Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2521
Revised: May 24, 2024
Accepted: July 4, 2024
Published online: August 27, 2024
Processing time: 137 Days and 1.5 Hours
Gastric cancer is one of the most common malignant tumors worldwide, and surgical resection is one of the main ways to treat gastric cancer. However, the immune status of postoperative patients is crucial for prognosis and survival, and immune cells play an important role in this process. Therefore, it is helpful to understand the immune status of postoperative patients by evaluating the levels of peripheral blood immune cells, especially total T cells (CD3+), helper T cells (CD3+CD4+), and suppressor T cells (CD3+CD8+), and its relationship to sur
To analyzed the immune cells in peripheral blood of patients with gastric cancer after surgery, detect the levels of total T cells, helper T cells and suppressor T cells.
A total of 58 patients with gastric cancer who received surgical treatment were included in the retrospective study. Flow cytometry was used to detect the level of peripheral blood immune cells and analyze the correlation between total T cells, helper T cells and inhibitory T cells. To explore the relationship between these immune markers and patient survival.
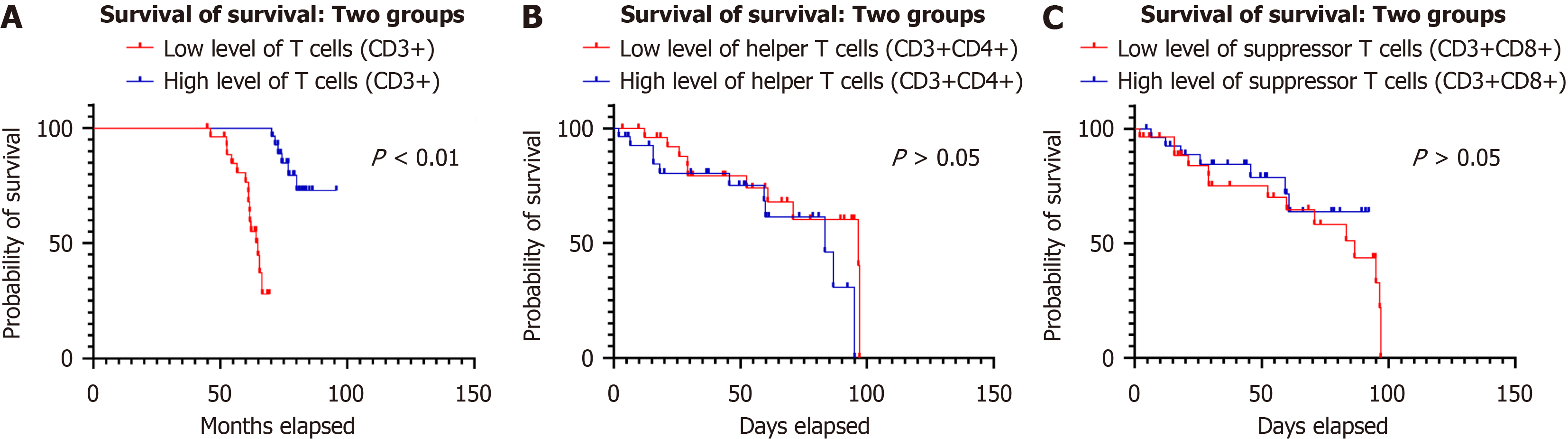
The results showed that the levels of total T cells, helper T cells, and suppressor T cells changed in patients after gastric cancer surgery. There was a significant positive correlation between total T cells, helper T cells and suppressor T cells (r = 0.35, P < 0.01; r = 0.56, P < 0.01). However, there was a negative correlation between helper T cells and suppressor T cells (r = -0.63, P < 0.01). Follow-up showed that the survival rate of patients in the high-level total T cell group was significantly higher than that in the low-level group (28.87 ± 24.98 months vs 18.42 ± 16.21 months). The survival curve shows that the curve of patients in the high-level group is shifted to the upper right, and that of the low-level group is shifted downward. There was no significant difference between the levels of helper T cells and suppressor T cells and patient survival time.
By detecting peripheral blood immune cells with flow cytometry, we can initially evaluate the immune status of patients after gastric cancer surgery and initially explore its relationship with patient survival.
Core Tip: Gastric cancer is one of the most common malignancies worldwide and one of the leading causes of cancer-related death. To detect immune cells in the peripheral blood of patients after gastric cancer surgery by flow cytometry, evaluate the levels of total T cells (CD3+), helper T cells (CD3+CD4+), and suppressor T cells (CD3+CD8+), and analyze them. By detecting peripheral blood immune cells with flow cytometry, we can initially evaluate the immune status of patients after gastric cancer surgery and initially explore its relationship with patient survival. This provides a certain clinical basis for personalized immune monitoring and intervention, and helps guide patient treatment and management.
- Citation: Wang QW, Zhu JW, Gong LZ. Clinical significance of peripheral blood immune cells in patients with gastric cancer after surgery. World J Gastrointest Surg 2024; 16(8): 2521-2527
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2521.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2521
Gastric cancer is one of the most common malignancies worldwide and one of the leading causes of cancer-related death[1,2]. Although surgical resection is still one of the most important treatments for gastric cancer[3,4], the prognosis of postoperative patients still faces challenges[5,6]. Immune cells play a crucial role in tumor initiation, development, and prognosis[7,8]. Therefore, it is of important clinical significance to study the immune status of patients after gastric cancer surgery and its impact on survival rate[9,10]. Treatment strategies for gastric cancer have been continuously developed and improved, including surgery, chemotherapy, radiotherapy and other comprehensive treatments[11]. However, although these treatments can effectively control tumor development to a certain extent[12,13], postoperative recurrence and metastasis are still one of the main factors affecting patient survival[14,15]. The role of immune monitoring in the postoperative management of gastric cancer has received increasing attention because the activity and functional status of immune cells are crucial to tumor control and prognosis[16].
As an efficient and accurate cell analysis technology, flow cytometry plays a vital role in the field of immunology research[17]. It uses laser irradiation and a multi-parameter detection system to analyze thousands of cells simultaneously, quickly and accurately identifying their surface markers and intracellular components[18,19]. Through flow cytometry, detailed quantitative analysis can be performed on the type, number, distribution, and functional status of immune cells, thereby providing an in-depth understanding of the status and function of the immune system. The immune status of patients after gastric cancer surgery is crucial for prognosis and survival. Immune cells play a key role in tumor initiation, development, and treatment, including the regulation of anti-tumor immune responses, the shaping of the tumor microenvironment, and the avoidance of postoperative immune suppression[20]. Therefore, detecting immune cell levels in peripheral blood of postoperative patients through flow cytometry can provide important information for assessing the patient's immune status.
Specifically, flow cytometry can accurately detect and quantitatively analyze immune cells such as total T cells (CD3+), helper T cells (CD3+CD4+), and suppressor T cells (CD3+CD8+)[21]. These cell subsets play different roles in the tumor immune response. Total T cells are involved in regulating the overall balance of the immune response, helper T cells promote the initiation and maintenance of anti-tumor immune responses, and suppressor T cells may contribute to immune response[22]. The reaction produces an inhibitory effect, thereby affecting tumor growth and prognosis. There
This study aims to detect immune cells in the peripheral blood of patients after gastric cancer surgery, paying special attention to the levels of total T cells (CD3+), helper T cells (CD3+CD4+) and suppressor T cells (CD3+CD8+), and analyze Correlations between them and their relationship to patient survival. Through this study, we hope to gain an in-depth understanding of the changes in the immune status of patients after gastric cancer surgery and provide more effective clinical guidance for improving the prognosis and survival rate of patients.
This study included 58 patients who underwent surgical treatment of gastric cancer in the hospital from 2021 to 2023. All patients received a clear preoperative diagnosis of gastric cancer and underwent radical surgery, and postoperative pathology confirmed gastric adenocarcinoma. Pathological staging was based on the eighth edition of gastric cancer staging criteria. This study was approved by the hospital ethics committee and informed consent was obtained from the patients. This study was retrospective. We collected peripheral blood samples from patients 1 month after surgery, detected the levels of immune cells in them using flow cytometry, and analyzed them. Patients were divided into high-level and low-level groups based on the median test results to evaluate the relationship between immune cell levels and patient survival.
Inclusion criteria: Gastric cancer patients over 18 years old; surgical treatment and pathological diagnosis of gastric adenocarcinoma; no severe immune system diseases or autoimmune diseases before surgery; no radiotherapy, che
Exclusion criteria: The presence of severe cardiovascular disease, liver and kidney insufficiency, or other serious underlying diseases; the presence of severe infectious or inflammatory diseases before surgery; the presence of known immunosuppressive factors, such as long-term use of immunosuppressants; there are other primary tumors or active secondary tumors; postoperative complications seriously affect survival or the conduct of experiments.
According to the median test results, patients were divided into high-level and low-level groups of three subpopulations: total T cells (CD3+), helper T cells (CD3+CD4+), and suppressor T cells (CD3+CD8+).
The specific groups are as follows: High-level group: The test results of total T cells, helper T cells, and suppressor T cells are higher than the median; low-level group: The test results of total T cells, helper T cells, and suppressor T cells Below the median.
This study was retrospective and no special intervention was performed on the patients. The patient only underwent standard surgical treatment and did not receive postoperative radiotherapy, chemotherapy or immunotherapy. Only peripheral blood samples from patients before surgery and 1 month after surgery were collected for flow cytometry detection.
The main observation indicators of this study were the levels of total T cells (CD3+), helper T cells (CD3+CD4+), and suppressor T cells (CD3+CD8+) in the peripheral blood of patients after surgery. At the same time, the patient's clinical data and survival data were also collected.
SPSS statistical software was used for data analysis. Continuous variables were compared using t test or Wilcoxon rank sum test; categorical variables were compared using chi-square test. Survival analysis was performed using Kaplan-Meier survival curves, and Log-rank test was used to compare survival curves. The results were statistically significant, P value < 0.05.
A total of 58 patients who underwent surgical treatment for gastric cancer were included in this study, including 37 males (58.6%) and 21 females (41.4%). The average age of the patients was 63.43 ± 7.83 years old. Before surgery, all patients received surgical treatment for gastric cancer and recovered well after surgery without obvious complications.
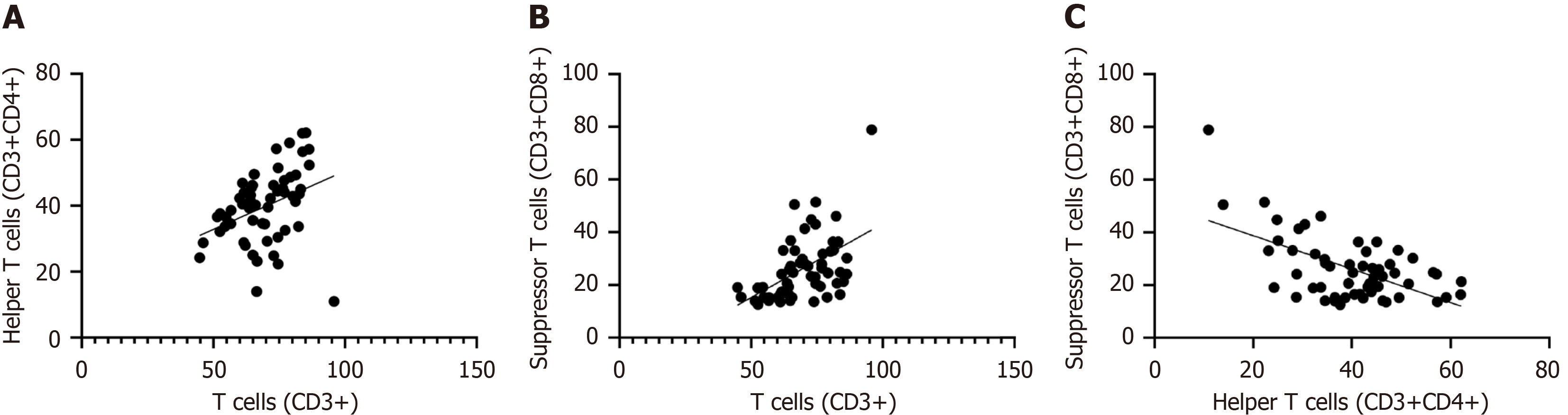
Further correlation analysis showed that there was a significant positive correlation between total T cell levels and helper T cell levels (r = 0.35, P < 0.01). This shows that as the level of total T cells increases, the level of helper T cells also increases correspondingly, suggesting that there may be some functional synergy or common regulatory mechanism between them. In addition, there was also a significant positive correlation between total T cell levels and inhibitory T cell levels (r = 0.56, P < 0.01). This finding suggests that an increase in total T cells may be associated with an increase in suppressor T cell levels, which may involve the immune system's regulation of the inflammatory response, or other related immune regulatory mechanisms. Interestingly, there was a negative correlation between helper T cell levels and suppressor T cell levels (r = -0.63, P < 0.01). This means that, within a sample, an increase in helper T cell levels is accompanied by a decrease in suppressor T cell levels, and vice versa. This may reflect the existence of some kind of balancing mechanism in the immune system, in which helper T cells and suppressor T cells play opposing but complementary roles in the immune response (Table 1). We further confirmed the trends in these relationships through visual displays in Figure 1, which show correlations between total T cell levels, helper T cell levels, and suppressor T cell levels.
| Group | R | 95% confidence interval | P value |
| Helper T cells (CD3+CD4+) and T cells (CD3+) | 0.35 | 0.1001-0.5642 | < 0.01 |
| Suppressor T cells (CD3+CD8+) and T cells (CD3+) | 0.56 | 0.3017-0.8200 | < 0.01 |
| Helper T cells (CD3+CD4+) and suppressor T cells (CD3+CD8+) | -0.63 | -0.8793 to -0.3906 | < 0.01 |
During the follow-up period, the survival rate of patients in the high-level group of total T cells (CD3+) was significantly better than that of patients in the low-level group. The median survival time of patients in the high-level group was 28.87 ± 24.98 months, while the median survival time of patients in the low-level group was only 18.42 ± 16.21 months. The survival analysis results showed that the survival curve of patients in the high-level group was significantly shifted to the upper right, while the survival curve of patients in the low-level group was shifted downward. The levels of helper T cells (CD3+CD4+) and suppressor T cells (CD3+CD8+) seem to have no difference with patient survival time (Figure 2).
Gastric cancer is one of the most common malignant tumors worldwide, accounting for the majority of cancer-related deaths. Although great progress has been made in the field of tumor treatment, including the application of multiple comprehensive treatments such as surgery, chemotherapy, and radiotherapy, the prognosis of gastric cancer is still poor, and postoperative recurrence and metastasis are one of the main factors affecting patient survival rate. The immune system plays a key role in the anti-tumor process. Immune cells play an important role in anti-tumor by identifying and eliminating abnormal cells and maintaining the body's immune balance. However, the development of tumors may lead to immune escape and immunosuppression, which in turn affects the activity and functional status of immune cells and reduces the body's resistance to tumors.
In recent years, with the development and application of immunotherapy, the role of immune monitoring in tumor treatment has gradually received attention. Immune monitoring assesses the patient's immune status by detecting the level and functional status of immune cells in the patient's body, providing an important basis for individualized treatment and management. As an efficient and accurate cell analysis technology, flow cytometry can quickly and accurately detect various types of immune cells and quantitatively evaluate their number and functional status. It has been widely used in the field of immune monitoring. However, current research on immune cell levels in the peripheral blood of patients with gastric cancer surgery is still limited. Accurate assessment and monitoring of the immune status of postoperative patients are of great significance for guiding treatment and prognosis assessment. Therefore, this study aims to detect immune cell levels in peripheral blood of patients with gastric cancer after surgery through flow cyto
This study aimed to detect immune cell levels in peripheral blood of postoperative patients through flow cytometry, evaluate its relationship with patient survival rate, and explore its clinical significance. In our study, we observed that total T cells, helper T cells, and suppressor T cell levels were closely related to the survival rate of gastric cancer patients after surgery. We found that there are certain changes in the levels of total T cells, helper T cells, and suppressor T cells in patients after gastric cancer surgery. This is consistent with previous research suggesting that surgical treatment of gastric cancer may have some impact on patient's immune status. Surgical removal of tumors may stimulate or suppress the immune system, resulting in changes in the number and function of immune cells. Therefore, it is crucial to monitor the immune system of postoperative patients and adjust the treatment plan promptly.
We observed that total T cell and helper T cell levels were positively correlated with patient survival, whereas suppressor T cell levels were negatively correlated with survival. This suggests that immune cells play an important role in the prognosis of postoperative patients with gastric cancer. Helper T cells are an important part of the immune system and can promote the initiation and maintenance of anti-tumor immune responses. Therefore, increasing their levels may be beneficial to controlling tumor growth and metastasis and improving patient survival rates. As the dominant cell group in the immune response, the increase in total T cells may reflect the activation status of the immune system and help improve the body's immune surveillance and elimination capabilities against tumors. On the contrary, an increase in suppressor T cells may indicate an aggravation of the immunosuppressive state, thereby weakening the body's immune response to tumors and resulting in reduced patient survival. Furthermore, we found a significant positive correlation between total T cells and helper T cell levels, as well as a positive correlation with suppressor T cell levels. This is consistent with the regulatory mechanism of the immune system. In immune responses, total T cells regulate and coordinate different types of immune cells, some of which differentiate into helper T cells to promote the initiation and maintenance of immune responses. Suppressive T cells play a negative regulatory role in immune responses, helping to maintain immune balance and prevent excessive immune responses. Therefore, increased levels of total T cells and helper T cells may reflect an activated state of the immune system, whereas increased levels of suppressive T cells may indicate an exacerbation of an immunosuppressive state.
In this study, we used flow cytometry as a tool for immune monitoring, and its accuracy and sensitivity were fully guaranteed. By conducting regular immune monitoring of postoperative patients and making individualized treatment adjustments based on the monitoring results, the patient's immune status can be better assessed, immune abnormalities can be discovered and dealt with in a timely manner, thereby improving the patient's prognosis and survival rate. However, this study also has some limitations. First, the sample size was relatively small, which may have affected the reliability of the results. Secondly, this study is a single-center observational study, and there is the possibility of selection bias and information bias. Therefore, it is necessary to further expand the sample size and conduct multicenter, ran
In summary, this study found that the levels of total T cells, helper T cells, and suppressor T cells are closely related to the survival rate of gastric cancer patients after surgery, providing important clinical basis for individualized immune monitoring and intervention. Further research will help to gain a deeper understanding of the mechanism of the immune system in tumor treatment and provide more effective strategies for improving patient prognosis and survival rates.
The author thanks to the approve and assistance of all friends and colleagues.
| 1. | Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y, Li B, Li H. The effects of TNF-α/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int J Cancer. 2022;150:1373-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Yang L, Zhao KL, Qin L, Ji DX, Zhang B, Zheng PF, Qin YM. Notch signaling pathway regulates CD4(+)CD25(+)CD127(dim/-) regulatory T cells and T helper 17 cells function in gastric cancer patients. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Chen X, Wang Y, Wang J, Wen J, Jia X, Wang X, Zhang H. Accumulation of T-helper 22 cells, interleukin-22 and myeloid-derived suppressor cells promotes gastric cancer progression in elderly patients. Oncol Lett. 2018;16:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Shan ZG, Chen J, Liu JS, Zhang JY, Wang TT, Teng YS, Mao FY, Cheng P, Zou QM, Zhou WY, Peng LS, Zhao YL, Zhuang Y. Activated neutrophils polarize protumorigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer. Clin Transl Med. 2021;11:e484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Lavu H, Potluri V. Proinflammatory T-helper 17 cells and interleukin 17 in gastric cancer. J Surg Res. 2013;185:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, Wang H, Li Z. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. J Natl Cancer Inst. 2019;111:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Kang W, Li O, Qi F, Wang J, You Y, He P, Suo Z, Zheng Y, Liu HM. Abrogation of USP7 is an alternative strategy to downregulate PD-L1 and sensitize gastric cancer cells to T cells killing. Acta Pharm Sin B. 2021;11:694-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 8. | Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018;109:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Mahajan R, El-Omar EM, Lissowska J, Grillo P, Rabkin CS, Baccarelli A, Yeager M, Sobin LH, Zatonski W, Channock SJ, Chow WH, Hou L. Genetic variants in T helper cell type 1, 2 and 3 pathways and gastric cancer risk in a Polish population. Jpn J Clin Oncol. 2008;38:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Qiu XT, Song YC, Liu J, Wang ZM, Niu X, He J. Identification of an immune-related gene-based signature to predict prognosis of patients with gastric cancer. World J Gastrointest Oncol. 2020;12:857-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Zhou X, Fang D, Liu H, Ou X, Zhang C, Zhao Z, Zhao S, Peng J, Cai S, He Y, Xu J. PMN-MDSCs accumulation induced by CXCL1 promotes CD8(+) T cells exhaustion in gastric cancer. Cancer Lett. 2022;532:215598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 12. | Poyrazoglu B, Akyildiz H. Regulatory T cells and monocytes crosstalk in patients with gastric cancer. Ann Ital Chir. 2022;93:544-549. [PubMed] |
| 13. | Nose Y, Saito T, Yamamoto K, Yamashita K, Tanaka K, Yamamoto K, Makino T, Takahashi T, Kawashima A, Haruna M, Hirata M, Ueyama A, Iwahori K, Satoh T, Kurokawa Y, Eguchi H, Doki Y, Wada H. The tissue-resident marker CD103 on peripheral blood T cells predicts responses to anti-PD-1 therapy in gastric cancer. Cancer Immunol Immunother. 2023;72:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 14. | Entezam M, Sanaei MJ, Mirzaei Y, Mer AH, Abdollahpour-Alitappeh M, Azadegan-Dehkordi F, Bagheri N. Current progress and challenges of immunotherapy in gastric cancer: A focus on CAR-T cells therapeutic approach. Life Sci. 2023;318:121459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Negura I, Pavel-Tanasa M, Danciu M. Regulatory T cells in gastric cancer: Key controllers from pathogenesis to therapy. Cancer Treat Rev. 2023;120:102629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Liang K, Zhou X, Zhang X, Xu H, Dai H, Song X, Yang X, Liu B, Shi T, Wei J. Combination therapy of DKK1 inhibition and NKG2D chimeric antigen receptor T cells for the treatment of gastric cancer. Cancer Sci. 2023;114:2798-2809. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Yu K, Gu Y, Zhang P, Fang H, Cao Y, Wang J, Lin C, Liu H, Zhang H, He H, Li R, Qin J, Li H, Xu J. Intratumoral PD-1(+)CD8(+) T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer. Br J Cancer. 2022;127:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 18. | Huang H, Huang Z, Ge J, Yang J, Chen J, Xu B, Wu S, Zheng X, Chen L, Zhang X, Jiang J. CD226 identifies functional CD8(+)T cells in the tumor microenvironment and predicts a better outcome for human gastric cancer. Front Immunol. 2023;14:1150803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Jiang S, Zhang Y, Zhang X, Lu B, Sun P, Wu Q, Ding X, Huang J. GARP Correlates With Tumor-Infiltrating T-Cells and Predicts the Outcome of Gastric Cancer. Front Immunol. 2021;12:660397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | He Q, Huangfu L, Fan B, Zhuang Q, He L, Li L, You W, Xing X. T-cells infiltration mediates the association between neutrophil/lymphocyte ratio and survival in gastric cancer. Cancer Med. 2023;12:15893-15902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Zhou Z, Li J, Hong J, Chen S, Chen M, Wang L, Lin W, Ye Y. Interleukin-15 and chemokine ligand 19 enhance cytotoxic effects of chimeric antigen receptor T cells using zebrafish xenograft model of gastric cancer. Front Immunol. 2022;13:1002361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Zheng L, Lin L, Song J, Huang S, Chen L, Li H, Ma N, Chen Q, Chen Y. Prognostic values of regulatory T cells (Tregs) and Treg-related genes in gastric cancer. Cent Eur J Immunol. 2023;48:14-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Qian S, Villarejo-Campos P, García-Olmo D. The Role of CAR-T Cells in Peritoneal Carcinomatosis from Gastric Cancer: Rationale, Experimental Work, and Clinical Applications. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Choo J, Kua LF, Soe MY, Asuncion BR, Tan BKJ, Teo CB, Tay RYK, So J, Shabbir A, Guowei K, Tan HL, Chan G, Ma H, Ramachandran GK, Lum JHY, Chee CE, Sridharan S, Tan P, Sundar R, Yong WP. Clinical relevance of PD-1 positive CD8 T-cells in gastric cancer. Gastric Cancer. 2023;26:393-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Shaopeng Z, Yang Z, Yuan F, Chen H, Zhengjun Q. Regulation of regulatory T cells and tumor-associated macrophages in gastric cancer tumor microenvironment. Cancer Med. 2024;13:e6959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |