Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2546
Revised: May 29, 2024
Accepted: June 27, 2024
Published online: August 27, 2024
Processing time: 101 Days and 3.7 Hours
Hepatocellular carcinoma (HCC) recurrence is highly correlated with increased mortality. Microvascular invasion (MVI) is indicative of aggressive tumor biology in HCC.
To construct an artificial neural network (ANN) capable of accurately predicting MVI presence in HCC using magnetic resonance imaging.
This study included 255 patients with HCC with tumors < 3 cm. Radiologists annotated the tumors on the T1-weighted plain MR images. Subsequently, a three-layer ANN was constructed using image features as inputs to predict MVI status in patients with HCC. Postoperative pathological examination is considered the gold standard for determining MVI. Receiver operating characteristic analysis was used to evaluate the effectiveness of the algorithm.
Using the bagging strategy to vote for 50 classifier classification results, a prediction model yielded an area under the curve (AUC) of 0.79. Moreover, correlation analysis revealed that alpha-fetoprotein values and tumor volume were not significantly correlated with the occurrence of MVI, whereas tumor sphericity was significantly correlated with MVI (P < 0.01).
Analysis of variable correlations regarding MVI in tumors with diameters < 3 cm should prioritize tumor sphericity. The ANN model demonstrated strong predictive MVI for patients with HCC (AUC = 0.79).
Core Tip: Constructing an artificial neural network (ANN) using magnetic resonance imaging can accurately predict the presence of microvascular invasion (MVI) in patients with hepatocellular carcinoma (HCC) with tumors < 3 cm. Tumor sphericity has emerged as a significant factor associated with MVI occurrence, emphasizing its importance in MVI analysis. The strong predictive capability of the ANN model, with an area under the curve of 0.79, shows its potential to enhance MVI prediction accuracy and aid in the management of patients with HCC.
- Citation: Xu JY, Yang YF, Huang ZY, Qian XY, Meng FH. Preoperative prediction of hepatocellular carcinoma microvascular invasion based on magnetic resonance imaging feature extraction artificial neural network. World J Gastrointest Surg 2024; 16(8): 2546-2554
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2546.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2546
Hepatocellular carcinoma (HCC) is the fifth and seventh most prevalent tumor in men and women, respectively. However, its incidence is rising continuously[1]. Surgical resection remains the most effective treatment for HCC. Advances in imaging technology have augmented the detection rate of early-stage HCC, consequently enhancing overall survival rates[2,3]. Nevertheless, the persistently high recurrence rate after surgical intervention continues to significantly affect the prognosis of patients with HCC[4]. Microvascular invasion (MVI) in HCC denotes the presence of a cluster of cancer cells within the lumen of endothelial cell-lined vessels, as observed by pathologists under a microscope. MVI is commonly considered positive when the number of cancer cells suspended in the vascular lumen exceeds 50[5]. The presence of MVI in HCC is widely acknowledged to indicate a higher likelihood of tumor spread to adjacent tissues, signifying a more aggressive disease phenotype. MVI is a significant risk factor for poor disease-free survival and overall survival in patients with HCC[6]. A previous study highlighted the strong correlation between MVI and HCC recurrence within the first two years of surgical treatment[7]. It is reported a 5-year disease-specific survival rate of 59.3% for patients with MVI compared to 92.0% for those without MVI after curative resection for HCC[8,9]. Additionally, in a postope
However, the diagnosis of MVI in HCC currently relies solely on the pathological examination of the HCC tissue acquired during surgery. Unfortunately, radiologists cannot directly identify MVI by visual inspection using computed tomography (CT) or magnetic resonance imaging (MRI). Consequently, the strategy for treating patients with HCC often necessitates the commencement of liver resection, limiting the diagnostic value of MVI. Tumor biopsy is suggested for diagnosing MVI; however, its clinical utility has not been validated for several reasons. First, the amount of tissue obtained via biopsy is typically insufficient to ensure an accurate diagnosis. Second, tumor biopsy poses the risk of tumor implantation along the needle tract[11].
Therefore, demand is evident concerning a noninvasive preoperative radiological method to determine MVI in HCC, which can facilitate informed personalized treatment planning for patients. However, the complexity and nonlinearity of the information contained in MRI images pose challenges for quantification. Therefore, distinguishing between feature categories is difficult using a traditional linear discriminant analysis. However, an artificial neural network (ANN) has demonstrated the ability to outperform traditional discriminant analysis methods in calculating these features[12].
Currently, studies on MVI rely predominantly on traditional statistical methods that require additional imaging sequences. To the best of our knowledge, a notable gap exists in studies focusing on patients with small HCC tumors measuring < 3 cm in diameter, particularly in terms of feature analysis and MVI prediction based on MRI T1 plain scans[13]. This retrospective study aimed to address this gap by screening features from MRI T1 plain scan images that were significantly associated with MVI in HCC tumors < 3 cm in diameter. We constructed an ANN model based on MRI texture features and evaluated its performance in predicting the presence of MVI in HCC.
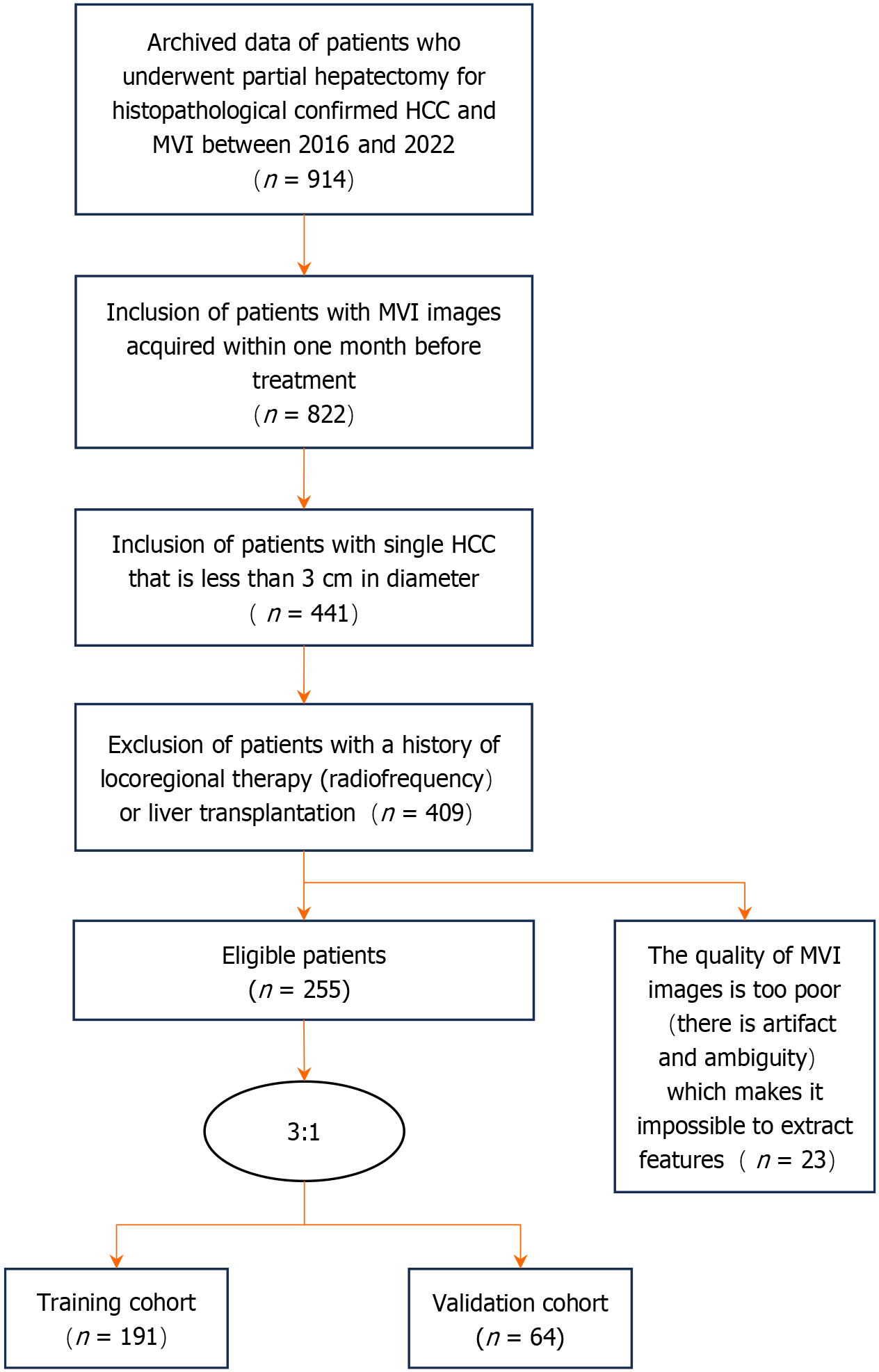
Between October 2016 and October 2022, data from patients meeting the following inclusion criteria were collected at Beijing Tsinghua Changgung Hospital: (1) Diagnosed with HCC; (2) Presence of a single lesion meeting the Milan criteria; (3) Lesion diameter < 3 cm; (4) Underwent MRI examinations within 1 month prior to surgery; (5) No prior treatment before the MRI examination; and (6) No history of other malignancies. Further exclusions included the following: (1) Patients who did not undergo hepatectomy after MRI examination; (2) Poor-quality MR images; and (3) Patients with contraindications related to MR examination (such as metal implants and claustrophobia). A total of 255 patients were included in this study, and the screening process is illustrated in Figure 1.
Among the included patients, 198 (77.6%) were men. The mean patient age was 52 years. Of the 255 patients diagnosed with HCC, 150 exhibited MVI, representing a ratio of 58.8%. The additional clinical parameters of these patients are presented in Table 1.
| Variables | MVI present (n = 150) | MVI absent (n = 105) | P value |
| Age | 52.1 | 52.0 | 0.8551 |
| Male sex, n (%) | 118 (78.70) | 80 (76.20) | 0.3822 |
| Basic condition | 0.1712 | ||
| Hepatitis C | 5 | 6 | |
| Hepatitis B | 132 | 86 | |
| Alcoholic liver | 1 | 1 | |
| Multiple | 12 | 12 | |
| AFP values | 193.8 | 195.9 | 0.1661 |
| AFP grade (+), n (%)3 | 68 (45.30) | 44 (41.20) | 0.5642 |
| Differentiation | 0.9882 | ||
| Low | 6 | 1 | |
| Medium-low | 1 | 2 | |
| Medium | 137 | 77 | |
| Medium-high | 3 | 8 | |
| High | 3 | 17 | |
| MVI positive, n (%) | 111 (58.12) | 40 (62.50) | 0.5602 |
Patients underwent MRI using 1.5T Signa HDx and 3.0T Signa HDxt magnetic resonance scanners (GE HealthCare Technologies, Chicago, IL, United States). The following sequence scan parameters corresponded to 1.5T and 3.0T, respectively: T1-weighted imaging (T1WI) plain LAVA Mask and three-dimensional dynamic enhanced scanning, with enhanced equilibrium phase coronal position: TR 3.0-4.0 ms, TE 1.5-2.0 ms, layer thickness 5.0 mm, layer spacing -2.5 mm; TR 3.0-4.0 ms, TE 1.0-1.5 ms, layer thickness 4 mm, layer spacing -2.0 mm. Notably, T1WI plain scan images were primarily used in our study. Following hepatectomy, the lesion tissue sections were examined microscopically to determine the presence of MVI based on the pathology. MVI was considered present when the number of cancer cells within the vascular lumen was ≥ 50. Subsequently, patients were categorized based on their MCI status, with those without MVI marked as 0 and those with MVI labeled as 1. All 255 patients were assessed by radiologists, and their original images were registered to facilitate feature extraction.
Radiomics entails the conversion of medical images by delineating regions of interest into high-dimensional characteristic data for analysis using computer image-processing techniques[14,15]. Through this transformation, valuable information regarding the diagnosis of MVI can be obtained.
Radiomics can quantify image features, including shapes and textures, which may not be quantifiable using other methods. Additionally, it can reflect biological characteristics such as internal and spatial heterogeneity.
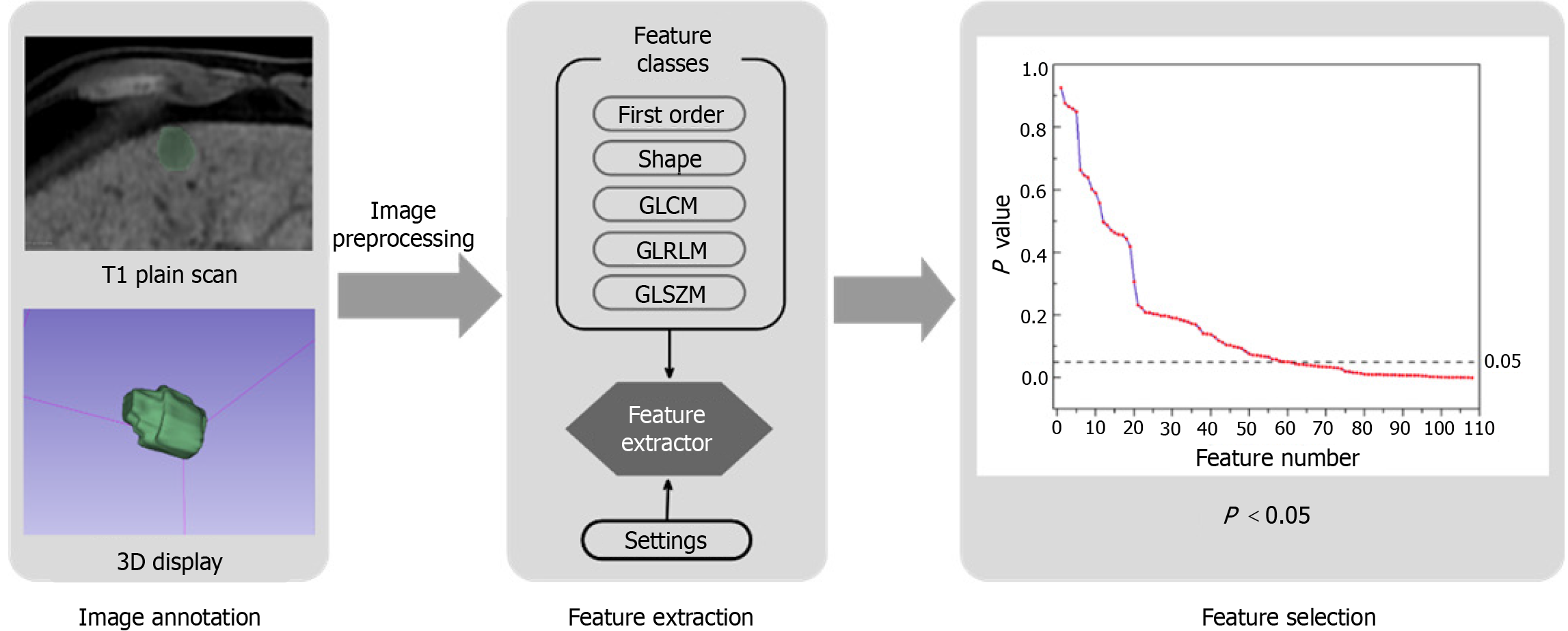
In this study, PyRadiomics (https://pyradiomics.readthedocs.io) was used for the high-throughput extraction of quantitative features. PyRadiomics is an open-source software program based on Python specifically designed for extracting radiological data from medical images[16]. Our research employed the PyRadiomics algorithm to facilitate the extraction of radiomics features. First, image loading and preprocessing tasks, such as resampling and clipping, were performed using SimpleITK. Subsequently, the loaded data were converted into a Numpy array for further evaluation using multiple classes of elements. In addition, an optional filter was integrated into the process. Notably, PyRadiomics has been successfully applied to the study of various cancers[17].
In our study, PyRadiomics facilitated the extraction of 110 dimensional features from MR images, which were classified into seven categories: First-order statistics, shape-based, gray-level co-occurrence matrix, gray-level run length matrix, gray-level size zone matrix, neighboring gray-tone difference matrix, and gray-level dependence matrix.
For the extracted features, data analysis initially involved identifying abnormal values, followed by normalization of features that met specific criteria to facilitate the training of the ANN. A bivariate Spearman test was conducted for each feature with respect to MVI to calculate the correlation and corresponding p-values. Features with P values < 0.05 were deemed significantly correlated with the MVI classification. Following this procedure on the 110-dimensional feature set, we found that 47-dimensional features exhibited a significant correlation with MVI. The screening process for the entire feature set using methods 2-2 to 2-4 can be referred to in Figure 2.
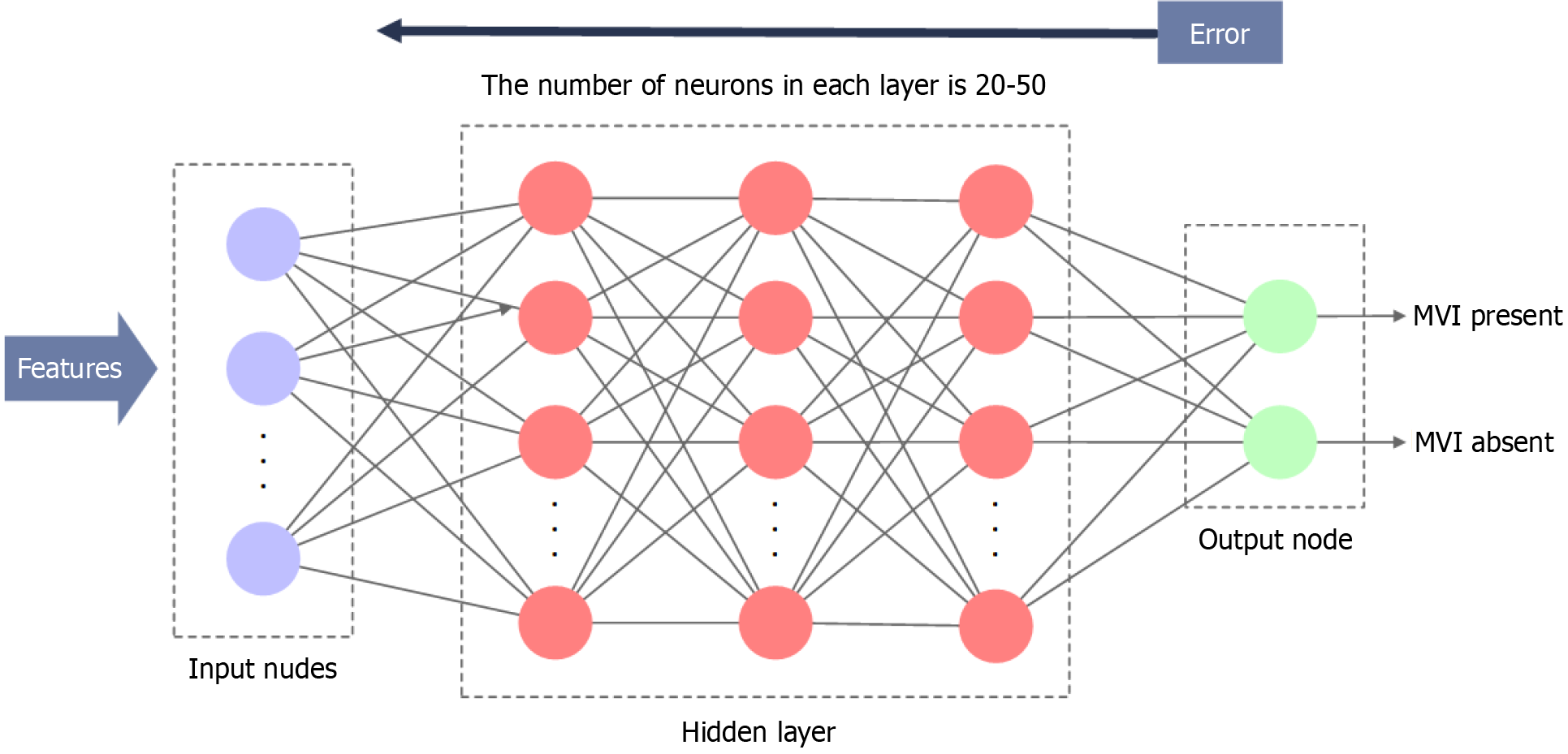
Variable features considered to be significantly correlated with the MVI in the above analysis were regarded as the input of the ANN. In the data assignment process, 5-fold cross-validation was performed. Each fold set consisted of 255 patients randomly assigned to two groups: Group A (for training, 191 patients, 75%) and Group B (for testing, 64 patients, 25%). To enhance the reliability of the results, training was performed five times, and the average area under the curve (AUC) was calculated. Moreover, an ANN comprising three hidden layers was established. Each classifier contained 47 random input neurons, and the number of neurons in each hidden layer ranged from 20 to 50 (Figure 3). This approach effectively increases the randomness of individual classifiers by biasing them toward different features. In this study, the learning rule employed was the back-propagation of errors, allowing for the adjustment of internal network parameters during repeated training cycles to reduce the overall error. Training was terminated when the sum of the squared errors reached a minimum. Subsequently, the trained neural network model was verified using a test group. Sensitivity, specificity, and the AUC were calculated and obtained as evaluation metrics.
The bagging strategy is a common algorithm used in ensemble learning that combines the predictions of multiple weak classifiers without strong dependencies. This method can enhance classifier classification results and significantly improve the generalization ability and accuracy of the model. In this study, 50 weak classifiers were trained using the ANN described in Section 2.5. Each classifier iterates the input data 1000 times to minimize the loss function. Random numbers were introduced to determine the number of training sets and neurons in each hidden layer, thereby enhancing the randomness of each classifier. Subsequently, the bagging process involves voting among the weak classifiers. If more than half of the classifier results indicated MVI (i.e., the patient was judged to have MVI), the bagging result was classified as 1; otherwise, it was classified as 0.
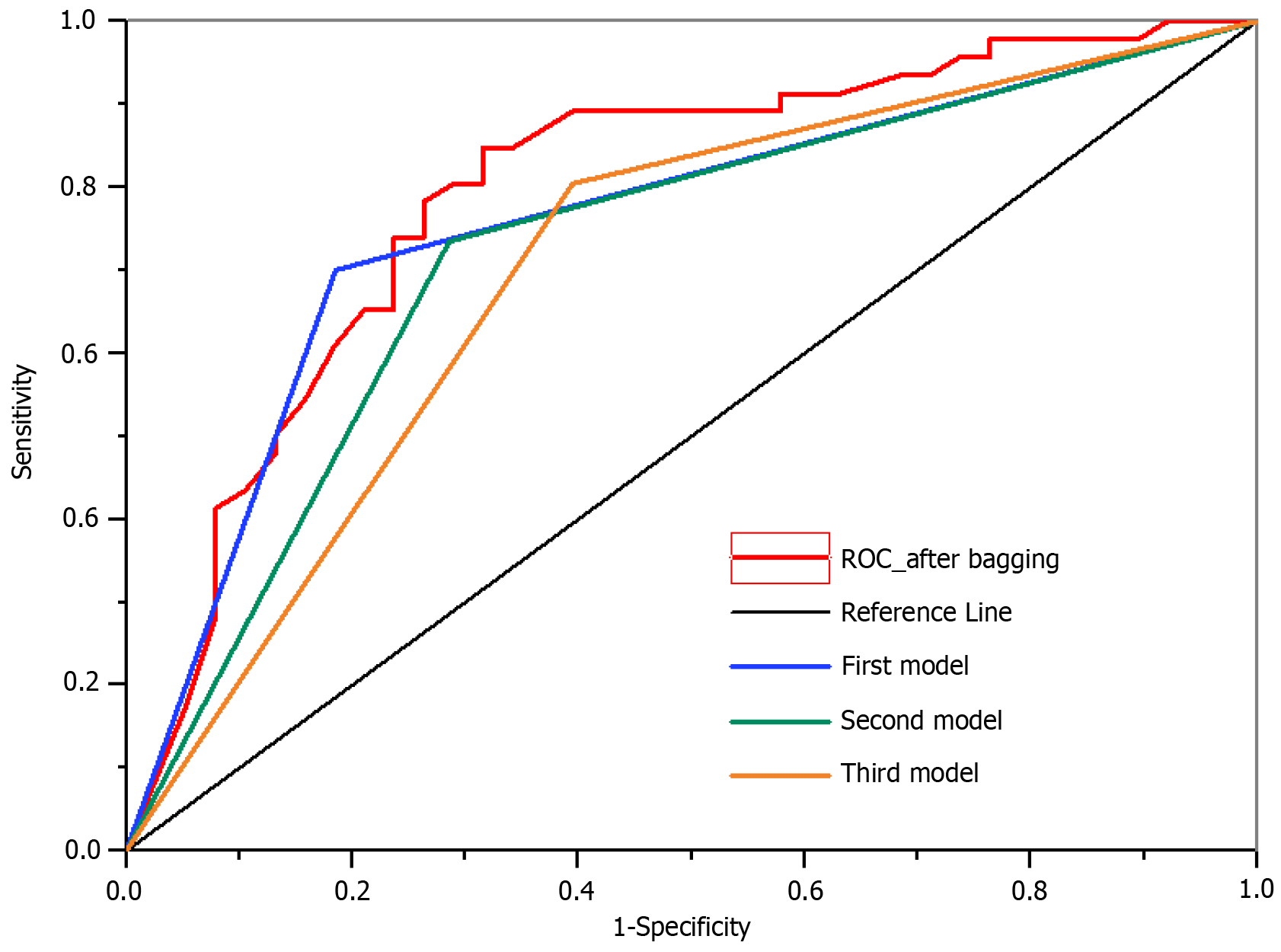
After applying the bagging technique, the AUC reached 0.79, whereas the AUC values of the top three neural network models reached 0.74, 0.73, and 0.71, respectively. Evidently, employing the bagging technique increased AUC values.
Figure 4 illustrates the results obtained after applying the bagging strategy, including metrics such as the AUC. The top three classifiers with the highest accuracies are shown in the figure.
Table 2 presents the correlations between the various indicators and the MVI. Surprisingly, tumor volume, alpha-fetoprotein (AFP) levels, and AFP classification did not correlate with MVI, contrary to the findings of previous studies. However, tumor non-roundness, calculated using the formula, where V represents tumor volume and A represents tumor surface area obtained through definite integration of the tumor-labeled mask, showed a significant correlation with MVI (P < 0.01). A non-roundness value of 1 indicates standard sphericity, with smaller values indicating greater tumor non-roundness. Moreover, non-roundness was the only relevant variable among the 16-dimensional features extracted from tumor shape. Therefore, in predicting MVI in tumors < 3 cm in diameter, more attention should be focused on tumor non-roundness than on tumor volume.
| Variables | Mean | P value |
| Male, n (%) | 198 (77.6) | 0.001a |
| Age | 52 | 0.619 |
| AFP values | 194 | 0.606 |
| AFP grades, n (%) | 112 (43.9) | 0.495 |
| Original_shape_MeshVolume | 6819.940 (mm3) | 0.104 |
| Original_shape_Sphericity | 0.734 | 0.008 |
| Original_shape_MajorAxisLength | 25.33151 (mm) | 0.113 |
HCC is the third leading cause of cancer-related mortality worldwide. The global incidence of HCC is 16 cases per 100000 individuals. Surgical resection remains the most effective treatment option for patients with HCC and has the best prognosis. However, even after successful resection, patients continue to face a high risk of postoperative HCC recurrence[18].
MVI is a powerful tool for predicting HCC recurrence after hepatic resection and transplantation[19-21]. However, MVI can only be confirmed through microscopic examination because its diagnosis surpasses the resolution of conventional imaging data. The incidence of MVI varies significantly among patients, ranging from 15.0%-57.1%. The ability of MVI to infiltrate into other branches of the portal venous system can alter tumor perfusion, thereby increasing the likelihood of metastasis and reducing progression-free survival[22,23].
Therefore, the development of a feasible, non-invasive method to diagnose MVI preoperatively is imperative, enabling surgeons to determine optimal treatment strategies for patients with HCC[24]. However, a unified clinical method for predicting MVI before surgery currently remains unavailable.
As a non-invasive preoperative examination, a computer-aided MVI diagnostic algorithm can mitigate the risks associated with invasive biopsy procedures. Moreover, it relieves psychological stress and aids doctors in formulating targeted surgical plans.
Currently, various methods are available for predicting MVI in published studies. These methods include investigating non-smooth margins on multiphasic CT images[25], observing the correlation between MVI and peritumoral hyperintensity observed on hepatobiliary phase images of gadoxetate disodium-enhanced MRI[26,27], assessing MVI in patients with small HCC using liver CT perfusion[28], and measuring apparent diffusion coefficients for lesions[29].
However, determining whether a tumor margin is smooth often relies on manual judgment, making the criteria subjective. In this study, we integrated a mask matrix with tumor labeling to obtain the volume and surface area of the tumor. Subsequently, the out-of-roundness was calculated using these parameters. This method offers greater objectivity and quantifiability in data analysis.
Moreover, gadoxetate disodium-enhanced MRI and perfusion liver CT require additional sequences, leading to extended examination times. Furthermore, the processing of such data requires complex algorithms. In contrast, our study used routine MRI T1 plain scan sequences without supplementary specialized sequences.
Jonas et al[19] reported a correlation among tumor diameter, nodule number, and histopathological classification, albeit with limited predictability for HCC vascular invasion exceeding 5 cm. Currently, studies of MVI predominantly use traditional statistical methods and require additional imaging sequences. To the best of our knowledge, studies focusing on patients with HCC with tumors < 3 cm, as well as on feature analysis and MVI prediction based solely on MRI T1 plain scan, remain lacking.
Despite the merits of this study, it also had a few limitations. This study gathered data from a single center (Beijing Tsinghua Changgung Hospital), which may affect the robustness of the constructed ANN. However, the ANN was superior to other linear models in the diagnosis of MVI. Moreover, the inherent learning ability of neural networks suggests that their performance can be enhanced by incorporating new data from more patients with HCC.
In summary, this study employed a radiomics model to extract and quantify 110-dimensional features from T1 plain MRI scans of 255 patients. Following feature analysis and screening, 47-dimensional features were identified and retained as inputs for the ANN. Subsequently, an ANN model was established to predict the MVI, achieving an AUC of 0.79, thereby exhibiting strong generalization ability owing to the bagging strategy.
| 1. | Ding J, Wang H. Multiple interactive factors in hepatocarcinogenesis. Cancer Lett. 2014;346:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 2. | Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K, Yamasaki S. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 944] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 4. | Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 6. | Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, Kim SY, Sinn DH, Kim JM, Kim K, Ha SY. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann Surg. 2021;273:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 7. | Renzulli M, Buonfiglioli F, Conti F, Brocchi S, Serio I, Foschi FG, Caraceni P, Mazzella G, Verucchi G, Golfieri R, Andreone P, Brillanti S. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. Eur Radiol. 2018;28:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Zheng J, Seier K, Gonen M, Balachandran VP, Kingham TP, D’Angelica MI, Allen PJ, Jarnagin WR, DeMatteo RP. Utility of Serum Inflammatory Markers for Predicting Microvascular Invasion and Survival for Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2017;24:3706-3714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Liu M, Wang L, Zhu H, Rong W, Wu F, Liang S, Xu N, Wu J. A Preoperative Measurement of Serum MicroRNA-125b May Predict the Presence of Microvascular Invasion in Hepatocellular Carcinomas Patients. Transl Oncol. 2016;9:167-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 12. | Yadav N, Yadav A, Kumar M, Kim JH. An efficient algorithm based on artificial neural networks and particle swarm optimization for solution of nonlinear Troesch’s problem. Neural Comput Appl. 2017;28:171-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Wang F, Yan CY, Qin Y, Wang ZM, Liu D, He Y, Yang M, Wen L, Zhang D. Multiple Machine-Learning Fusion Model Based on Gd-EOB-DTPA-Enhanced MRI and Aminotransferase-to-Platelet Ratio and Gamma-Glutamyl Transferase-to-Platelet Ratio to Predict Microvascular Invasion in Solitary Hepatocellular Carcinoma: A Multicenter Study. J Hepatocell Carcinoma. 2024;11:427-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 3846] [Article Influence: 295.8] [Reference Citation Analysis (2)] |
| 15. | van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 3897] [Article Influence: 487.1] [Reference Citation Analysis (0)] |
| 16. | Xiao DD, Yan PF, Wang YX, Osman MS, Zhao HY. Glioblastoma and primary central nervous system lymphoma: Preoperative differentiation by using MRI-based 3D texture analysis. Clin Neurol Neurosurg. 2018;173:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Lai SL, Chen J, Xie D, Wu FX, Jin GQ, Su DK. Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J Gastroenterol. 2017;23:6467-6473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, Regimbeau JM, Nagorney DM, Do KA, Ellis LM, Curley SA, Pollock RE, Vauthey JN. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722-30; discussion 730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 20. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. [PubMed] |
| 21. | Simpson-Herren L, Noker PE, Wagoner SD. Variability of tumor response to chemotherapy. I. Contribution of host heterogeneity. Cancer Chemother Pharmacol. 1987;20:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 563] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014;203:W253-W259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 25. | Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 26. | Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Wu D, Tan M, Zhou M, Sun H, Ji Y, Chen L, Chen G, Zeng M. Liver computed tomographic perfusion in the assessment of microvascular invasion in patients with small hepatocellular carcinoma. Invest Radiol. 2015;50:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol. 2014;29:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Zhou M, Shan D, Zhang C, Nie J, Wang G, Zhang Y, Zhou Y, Zheng T. Value of gadoxetic acid-enhanced MRI for microvascular invasion of small hepatocellular carcinoma: a retrospective study. BMC Med Imaging. 2021;21:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |