Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2528
Revised: May 12, 2024
Accepted: July 11, 2024
Published online: August 27, 2024
Processing time: 151 Days and 11.7 Hours
The abdominal perineal resection (APR), historically referred to as Mile’s proce
To evaluate the value of Lone-Star retractor (LSR) perineal exposure method in the treatment for laparoscopic APR of rectal cancer.
We reviewed the records of 38 patients with rectal cancer at Anqing Municipal Hospital from January 2020 to December 2023, including 20 patients who underwent the APR procedure with a LSR perineal exposure method (LSR group) and 18 patients who underwent the APR procedure with a conventional perineal exposure method (control group). In the LSR group, following incision of the skin and subcutaneous tissue, the LSR was placed and dynamically adjusted according to the surgical plane to fully expose the perineal operative field.
A total of 38 patients underwent laparoscopic APR, none of whom were found to have distant metastasis upon intraoperative exploration. Perineal blood loss, the postoperative hospital stays and the wound pain scores in the LSR group were significantly lower than those in the control group. A single surgeon completed the perineal operation significantly more often in the LSR group than in the control group (P < 0.05). The incidence of infection via the perineal incision in the LSR group was significantly lower than that in the control group (P < 0.05). No cases of distant metastasis or local recurrence were found among the patients at the postoperative follow-up.
The application of the LSR technique might be helpful for performing perineal exposure during APR for rectal cancer and could reduce the incidence of perineal complications, shorten the postoperative hospital stay, improve postoperative pain, and allow one surgeon to perform the perineal operation.
Core Tip: Abdominal perineal resection (APR) is an important method for the treatment of low rectal cancer. However, optimal perineal exposure stands as a pivotal phase in APR, given that the precision of this maneuver is directly correlated with surgical safety and long-term prognosis. Herein, we introduced the application of the Lone-Star retractor (LSR) method in APR. According to our study, the application of the LSR technique might be helpful for perineal exposure during APR for rectal cancer, which could reduce the incidence of perineal complications, shorten the postoperative hospital stay, improve postoperative pain, and achieve a one-person perineal operation.
- Citation: Ma J, Tang DB, Tang YQ, Wang DT, Jiang P, Zhang YM. Lone-Star retractor perineal exposure method for laparoscopic abdominal perineal resection of rectal cancer. World J Gastrointest Surg 2024; 16(8): 2528-2537
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2528.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2528
Colorectal cancer ranks as the fourth leading cause of cancer-related mortality globally, claiming nearly 900 thousand lives annually[1]. Notably, there has been a concerning rise in the incidence of rectal cancer among younger demographics[2]. The abdominal perineal resection (APR), historically referred to as Mile’s procedure, stands as a time-honored surgical intervention for rectal cancer management[3]. Advancements in surgical techniques and the advent of neoadjuvant therapies have significantly improved the rate of sphincter preservation among patients afflicted with rectal cancer, including those with ultralow rectal cancer[4-8]. Despite these improvements, APR maintains its irreplaceable role in the clinical landscape, particularly for cases involving low rectal cancer with encroachment on the external anal sphincter or levator ani muscles, where achieving safe margins via low anterior resection (LAR) is not feasible.
The success of APR is fundamentally contingent upon achieving excellent perineal exposure. Initially, patients are positioned in lithotomy with complete hip abduction, a maneuver that facilitates full visualization of the operative site and enables a two-surgeon team to effectively conduct perineal procedures. Nonetheless, this positioning can be problematic for elderly male patients or those with hip pathologies, who may find it challenging to achieve the necessary leg abduction. In some medical institutions, surgeons may opt to reposition the patient to a prone jackknife position during perineal operations, an attempt to enhance exposure of the surgical field. However, this approach, while potentially beneficial, is viewed by the authors as cumbersome and as introducing additional time and the potential for increased perioperative risks. Finally, to expose the operative field, the assistant often needs to suspend his or her arms, which not only can lead to significant postoperative discomfort in the arms and shoulders but also prevents tractional continuity.
The Lone-Star retractor (LSR), manufactured by Cooper Surgical Inc. in Connecticut, United States, has earned a reputation for its user-friendly design and dependable performance in facilitating optimal exposure of pertinent surgical anatomy. Its versatility has been harnessed across a spectrum of medical disciplines, including pediatric surgery, hemorrhoidectomy, transanal Total Mesorectal Excision, intersphincteric resection, Natural Orifice Specimen Extraction Surgery, stoma reduction, and neurosurgery[9-15]. Despite its widespread application, the utilization of the LSR for APR has not yet been documented in the literature. In this context, we present a novel application of the LSR method for perineal exposure.
We conducted a comprehensive review of medical records from 38 rectal cancer patients who underwent APR at Anqing Municipal Hospital, encompassing a period from January 2020 through December 2023, among whom 20 patients had their perineum exposed using a LSR method (LSR group); the other 18 patients with rectal cancer underwent the APR procedure with a conventional perineal exposure method and were included as controls (control group).
Our research methodology was designed to honor the principles outlined in the 1964 Declaration of Helsinki, as well as its subsequent revisions. Prior to the commencement of the study, it received formal approval from the Medical Ethics Committee of Anqing Municipal Hospital, as evidenced by the reference number 2023.058. Informed consent was obtained from all participants.
Each patient enrolled in the study underwent a battery of standard preoperative assessments to ensure accurate tumor staging, diagnosis, and localization. This comprehensive evaluation included a colonoscopy to visually inspect the colon and rectum, magnetic resonance imaging for detailed soft tissue assessment, and computed tomography to scan for potential metastatic spread.
The definitive diagnosis of rectal cancer was established through a pathological examination that provided histological confirmation. Furthermore, an intraoperative exploration was conducted to rigorously exclude the presence of distant metastases, including those involving the liver or the peritoneum.
The inclusion criteria included a confirmed diagnosis of low rectal cancer, ascertained through a combination of pathological assessment and imaging studies, with the stipulation that the inferior margin of the lesion must be within a distance of less than 6 cm from the anal verge. Furthermore, candidates had to meet at least one of the following conditions to be included in the study: (1) Advanced local tumor staging coupled with a patient’s decision to decline neoadjuvant therapy; (2) A positive distal resection margin following LAR; and (3) The presence of a lesion that invades or is in close proximity to the external anal sphincter or levator ani muscles.
The exclusion criteria were as follows: (1) General conditions or other comorbidities that rendered a patient intolerant to general anesthesia and unsuitable for surgical treatment; (2) Age less than 18 years; (3) Preoperative examination and intraoperative exploration of distant tumor metastases that was unable to achieve radical resection; (4) Simultaneous combined organ resection or pelvic lymph dissection; (5) The need to undergo emergency surgery for obstruction or bleeding; and (6) A previous history of major abdominal or perineal surgery.
The following clinicopathological parameters were retrieved: Patient age, sex, body mass index (BMI), maximum tumor diameter, distance of the tumor from the anal verge, neoadjuvant therapy, preoperative hemoglobin, preoperative prealbumin, preoperative comorbidities, tumor stage (both clinical and pathological), number of dissected lymph nodes, operation time, intraoperative blood loss, postoperative anal exhaust time, postoperative hospitalization time, specimen perforation, circumferential resection margin (CRM), number of operators needed to perform the perineal operations and postoperative complications. BMI was calculated as weight/height squared and is depicted in kg/m2. Preoperative comorbidities included hypertension, diabetes, chronic obstructive pulmonary disease, heart disease, cerebrovascular disease, and immune system diseases. The numerical rating scale score was used for the wound pain score. Tumor staging was performed with the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system[16].
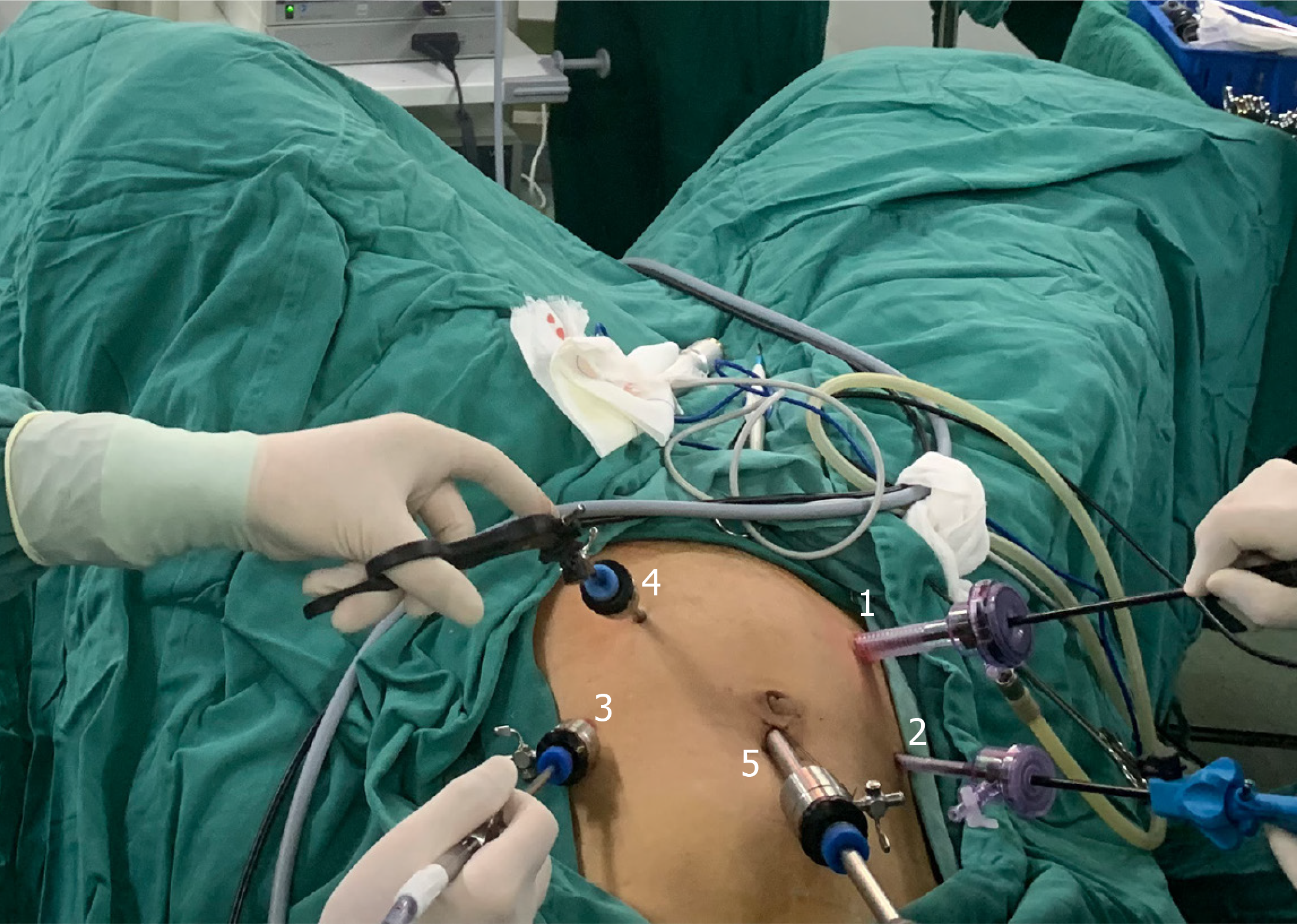
Following the induction of general anesthesia, patients were carefully positioned in the lithotomy posture, a standard practice that facilitates the abdominal phase of the procedure. The surgical approach was executed using the conventional five-hole technique, as depicted in Figure 1.
The surgeon laparoscopically detached the left Toldt’s fascia and ligated and severed the inferior mesenteric artery at its root and the inferior mesenteric vein distally from the junction of the left colonic vein.
Subsequently, the sigmoid mesentery was dissected along the course of the marginal vascular arch, extending 10 centimeters above the tumor margin. Following this, the rectum underwent posterior dissection, meticulously following the plane of the posterior rectal space, extending all the way to the tip of the coccyx according to the TME principle.
The peritoneal incision was executed anteriorly, forming an arcuate incision 1 cm superior to the peritoneal reflex line. This precise incision facilitated the subsequent dissection, which was carried out to the lower boundary of the seminal vesicle glands in males and to the inferior edge of the uterine cervix in females.
The lateral rectum was next dissected to the inferior border of the pelvic autonomic nerves bilaterally. Finally, the rectum was dissected to the striated muscles of the levator ani, and the proximal sigmoid colon was excised with one firing of a 60 mm linear stapler.
Continuing the surgical procedure, the lateral aspects of the rectum were carefully dissected down to the lower boundary of the pelvic autonomic nerves on both sides. Finally, the rectum was dissected to the striated muscles of the levator ani, and the proximal sigmoid colon was excised with one firing of a 60 mm linear stapler.
After the sigmoid colon was excised, the surgeon moved to the perineum to perform the perineal operation, while two other assistants completed closure of the pelvic floor peritoneum and the colostomy.
The anus was closed using the purse-string suture method. Then, a circular incision was made along the anus from the tip of the coccyx at the posterior border to the perineal center point anteriorly and 2 cm from the anal verge laterally.
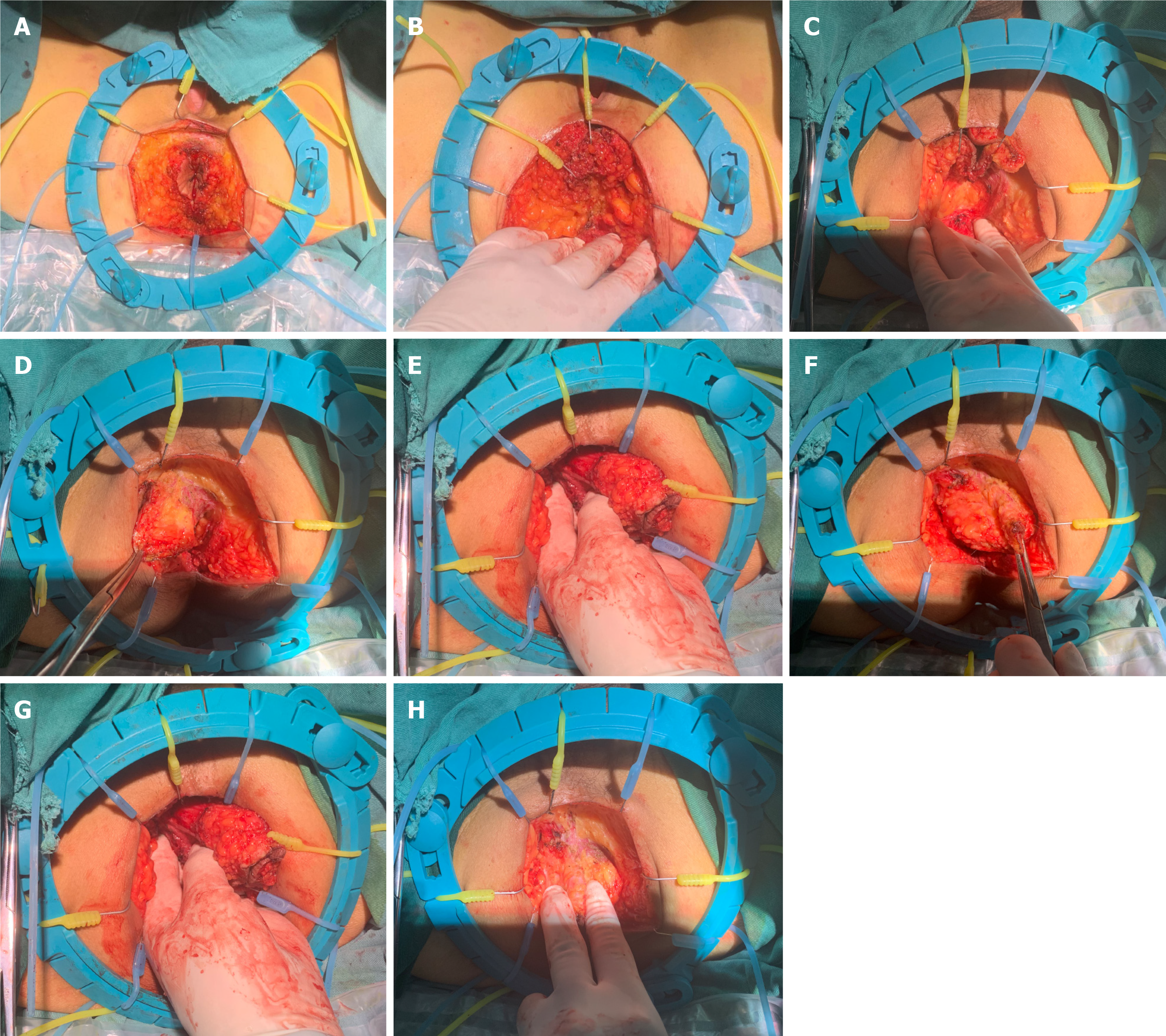
Following the initial incision through the skin and subcutaneous layers, the LSR system was strategically positioned to optimize exposure of the surgical field. The incision margins were then effectively retracted using eight small hooks, which were evenly distributed around the incision site (Figure 2A).
When dissecting the posterior tissues to the caudal anal ligament, three small anterior hooks were employed to retract the anal canal tissue toward 12 o’clock position. Subsequently, the surgeon executed a symmetrical retraction of the posterior fat tissue in the opposing direction to fully expose the posterior surgical field (Figure 2B and C).
Upon incising the caudal anal ligament to establish continuity with the pelvic cavity, the surgeon introduced the left hand into the pelvic space. Employing the index and middle fingers as a reliable anatomical landmark, the surgeon continued the incision along the levator ani muscles.
In our standard surgical approach, we systematically initiate the dissection with the left levator ani muscle before proceeding to the right levator ani. This sequential strategy is predicated on the ergonomic advantage it offers: During the dissection of the left levator ani, the surgeon’s left thumb can effectively push the rectal tissue towards 9 o’clock position, thereby enhancing visibility and facilitating the execution of the surgical technique (Figure 2D and E).
Having completed the dissection of the posterior and left-sided tissues, the surgeon advanced to the incision of the right levator ani, as depicted in Figure 2F and G. It is of particular importance to address the vascular structures located at the 1 and 11 o’clock positions prior to the incision.
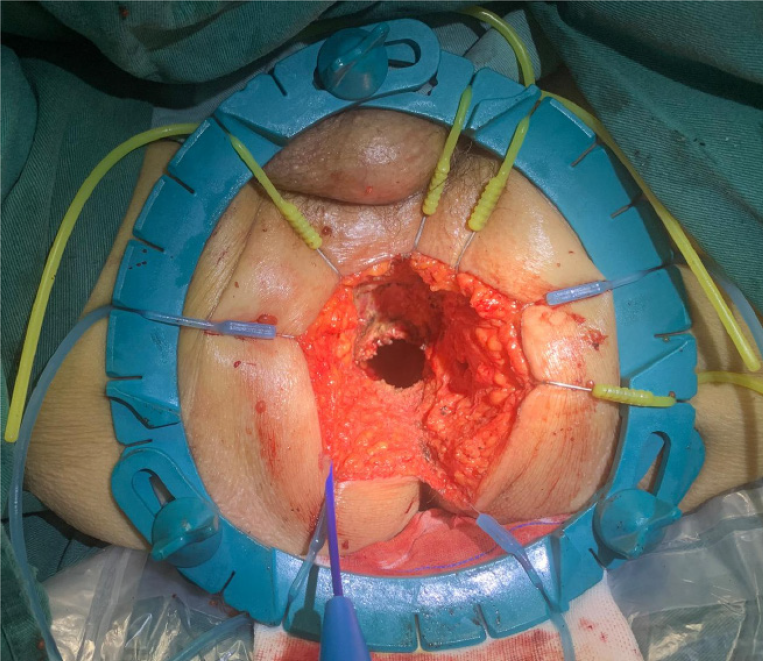
In the final phase of the procedure, the detachment of the anterior rectal wall tissue was the sole remaining task, as illustrated in Figure 2H. The surgeon carefully positioned the residual attached tissue between the index finger and thumb of the left hand, exerting gentle traction to orient the tissue towards the 6 o’clock position. This maneuver facilitated the completion of the anterior dissection with precision. Upon the extraction of the specimen, as shown in Figure 3, we flushed and placed the drainage tube. Subsequently, the perineal incision was closed.
After the sigmoid colon was excised, the surgeon and an assistant moved to the perineum to perform the perineal operation, while two other assistants completed closure of the pelvic floor peritoneum and the colostomy.
In the control group, the operation needed to be performed by two people; otherwise, the perineal skin and subcutaneous tissue incisions were the same as those in the LSR group, and the surgical dissection was performed from the posterior wall to the lateral wall and finally to the anterior wall. First, the posterior wall was aligned with the tip of the tailbone, and the adipose tissue was incised to reach the tip of the tailbone, which was freed from the abdominal cavity. Second, the lateral wall was dissected with an electric scalpel to incise the tissues of the ischiorectal fossa, and then the tissues were dissected upward along the inner side of the gluteus maximus muscle. Finally, the anterior wall was dissected to incise the superficial transversus and deep transversus of the perineum muscle; the incision was then advanced to reach the urethral bulb followed by the lower edge of the prostate gland, completing the circumferential dissection of the pelvic diaphragm. During the above operations, the assistant used their fingers, vascular forceps and an army-navy retractor to cooperate with the surgeon’s maneuvers for exposing the perineum, completing the perineal excision, and finally, flushing and placing the drainage tube.
Data analysis was performed utilizing SPSS software, version 26.0. Continuous variables exhibiting normal distribution were reported as the mean ± SD, whereas data that deviated from normality were presented as the median with interquartile ranges (IQR). For the statistical comparison of continuous variables between the two groups, we employed the independent samples t-test for parametric data and the rank-sum test (Mann-Whitney U test) for nonparametric data, based on their distribution characteristics. Categorical data were displayed as frequency counts and percentages, with group comparisons made using the chi-square test or Fisher’s exact test, where appropriate. The threshold for statistical significance was set at P < 0.05.
The purpose of this study was to evaluate whether the LSR exposure method could allow a single person to perform the perineal operation. The sample size was determined based on the results of a pilot study. For a power of 80% and a type I error of 0.05, our calculations indicated that at least 11 subjects were required for each group to detect a significant difference in the completion rate for a single-person perineal operation.
A comparative analysis of the demographic and clinicopathological characteristics of the study participants, categorized into two groups, is detailed in Table 1. An initial assessment of the baseline data revealed no statistically significant disparities between the groups in terms of age, gender, tumor stage, or other clinical parameters (P > 0.05 for all comparisons). A total of 38 patients underwent laparoscopic APR (20 patients in the LSR group, 18 patients in the control group), none of whom had ascites, peritoneal metastasis or liver metastasis according to the intraoperative exploration
| LSR group | Control group | Value of statistics | P value | |
| n = 20 | n = 18 | |||
| Age (year) | 67.00 ± 9.11 | 66.89 ± 9.46 | t = 0.037 | 0.971 |
| Sex, n (%) | F | 1.000 | ||
| Male | 14 (70.0) | 13 (72.2) | ||
| Female | 6 (30.0) | 5 (27.8) | ||
| Body mass index (kg/m2) | 23.19 ± 3.81 | 22.45 ± 3.62 | t = 0.619 | 0.540 |
| Distance of the tumor from the anal verge (cm) | 3.00 (2.00, 4.00) | 3.75 (3.00, 4.00) | z = 1.634 | 0.102 |
| Tumor size (cm) | 4.25 (3.58, 4.25) | 4.00 (3.00, 6.00) | z = 0.266 | 0.791 |
| Neoadjuvant therapy, n (%) | F | 0.606 | ||
| Yes | 3 (15.0) | 1 (5.6) | ||
| No | 17 (85.0) | 17 (94.4) | ||
| Clinical stage, n (%) | χ2 = 1.826 | 0.401 | ||
| I | 5 (25.0) | 8 (44.4) | ||
| II | 9 (45.0) | 5 (27.8) | ||
| III | 6 (30.0) | 5 (27.8) | ||
| IV | 0 (0.0) | 0 (0.0) | ||
| Preoperative comorbidities, n (%) | - | 0.516 | ||
| Yes | 12 (60.0) | 8 (44.4) | ||
| No | 8 (40.0) | 10 (55.6) | ||
| Preoperative hemoglobin | 114.05 ± 17.18 | 120.89 ± 16.26 | t = 1.257 | 0.217 |
| Preoperative prealbumin | 256.40 ± 67.50 | 227.18 ± 59.39 | t = 1.410 | 0.167 |
A comprehensive comparison of the surgical outcomes between the LSR and control groups is presented in Table 2. The LSR group demonstrated significantly reduced perineal blood loss, shorter postoperative hospital stays, and lower wound pain scores, indicating an enhanced recovery profile compared to the control group (P < 0.05). Furthermore, the LSR group achieved a higher rate of single-operator perineal surgery completion, underscoring the efficiency of the LSR method (P < 0.05). No significant intergroup differences were observed regarding operation time, perineal operation duration, total blood loss, lymph node yield, timing of the first postoperative bowel movement, specimen perforation rate, pathological staging, or the incidence of positive CRMs (all P > 0.05).
| LSR group | Control group | Value of statistics | P value | |
| n = 20 | n = 18 | |||
| First postoperative bowel movement (days) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | z = 0.121 | 0.904 |
| Total blood loss | 75.00 (50.00, 100.00) | 100.00 (87.50, 200.00) | z = 1.647 | 0.100 |
| Perineal blood loss | 30.00 (20.00, 50.00) | 50.00 (35.00, 132.50) | z = 2.051 | 0.040 |
| Postoperative hospital length of stay (days) | 9.00 (7.00, 11.75) | 13.00 (10.00, 17.00) | z = 2.953 | 0.003 |
| Operation time (minutes) | 250.00 ± 68.71 | 261.94 ± 34.73 | t = 0.664 | 0.511 |
| Perineal operation time (minutes) | ||||
| Number of harvested lymph nodes | 14.05 ± 5.28 | 12.06 ± 4.76 | t = 1.218 | 0.231 |
| Wound pain score | 4.00 (3.00, 5.00) | 6.00 (4.75, 6.00) | z = 2.914 | 0.004 |
| Specimen perforation | F | 0.474 | ||
| Yes | 0 (0.0) | 1 (5.6) | ||
| No | 20 (100.0) | 17 (94.4) | ||
| Number of people needed for the perineal operation | F | 0.000 | ||
| One | 18 (90.0) | 0 (0.0) | ||
| Two | 2 (10.0) | 18 (100.0) | ||
| Pathological stage, n (%) | χ2 = 2.712 | 0.258 | ||
| I | 5 (25.0) | 9 (50.0) | ||
| II | 8 (40.0) | 4 (22.2) | ||
| III | 7 (35.0) | 5 (27.8) | ||
| IV | 0 (0.0) | 0 (0.0) | ||
| CRM | F | 0.474 | ||
| Positive | 0 (0.0) | 1 (5.6) | ||
| Negative | 20 (100.0) | 17 (94.4) |
The comparison of postoperative complications between the observation and control groups is summarized in Table 3. The incidence of infection via the perineal incision in the LSR group was significantly lower than that in the control group (P < 0.05). There were no significant differences between the two groups in terms of the overall incidence of postoperative complications, perineal wound dehiscence, pelvic infection, intestinal obstruction, stoma mucocutaneous separation or pneumonia (all P > 0.05).
| LSR group | Control group | Value of statistics | P value | |
| n = 20 | n = 18 | |||
| Overall postoperative complications1, n (%) | 2 (10.0) | 7 (38.9) | F | 0.058 |
| Perineal wound infection | 0 (0.0) | 5 (27.8) | 0.017 | |
| Perineal wound dehiscence | 0 (0.0) | 2 (11.1) | 0.218 | |
| Pelvic infection | 0 (0.0) | 1 (5.6) | 0.474 | |
| Intestinal obstruction | 0 (0.0) | 1 (5.6) | 0.474 | |
| Stoma mucocutaneous separation | 1 (5.0) | 0 (0.0) | 1.000 | |
| Pneumonia | 1 (5.0) | 0 (0.0) | 1.000 |
All patients were subjected to a series of blood tests and imaging studies at intervals of three to six months. The most recent follow-up data, collected as of January 2024, indicate that there have been no detected cases of either metastasis or tumor recurrence among the patient’s cohort postoperatively.
The advent of self-retaining retractors in surgical practice dates back to 1964, marking a significant advancement in the field with their initial application aimed at enhancing exposure of the abdominal cavity. These retractors have since been valued for their sustained performance, economic viability, and their capacity to provide optimal visualization of the surgical field[17]. The LSR, with its technological refinements, has expanded its utility across a spectrum of surgical interventions. In our clinical experience, the LSR method has emerged as a key technique for achieving perineal exposure in APR procedures.
The LSR method offers distinct advantages for perineal exposure during rectal cancer resection, which are delineated as follows: (1) The retractor’s adjustability is a key feature, enabling surgeons to achieve comprehensive visualization of the operative field throughout the perineal phase of the surgery. This adaptability is particularly advantageous for complex pelvic surgeries, including those involving male patients, as it aids in the precise intraoperative localization of critical structures. This precision significantly mitigates the risk of surgical complications and the occurrence of anterior wall perforations. In our study, there was significantly less intraoperative perineal bleeding in the LSR group than in the control group, and no perforated specimens were obtained in the LSR group, demonstrating the superiority of the LSR method; (2) The LSR exposure method offers a significant logistical advantage in the surgical workflow: It enables two assistants to undertake the colostomy procedure following the completion of the abdominal phase. Concurrently, the surgeon can seamlessly transition to the perineal operation without necessitating the presence of additional surgical personnel. The LSR exposure method not only enhances the operational efficiency but also streamlines the surgical team’s requirements, thereby optimizing both time management and resource allocation within the operating theater; and (3) The LSR exposure method has demonstrated its utility in enabling perineal surgery to be conducted by a single operator. In our study, the LSR group exhibited a notable trend: 18 procedures were adeptly completed by a single surgeon, with only two instances requiring the assistance of a second operator due to intraoperative bleeding. Our analysis indicates that the intraoperative ligation of the lateral anal artery is a critical step, serving as a pivotal strategy to mitigate active hemorrhage.
There are several advantages of single-person perineal operations. Firstly, because of the confined operating space, the necessity for assistants to maintain arm suspension over prolonged periods can lead to significant postoperative discomfort in the arms and shoulders. By eliminating the need for a second assistant, single-operator procedures alleviate this issue. Secondly, in cases where patients are unable to achieve full leg abduction, the limited perineal surgical area accommodates only a single operator, thereby optimizing the utilization of the LSR. Lastly, the LSR exposure method eliminates the need for intraoperative repositioning, thereby avoiding the surgical and cardiovascular risks associated with position changes and significantly reducing the overall operative duration.
In the LSR group, no positive CRMs or perforated specimens were obtained, and no severe postoperative complications or events necessitating reoperation occurred. The number of harvested lymph nodes in the LSR group was similar to that in the control group, which showed that the LSR exposure method significantly reduced the incidence of surgical complications without compromising the quality of the procedure.
Because of the low incidence of postoperative perineal complications in the LSR group, the patients in this group had shorter postoperative hospital stays. The following reasons may explain the significantly lower incidence of perineal complications in the LSR group: (1) Good intraoperative exposure led to a clear field of vision, less intraoperative perineal bleeding, and less postoperative wound exudation, all of which reduced the chances of infection; and (2) In the control group, the need for two persons to perform the perineal operation limited the available operative space, which may have increased the risk of intraoperative contamination.
There were no visible abdominal incisions during laparoscopic APR, and postoperative pain mainly originated from the perineal incision. In our study, patients in the LSR group had lower postoperative pain scores. With the LSR exposure method, we considered the surgery easier to perform, with no need to repeatedly adjust the retractor or use a violent technique. As a result, patients had less intraoperative tissue damage and experienced less postoperative wound pain.
When employing the LSR exposure method in perineal surgery, several critical considerations must be observed to ensure procedural safety and efficacy. Firstly, the surgeon must exercise caution to prevent accidental injury due to the sharp head ends of the small hooks, particularly during the process of their placement and removal. Secondly, to minimize the exceedingly rare risk of tumor implantation from incision, care should be taken to avoid contact between the hook ends and the tumor tissue[18]. Lastly, to safeguard against potential intraoperative vascular injuries, surgeons should be vigilant in avoiding direct traction on blood vessels. In cases where additional protection is warranted, the application of a gauze overlay can provide a barrier against inadvertent side injuries.
The present study has several limitations. Long-term follow-up assessments are needed, and overall survival and disease-free survival rates need to be evaluated. Furthermore, we had hoped to control for confounders with multi
In a word, the application of the LSR technique might be helpful for performing perineal exposure during APR for rectal cancer and could reduce the incidence of perineal complications, shorten the postoperative hospital stay, improve postoperative pain, and allow one surgeon to perform the perineal operation.
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9919] [Article Influence: 4959.5] [Reference Citation Analysis (2)] |
| 2. | Lang D, Ciombor KK. Diagnosis and Management of Rectal Cancer in Patients Younger Than 50 Years: Rising Global Incidence and Unique Challenges. J Natl Compr Canc Netw. 2022;20:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 3. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1935] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 5. | Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients’ survival. Br J Surg. 1983;70:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 370] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Wolmark N, Fisher B. An analysis of survival and treatment failure following abdominoperineal and sphincter-saving resection in Dukes’ B and C rectal carcinoma. A report of the NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. Ann Surg. 1986;204:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys. 1998;42:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Marks JH, Myers EA, Zeger EL, Denittis AS, Gummadi M, Marks GJ. Long-term outcomes by a transanal approach to total mesorectal excision for rectal cancer. Surg Endosc. 2017;31:5248-5257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Knox A, Harrop AR. Lone star retractor for pediatric hand surgery. Plast Reconstr Surg. 2011;128:36e-37e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Durai R, Makhija R. Anal retraction sutures as an alternative to Lone Star® retractor. Ann R Coll Surg Engl. 2015;97:477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Carriero A, Dal Borgo P, Pucciani F. Stapled mucosal prolapsectomy for haemorrhoidal prolapse with Lone Star Retractor System. Tech Coloproctol. 2001;5:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Glaysher MA, Moran BJ. Reversal of ileostomy utilising Lone Star Retractor System™. Tech Coloproctol. 2014;18:1125-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Piatkowski J, Jackowski M, Nowak M, Szeliga J. TaTME: 2 Years of Experience of a Single Center. Surg Laparosc Endosc Percutan Tech. 2019;29:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Chen C, Chen H, Yang M, Wu X, Yuan X, Zhu C, Han Y, Yin L. Laparoscopy-Assisted Natural Orifice Specimen Extraction to Treat Tumors of the Sigmoid Colon and Rectum: The Short- and Long-Term Outcomes of a Retrospective Study. J Laparoendosc Adv Surg Tech A. 2019;29:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Piazza A, Ricciardi L, Trungu S, Forcato S, di Bartolomeo A, Scerrati A, Miscusi M, Raco A. The Lone Star Retractor System in Neurosurgery. World Neurosurg. 2021;153:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 17. | Thompson RC. Self-retaining retractor support. New applications. Calif Med. 1964;101:277-279. [PubMed] |
| 18. | van Lieshout AS, Grüter AAJ, Smits LJH, Tanis PJ, Tuynman JB. Local recurrence at the site of the Lone Star device through implantation of exfoliated cells during local excision for early rectal cancer: A case report. Int J Surg Case Rep. 2022;93:106891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |