative nutritional intake and recovery, prolonging hospitalization and recovery time[6,7]. Our study investigated this issue and established relevant predictive models to provide clinical guidance.
The results of this study showed no statistically significant difference in age, BMI, hypertension, and diabetes between the two groups in the training set (P > 0.05). However, we found statistically significant differences in gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction (P < 0.05). Furthermore, the results of the multivariate logistic regression analysis demonstrated that gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction were independent factors influencing preoperative gastric retention in ERCP patients (P < 0.05). The physiological structure of females differs from that of males, potentially leading to high susceptibility to gastric emptying disorders in certain situations, thereby increasing the risk of preoperative gastric retention[8]. Additionally, compared with males, females are typically more susceptible to psychological and emotional factors such as anxiety and depression, which may disrupt gastrointestinal function, including delayed gastric emptying, thereby increasing the risk of preoperative gastric retention[9]. Patients with malignant biliary and pancreatic tumors often have tumor masses, which may compress adjacent gastrointestinal tracts, thereby affecting gastrointestinal motility and emptying function. Moreover, tumor growth may directly invade the gastrointestinal tract, leading to stenosis or obstruction and increasing the risk of gastric retention[10]. Given the influence of the disease, patients with malignant biliary and pancreatic tumors often have impaired digestive function to varying degrees, leading to impaired secretion and excretion of bile and pancreatic juice, affecting food digestion and absorption, and potentially resulting in prolonged food retention in the stomach, thereby causing gastric retention[11]. The presence of a malignant tumor may cause changes in the body’s neuroendocrine system, including alterations in hormone levels, which may affect gastrointestinal motility and emptying function and increase the risk of gastric retention. Patients with malignant biliary and pancreatic tumors may undergo a series of treatments such as drug therapy, radiotherapy, and chemotherapy before surgery. These drugs and treatment methods may have side effects on the gastrointestinal tract, such as nausea, vomiting, and loss of appetite, increasing the risk of gastric retention. Jaundice is usually caused by biliary obstruction, which may not only affect the normal excretion of bile but also influence gastrointestinal motility and emptying function, leading to gastric retention[12,13]. Moreover, jaundice is a clear manifestation of impaired liver function. Impaired liver function may affect bile synthesis and secretion and gastrointestinal digestion function, leading to prolonged food retention in the stomach, thereby causing gastric retention[14].
Opioid drugs are mainly used to treat chronic and acute pain and have potent analgesic effects. However, these drugs may also inhibit gastrointestinal motility, leading to a decrease in gastrointestinal function. Slowing gastrointestinal motility prolongs food retention in the stomach, thereby increasing the risk of gastric retention. The common side effects of opioid drugs include nausea, vomiting, and constipation, which can affect the patient’s quality of life and exacerbate gastrointestinal functional issues. Nausea and vomiting may lead to delayed gastric emptying, whereas constipation may increase intestinal pressure, further affecting gastrointestinal motility and emptying[15]. Additionally, studies have[16] found that opioid drugs may increase gastric acid secretion and delay gastric emptying, leading to the accumulation of gastric acid in the stomach, which not only increases stimulation to the gastric mucosa but also exacerbates symptoms of gastric retention[17].
Gastrointestinal obstruction refers to narrowing or occlusion occurring at any part of the gastrointestinal tract from the mouth to the anus, which obstructs the normal passage of food and digestive juices[18]. When obstruction occurs in the upper gastrointestinal tract such as the stomach or duodenum, food retention in the stomach is prolonged, leading to gastric retention. Gastrointestinal obstruction is often accompanied with symptoms such as nausea and vomiting, which can exacerbate delayed gastric emptying. Vomiting causes reflux of gastric contents, whereas nausea may lead to reduced food intake, further affecting gastric emptying. The causes of gastrointestinal obstruction are diverse, and they include tumors, inflammation, and external pressure. These factors not only lead to obstruction but also affect gastrointestinal motility and secretion function[19]. For example, tumor growth may directly compress the gastrointestinal tract, and inflammation may cause gastrointestinal spasms or edema, both of which affect gastrointestinal emptying function.
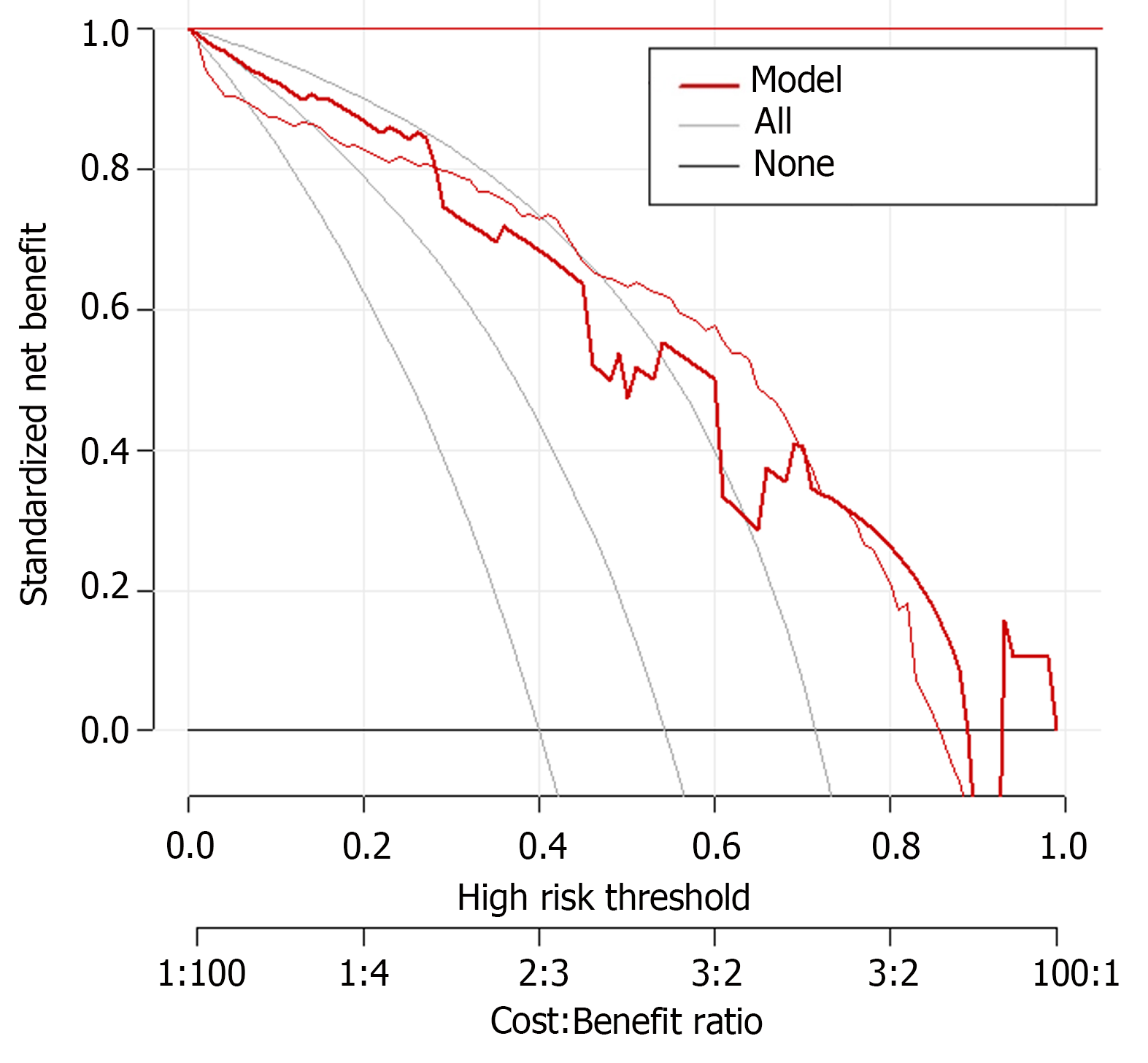
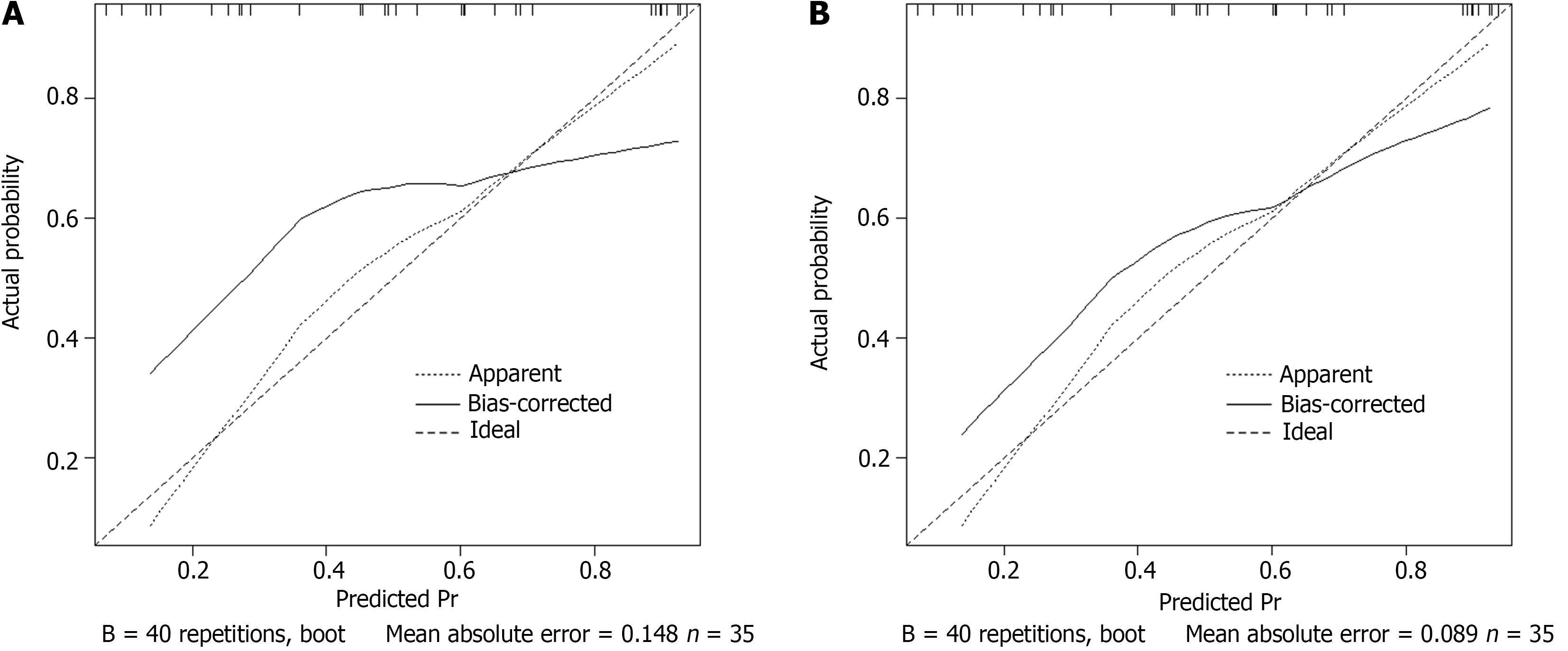
In previous studies, it was found that female, malignant tumor of gallbladder and pancreas and digestive tract obstruction were independent risk factors of gastric retention. In particular, in the prediction study on the risk of gastric retention before ERCP in patients with cholangiopancreatic diseases, the GCSI scale (gastroparesis main symptom index scale) was used as a predictive tool, and it was found that the best critical value was 25 points, the predictive sensitivity was as high as 87.5%, and the specificity was 91.4%. However, most of these studies focus on a single or a few factors, and the interaction between multiple factors and individual differences of patients are not considered enough. In the establishment of the prediction model, although the GCSI scale shows a high prediction accuracy, it still needs to be further optimized and improved. The model should be able to more comprehensively consider the individual differences of patients' age, body weight, underlying diseases, as well as the interaction between different factors, so as to improve the predictive efficiency. In this study, the prediction model is based on the influencing factors of single-factor and multi-factor analysis. The calibration curves in the training and validation sets showed a slope close to 1, indicating good predictive accuracy. ROC analysis results showed that the AUC of the predictive model reached 0.901 in the training set, demonstrating high predictive accuracy, with a standard error of 0.023 and a 95% confidence interval of 0.8264-0.9567, indicating the stability and reliability of the predictive results. When the optimal cutoff value was set to 0.71, the sensitivity of the model reached 87.5%, and the specificity was as high as 84.2%, indicating that the model had high predictive ability in predicting preoperative gastric retention in ERCP patients. The predictive performance of the model on the validation set was also impressive, with an AUC of 0.842, a standard error of 0.013, and a 95%CI of 0.8061-0.9216, demonstrating the stability and reliability of the model’s predictive ability. When the optimal cutoff value was set to 0.56, although the sensitivity was slightly lower at 56.2% compared with the training set, the specificity was as high as 100.0%, further demonstrating the value of the model.
This study successfully established a predictive model of high value. However, it had some limitations. In terms of data collection, there may be issues with insufficient sample size or inadequate sample selection[20], which may limit the applicability of the model to specific populations, reducing its generalizability and accuracy. To address this problem, future research could consider expanding the sample size, including a diverse patient population, to improve the model’s generalizability.
In summary, gender, primary disease, jaundice, opioid use, and gastrointestinal obstruction are important influencing factors for preoperative gastric retention in ERCP patients. Establishing a predictive model based on these influencing factors can help clinicians thoroughly assess patient risk and formulate personalized preoperative preparations and interventions, thereby reducing the incidence of preoperative gastric retention in ERCP patients and improving the safety and success rate of surgery.
| 1. | Tang Y, Wu Z, Deng X, Cheng Z, Zhong X. Removal of an impacted apricot pit from a scarring duodenal stenosis using endoscopic retrograde cholangiopancreatography (ERCP) stone extraction technique. Rev Esp Enferm Dig. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Camilleri M, Kuo B, Nguyen L, Vaughn VM, Petrey J, Greer K, Yadlapati R, Abell TL. ACG Clinical Guideline: Gastroparesis. Am J Gastroenterol. 2022;117:1197-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 3. | Hollenbach M, Hoffmeister A. Adverse events in endoscopic retrograde cholangiopancreaticography (ERCP): Focus on post-ERCP-pancreatitis. United European Gastroenterol J. 2022;10:10-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Natt N, Michael F, Michael H, Dubois S, Al Mazrou'i A. ERCP-Related Adverse Events in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol. 2022;2022:2372257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | van der Merwe SW, van Wanrooij RLJ, Bronswijk M, Everett S, Lakhtakia S, Rimbas M, Hucl T, Kunda R, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Perez-Miranda M, van Hooft JE. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:185-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 275] [Article Influence: 91.7] [Reference Citation Analysis (3)] |
| 6. | Ak Ç, Aykut H, Pala E, Sayar S, Tarikçi Kiliç E, Adali G, Kahraman R, Öztürk O, Özdil K. Post-ERCP Complication Analysis of an Experienced Center. Surg Laparosc Endosc Percutan Tech. 2022;32:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Lattanzi B, Ramai D, Gkolfakis P, Facciorusso A. Predictive models in EUS/ERCP. Best Pract Res Clin Gastroenterol. 2023;67:101856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Rivas A, Pherwani S, Mohamed R, Smith ZL, Elmunzer BJ, Forbes N. ERCP-related adverse events: incidence, mechanisms, risk factors, prevention, and management. Expert Rev Gastroenterol Hepatol. 2023;17:1101-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Cahyadi O, Tehami N, de-Madaria E, Siau K. Post-ERCP Pancreatitis: Prevention, Diagnosis and Management. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 10. | Solanki S, Kichloo A, Dahiya DS, Solanki D, Singh J, Wani F, Albosta M, Ghimire S, Haq KF, Khan HMA, Jafri SM, Siddiqui MA, Zuchelli T. Endoscopic Retrograde Cholangiopancreatography (ERCP) in Patients With Liver Cirrhosis: Analysis of Trends and Outcomes From the National Inpatient Sample Database. J Clin Gastroenterol. 2022;56:618-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Fukuda R, Hakuta R, Nakai Y, Hamada T, Takaoka S, Tokito Y, Suzuki Y, Oyama H, Kanai S, Noguchi K, Suzuki T, Ishigaki K, Saito K, Saito T, Takahara N, Mizuno S, Ito Y, Kogure H, Fujishiro M. Development and external validation of a nomogram for prediction of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreatology. 2023;23:789-796. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Sandru V, Ilie VC, Jamal AG, Panaitescu A, Plotogea OM, Rinja E, Minciuna CE, Vasilescu C, Constantinescu G, Lacatus M. Endoscopic Retrograde Cholangiopancreatography in Acute Biliary Pancreatitis: Urgent vs. Delayed and the Outcome of Same-Admission Cholecystectomy. Chirurgia (Bucur). 2022;117:22-29. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Qi S, Xu J, Yan C, He Y, Chen Y. Early versus delayed laparoscopic cholecystectomy after endoscopic retrograde cholangiopancreatography: A meta-analysis. Medicine (Baltimore). 2023;102:e34884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 14. | Osagiede O, Lukens FJ, Kumbhari V, Corral JE. Endoscopic Retrograde Cholangiopancreatography Performed by Trainees Is Not Associated with Increased Immediate Adverse Events or Technical Failure Rates. Dig Dis Sci. 2023;68:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Dubravcsik Z, Gyökeres T, Novák P, Budai A, Mohácsi S, Velkei T, Madácsy L. [Complications of endoscopic retrograde cholangiopancreatography]. Orv Hetil. 2022;163:911-919. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Jang DK, Kim J, Paik CN, Kim JW, Lee TH, Jang JY, Yoon SB, Lee JK. Endoscopic retrograde cholangiopancreatography-related adverse events in Korea: A nationwide assessment. United European Gastroenterol J. 2022;10:73-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Park CH. [The Latest Knowledge on Endoscopic Retrograde Cholangiopancreatography-related Pancreatitis]. Korean J Gastroenterol. 2022;79:195-198. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Azimaraghi O, Bilal M, Amornyotin S, Arain M, Behrends M, Berzin TM, Buxbaum JL, Choice C, Fassbender P, Sawhney MS, Sundar E, Wongtangman K, Leslie K, Eikermann M. Consensus guidelines for the perioperative management of patients undergoing endoscopic retrograde cholangiopancreatography. Br J Anaesth. 2023;130:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Reference Citation Analysis (0)] |
| 19. | Itoi T. Pancreatobiliary endoscopy: Diagnostic endoscopic retrograde cholangiopancreatography. Dig Endosc. 2022;34 Suppl 2:99-101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Ayres AM, Wozniak J, O'Neil J, Stewart K, Leger JS, Pasculle AW, Lewis C, McGrath K, Slivka A, Snyder GM. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound endoscope reprocessing: Variables impacting contamination risk. Infect Control Hosp Epidemiol. 2023;44:1485-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |