Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2503
Revised: July 5, 2024
Accepted: July 9, 2024
Published online: August 27, 2024
Processing time: 96 Days and 2.6 Hours
The effect of the number of lymph node dissections (LNDs) during radical resection for colorectal cancer (CRC) on overall survival (OS) remains controver
To investigate the association between the number of LNDs and OS in patients with tumor node metastasis (TNM) stage I–II CRC undergoing radical resection.
Patients who underwent radical resection for CRC at a single-center hospital between January 2011 and December 2021 were retrospectively analyzed. Cox regression analyses were performed to identify the independent predictors of OS at different T stages.
A total of 2850 patients who underwent laparoscopic radical resection for CRC were enrolled. At stage T1, age [P < 0.01, hazard ratio (HR) = 1.075, 95% confidence interval (CI): 1.019-1.134] and tumour size (P = 0.021, HR = 3.635, 95%CI: 1.210-10.917) were independent risk factors for OS. At stage T2, age (P < 0.01, HR = 1.064, 95%CI: 1.032-1.098) and overall complications (P = 0.012, HR = 2.297, 95%CI: 1.200-4.397) were independent risk factors for OS. At stage T3, only age (P < 0.01, HR = 1.047, 95%CI: 1.027-1.066) was an independent risk factor for OS. At stage T4, age (P < 0.01, HR = 1.057, 95%CI: 1.039-1.075) and body mass index (P = 0. 034, HR = 0.941, 95%CI: 0.890-0.995) were independent risk factors for OS. However, there was no association between LNDs and OS in stages I and II.
The number of LDNs did not affect the survival of patients with TNM stages I and II CRC. Therefore, insufficient LNDs should not be a cause for alarm during the surgery.
Core Tip: This retrospective cohort study aimed to investigate the association between the total number of lymph node dissections (LNDs) and overall survival (OS) in patients with tumor node metastasis stages I and II colorectal cancer who underwent laparoscopic radical resection. The results indicated that there was no association between the total number of LNDs and the OS in these patients. Therefore, insufficient LNDs should not be a cause for alarm during the surgery.
- Citation: He F, Qu SP, Yuan Y, Qian K. Lymph node dissection does not affect the survival of patients with tumor node metastasis stages I and II colorectal cancer. World J Gastrointest Surg 2024; 16(8): 2503-2510
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2503.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2503
Colorectal cancer (CRC) is the second leading cause of cancer-associated morbidity and the third leading cause of mortality worldwide[1,2]. More than 900000 people die due to CRC each year[3-5]. Radical resection is the most important form of treatment for patients with CRC; however, the rate of recurrence is approximately 40% in patients with tumor node metastasis (TNM) stage II[6-8]. The 5-year survival rate of patients with stage II CRC is 60%-80%[9]. Compared to patients in stage I, the decision to administer adjuvant chemotherapy to patients in stage II is complicated[10].
At present, the lymph node status during surgical resection is considered the strongest predictor of patient prognosis[11,12]. It is generally considered that an increase in the number of lymph nodes in CRC-resected specimens increases the possibility of identifying the involved lymph nodes[13]. Previous studies and guidelines have indicated that at least 12 Lymph nodes must be removed to ensure adequate sampling[14,15]. However, in clinical practice, the number of lymph nodes recovered varies greatly owing to many factors[16].
Some researchers have considered that extensive lymph node dissection (LND) might prolong survival, while others believe that it might increase the risk of postoperative complications without improving survival[14,17,18]. However, the effect of the number of LNDs on patient survival remains controversial.
Therefore, this study aimed to investigate the association between the total number of LNDs and overall survival (OS) in patients with TNM stages I and II CRC who underwent radical resection.
This study included 2850 patients who underwent radical resection for CRC between January 2011 and December 2021 at the First Affiliated Hospital of Chongqing Medical University.
The inclusion criteria were as follows: (1) Pathological diagnosis of colorectal adenocarcinoma; (2) Laparoscopic radical resection for CRC; (3) Postoperative pathological stage being TNM stages I and II; and (4) Age > 18 years.
The exclusion criteria were as follows: (1) Patients who received neoadjuvant radiochemotherapy; (2) Patients with severe cardiopulmonary disease; and (3) Patients with incomplete clinical data.
According to the clinical guidelines, this study enrolled all patients who underwent laparoscopic radical resection for CRC, including total mesorectal excision or complete mesocolic excision, which was pathologically confirmed as R0 resection. The patients were followed up via telephone reviews.
The TNM stage was determined according to the American Joint Committee on Cancer 8th Edition[19]. The complications were defined according to the Clavien-Dindo classification[20]. The time interval from the date of surgery to the time of the last follow-up or death was defined as the OS.
The baseline information included sex, age, smoking, drinking, body mass index (BMI), hypertension, type 2 diabetes mellitus, previous abdominal surgery, tumour location, T stage, tumour size, LNDs, and overall complications. All details were collected from medical records and telephone interviews.
Categorical variables are expressed as n (%), and continuous variables are expressed as the mean ± SD. Cox regression analyses were performed to identify the independent predictive factors for the OS. Data were analyzed using SPSS (version 22.0) statistical software. The level of statistical significance was set at P < 0.05.
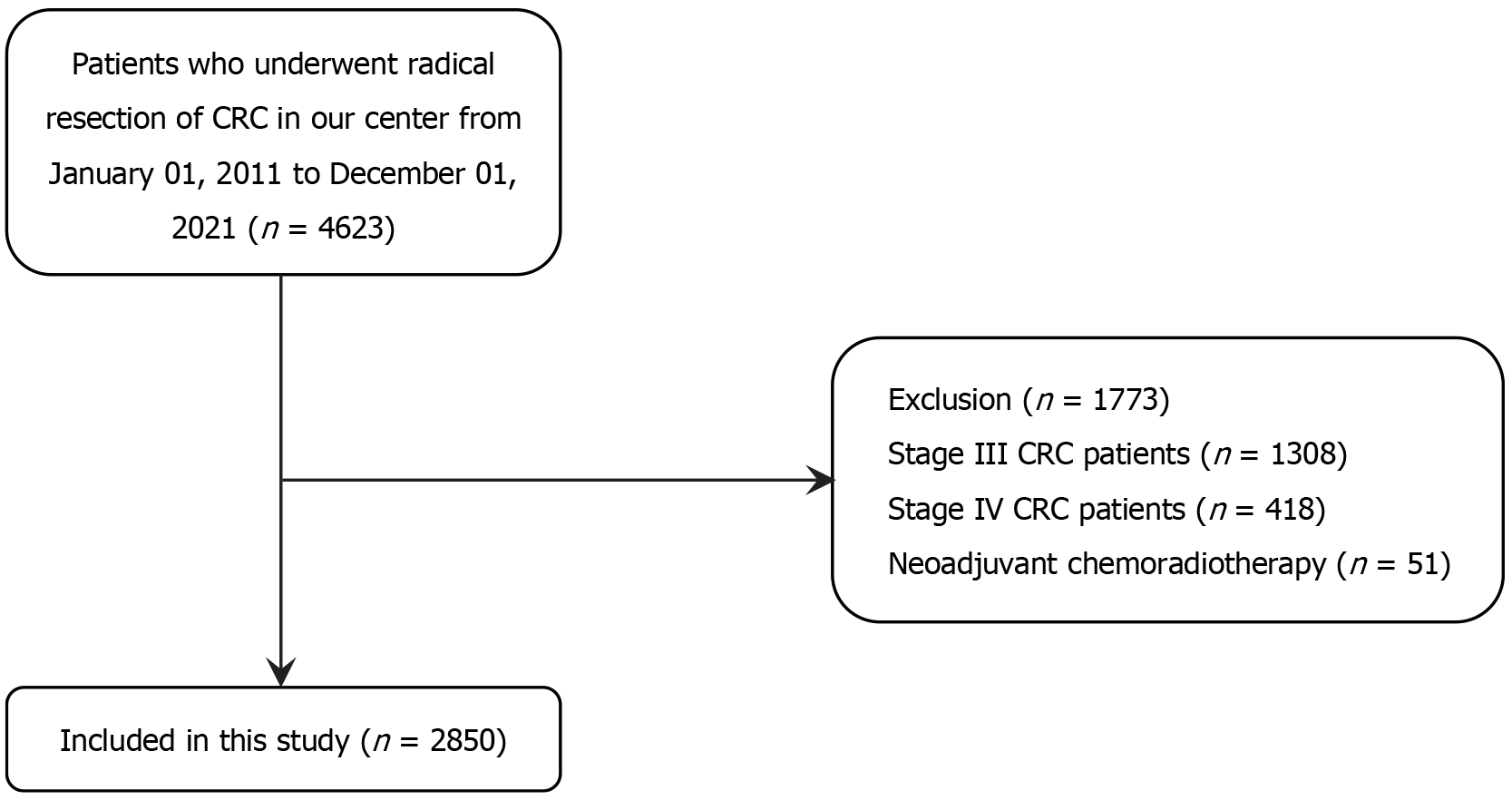
A total of 4623 patients with CRC who underwent laparoscopic radical resection for CRC and had complete medical information were included in this study. Patients with stages III or IV CRC (n = 1722) and those who received neoadjuvant radiochemotherapy (n = 51) were excluded (Figure 1). Finally, 2850 eligible patients with CRC were included in the study. The mean age was 62.8 ± 12.0 years, and 1713 (60.1%) of the patients were males. There were more cases of rectal cancers and a higher proportion of small tumours (< 5 cm). The number of patients in stages T1, T2, T3, and T4 was 270 (9.5%), 596 (20.9%), 725 (25.4%), and 1259 (44.2%), respectively. The average total number of dissected lymph nodes was 14.9 ± 7.8. Moreover, the median follow-up time was 38 (1-114) months (Table 1).
| Characteristics | n (%) |
| Age (years) | 62.8 ± 12.0 |
| Sex | |
| Male | 1713 (60.1) |
| Female | 1137 (39.9) |
| BMI (kg/m2) | 22.7 ± 3.2 |
| Smoking | 1110 (38.9) |
| Drinking | 898 (31.5) |
| Hypertension | 745 (26.1) |
| T2DM | 350 (12.3) |
| Surgical history | 688 (24.1) |
| Tumor location | |
| Colon | 1363 (47.8) |
| Rectum | 1487 (52.2) |
| T stage | |
| 1 | 270 (9.5) |
| 2 | 596 (20.9) |
| 3 | 725 (25.4) |
| 4 | 1259 (44.2) |
| Tumor size | |
| < 5 cm | 1702 (59.7) |
| ≥ 5 cm | 1148 (40.3) |
| Total lymph nodes | 14.9 ± 7.8 |
| Overall complications | 618 (21.7) |
| Follow-up (months) | 38 (1-114) |
The univariate and multivariate cox regression analyses revealed that age [hazard ratio (HR) = 1.075, 95% confidence interval (CI): 1.019-1.134, P < 0.01] and tumour size (HR = 3.635, 95%CI: 1.210-10.917, P = 0.021) were independent risk factors for the OS for patients with T1 stage CRC. However, the number of LNDs did not change the OS of these patients (HR = 0.989, 95%CI: 0.906-1.079, P = 0.807) (Table 2).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.045 (1.017-1.134) | 0.010a | 1.075 (1.019-1.134) | < 0.01a |
| Sex (male/female) | 0.592 (0.185-1.894) | 0.377 | ||
| BMI (kg/m2) | 0.814 (0.662-1.001) | 0.052 | ||
| T2DM (yes/no) | 2.696 (0.845-8.605) | 0.094 | ||
| Tumor location (colon/rectum) | 2.109 (0.734-6.060) | 0.166 | ||
| Surgical history (yes/no) | 0.467 (0.104-2.087) | 0.319 | ||
| Smoking (yes/no) | 0.920 (0.307-2.759) | 0.881 | ||
| Drinking (yes/no) | 1.046 (0.348-3.140) | 0.936 | ||
| Hypertension (yes/no) | 0.852 (0.236-3.068) | 0.806 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 3.418 (1.134-10.299) | 0.029a | 3.635 (1.210-10.917) | 0.021a |
| Lymph nodes | 0.989 (0.906-1.079) | 0.807 | ||
| Overall complications (yes/no) | 1.227 (0.381-3.944) | 0.732 | ||
Univariate and multivariate cox regression analyses were used to identify independent predictors of the OS for patients with T2 stage CRC. Age (HR = 1.064, 95%CI: 1.032-1.098, P < 0.01) and overall complications (HR = 2.297, 95%CI: 1.200-4.397, P = 0.012) were identified as independent risk factors for the OS. However, the number of LNDs did not change the OS (HR = 0.946, 95%CI: 0.893-1.002, P = 0.057) (Table 3).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.073 (1.040-1.107) | < 0.01a | 1.064 (1.032-1.098) | < 0.01a |
| Sex (male/female) | 0.718 (0.374-1.376) | 0.318 | ||
| BMI (kg/m2) | 0.947 (0.856-1.047) | 0.290 | ||
| T2DM (yes/no) | 2.309 (1.015-5.251) | 0.046a | 1.966 (0.862-4.485) | 0.108 |
| Tumor location (colon/rectum) | 1.058 (0.514-2.178) | 0.879 | ||
| Surgical history (yes/no) | 0.805 (0.369-1.757) | 0.586 | ||
| Smoking (yes/no) | 1.008 (0.516-1.971) | 0.981 | ||
| Drinking (yes/no) | 0.836 (0.383-1.825) | 0.653 | ||
| Hypertension (yes/no) | 1.405 (0.708-2.791) | 0.331 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 0.892 (0.392-2.027) | 0.785 | ||
| Lymph nodes | 0.946 (0.893-1.002) | 0.057 | ||
| Overall complications (yes/no) | 2.833 (1.498-5.355) | < 0.01a | 2.297 (1.200-4.397) | 0.012a |
In patients in the T3 stage, using univariate and multivariate cox regression analyses, we found that age (HR = 1.047, 95%CI: 1.027-1.066, P < 0.01) was an independent risk factor for the OS. However, the number of LNDs did not affect the OS of these patients (HR = 0.973, 95%CI: 0.946-1.002, P = 0.067) (Table 4).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.051 (1.031-1.070) | < 0.01a | 1.047 (1.027-1.066) | < 0.01a |
| Sex (male/female) | 0.840 (0.557-1.267) | 0.405 | ||
| BMI (kg/m2) | 0.946 (0.890-1.006) | 0.079 | ||
| T2DM (yes/no) | 2.040 (1.247-3.337) | < 0.01a | 1.470 (0.891-2.425) | 0.132 |
| Tumor location (colon/rectum) | 0.909 (0.612-1.351) | 0.638 | ||
| Surgical history (yes/no) | 0.598 (0.340-1.021) | 0.059 | ||
| Smoking (yes/no) | 1.177 (0.789-1.756) | 0.424 | ||
| Drinking (yes/no) | 1.252 (0.830-1.888) | 0.284 | ||
| Hypertension (yes/no) | 1.316 (0.849-2.040) | 0.220 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 0.969 (0.652-1.440) | 0.877 | ||
| Lymph nodes | 0.973 (0.946-1.002) | 0.067 | ||
| Overall complications (yes/no) | 1.777 (1.181-2.674) | < 0.01a | 1.487 (0.983-2.250) | 0.060 |
In these patients, we found that age (HR = 1.057, 95%CI: 1.039-1.075) and BMI (HR = 0.941, 95%CI: 0.890-0.995, P < 0.01, P = 0.034) were independent risk factors for the OS. However, the number of LNDs did not affect the OS (HR = 0.979, 95%CI: 0.955-1.003, P < 0.01) (Table 5).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.061 (1.044-1.079) | < 0.01a | 1.057 (1.039-1.075) | < 0.01a |
| Sex (male/female) | 0.848 (0.589-1.219) | 0.373 | ||
| BMI (kg/m2) | 0.915 (0.864-0.969) | < 0.01a | 0.941 (0.890-0.995) | 0.034a |
| T2DM (yes/no) | 1.139 (0.674-1.923) | 0.627 | ||
| Tumor location (colon/rectum) | 0.845 (0.599-1.192) | 0.337 | ||
| Surgical history (yes/no) | 1.187 (0.803-1.756) | 0.390 | ||
| Smoking (yes/no) | 1.309 (0.928-1.847) | 0.125 | ||
| Drinking (yes/no) | 1.096 (0.764-1.574) | 0.618 | ||
| Hypertension (yes/ no) | 1.772 (1.240-2.531) | < 0.01a | ||
| Tumor size (≥ 5 cm/< 5 cm) | 0.875 (0.620-1.234) | 0.447 | 0.855 (0.605-1.207) | 0.373 |
| Lymph nodes | 0.979 (0.955-1.003) | 0.084 | ||
| Overall complications (yes/no) | 1.766 (1.220-2.558) | < 0.01a | 1.432 (0.982-2.088) | 0.062 |
In this study, we found that age and tumour size were independent risk factors for the OS of patients with T1-stage CRC. In the T2 stage, age and overall complications were identified as independent risk factors for the OS. In the T3 stage, age was identified as an independent risk factor for the OS. Moreover, in the T4 stage, age and BMI were independent risk factors for the OS. Nevertheless, we did not find an association between the total number of LNDs and OS in patients with TNM stages I and II CRC.
Lymph node involvement is considered the most important factor affecting the prognosis of patients with CRC after radical surgery[21-23]. Ideally, all lymph nodes in the surgical specimen should be collected and examined to accurately determine the tumour stage. However, this approach is impractical. The results of large databases such as surveillance, epidemiology, and end results showed that only approximately 40% of patients underwent sufficient lymph node examinations[24,25]. Recently, the scope of lymph node examination has become an interesting topic; however, conside
The number of lymph nodes required to accurately determine the tumour stage remains controversial. Current guidelines from the Joint American Cancer Commission recommend that 12 or more lymph nodes should be evaluated for accurate staging[26]. Scott et al[27] found that more than 90% of specimens with lymph node metastasis could be identified when the examination was performed on at least 13 Lymph nodes. Choi et al[28] suggested that at least 21 Lymph nodes should be examined in patients with stage II disease to accurately determine the prognosis. Sarli et al[29] found that in stage II, the survival rates of patients with negative lymph nodes were similar to those of patients with one to three positive lymph nodes, and fewer than 10 Lymph nodes were removed. In a systematic review, Chang et al[30] reported that the survival rate of patients with stages II and III colon cancer increased as the number of lymph nodes increased. However, McDonald et al[18] presented with a different opinion. They believed that an increase in the number of lymph nodes would not affect the OS. Therefore, it is necessary to explore the relationship between the total number of LNDs and OS in patients with TNM stages I and II CRC.
In the Cox regression analyses, we found many factors affecting CRC survival, including age, tumour size, and overall complications. This finding is consistent with those of previous studies[31-33]. The average total number of LNDs was 14.9 ± 7.8. Nevertheless, we did not find that the total number of LNDs affected the OS in T1-4N0M0. The mechanism is unclear, but possible reasons may be as follows: First, T1-4N0M0 is the early stage of the tumour without lymph node metastasis. Second, for early tumours, surgeons may not be as aggressive as for advanced tumours, which may cause less damage to the body.
To the best of our knowledge, this study had the largest sample size to date to reveal the relationship between LNDs and OS. However, the study had some limitations. First, this was a retrospective, single-centre study. Moreover, we excluded patients with CRC who had received neoadjuvant radiochemotherapy before surgery. Previous studies have shown that the number of recovered lymph nodes decreases after neoadjuvant therapy[34,35]. Therefore, multicentre prospective randomised controlled trials should be conducted in the future.
We found that no association between the total number of LNDs and the OS of patients with TNM stages I and II CRC. Therefore, an insufficient number of LNDs should not be a cause for alarm during surgery.
We acknowledge all the authors whose publications are referred to in our article.
| 1. | Magwenyane AM, Ugbaja SC, Amoako DG, Somboro AM, Khan RB, Kumalo HM. Heat Shock Protein 90 (HSP90) Inhibitors as Anticancer Medicines: A Review on the Computer-Aided Drug Discovery Approaches over the Past Five Years. Comput Math Methods Med. 2022;2022:2147763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Cheng YX, Liu XY, Kang B, Tao W, Wei ZQ, Peng D. Comparison of surgical and oncologic outcomes in very elderly patients (≥ 80 years old) and elderly (65-79 years old) colorectal cancer patients: a propensity score matching. BMC Gastroenterol. 2022;22:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1067] [Article Influence: 177.8] [Reference Citation Analysis (1)] |
| 4. | Cheng Y, Cheng YX, Liu XY, Kang B, Tao W, Peng D. The Effect of Type 2 Diabetes Mellitus on the Short-Term Outcomes and Prognosis of Stage I-III Colorectal Cancer: A Propensity Score Matching Analysis. Cancer Manag Res. 2022;14:205-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3015] [Article Influence: 502.5] [Reference Citation Analysis (3)] |
| 6. | Liu XY, Kang B, Cheng YX, Yuan C, Tao W, Zhang B, Wei ZQ, Peng D. The short-term and oncologic outcomes of younger VS older colorectal cancer patients undergoing primary surgery: a propensity score matching analysis. BMC Cancer. 2022;22:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, Peng D. Hypertension Remission after Colorectal Cancer Surgery: A Single-Center Retrospective Study. Nutr Cancer. 2022;74:2789-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Greenwood A, Keating J, Kenwright D, Shekouh A, Dalzell A, Dennett E, Danielson K. Brief report: Lymph node morphology in stage II colorectal cancer. PLoS One. 2021;16:e0249197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, Fakih MG, Fleshman J Jr, Fuchs C, Grem JL, Kiel K, Knol JA, Leong LA, Lin E, Mulcahy MF, Rao S, Ryan DP, Saltz L, Shibata D, Skibber JM, Sofocleous C, Thomas J, Venook AP, Willett C; National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Balducci G, Sederino MG, Laforgia R, Carbotta G, Minafra M, Delvecchio A, Fedele S, Tromba A, Carbone F, Palasciano N. Lymph node assessment in colorectal cancer surgery: laparoscopic versus open techniques. G Chir. 2017;38:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut. 2002;51:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | O'Shea A, Aly O, Parnaby CN, Loudon MA, Samuel LM, Murray GI. Increased lymph node yield in colorectal cancer is not necessarily associated with a greater number of lymph node positive cancers. PLoS One. 2014;9:e104991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Wong SL. Lymph node counts and survival rates after resection for colon and rectal cancer. Gastrointest Cancer Res. 2009;3:S33-S35. [PubMed] |
| 15. | Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg. 1989;76:1165-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Shen SS, Haupt BX, Ro JY, Zhu J, Bailey HR, Schwartz MR. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009;133:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Orsenigo E, Gasparini G, Carlucci M. Clinicopathological Factors Influencing Lymph Node Yield in Colorectal Cancer: A Retrospective Study. Gastroenterol Res Pract. 2019;2019:5197914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | McDonald JR, Renehan AG, O'Dwyer ST, Haboubi NY. Lymph node harvest in colon and rectal cancer: Current considerations. World J Gastrointest Surg. 2012;4:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25:1454-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 20. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8608] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 21. | Steup WH, Moriya Y, van de Velde CJ. Patterns of lymphatic spread in rectal cancer. A topographical analysis on lymph node metastases. Eur J Cancer. 2002;38:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Park YJ, Park KJ, Park JG, Lee KU, Choe KJ, Kim JP. Prognostic factors in 2230 Korean colorectal cancer patients: analysis of consecutively operated cases. World J Surg. 1999;23:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Afolabi H, Salleh SM, Zakaria Z, Ch'ng ES, Mohd Nafi SN, Abdul Aziz AAB, Al-Mhanna SB, Wada Y, Abdulrahman AS. The Prediction of Survival Outcome and Prognosis Factor in Association with Comorbidity Status in Patients with Colorectal Cancer: A Research-Based Study. Healthcare (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D; National Cancer Institute Expert Panel. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 27. | Scott KW, Grace RH, Gibbons P. Five-year follow-up study of the fat clearance technique in colorectal carcinoma. Dis Colon Rectum. 1994;37:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, Regina G, Roncoroni L. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 30. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 31. | Kido H, Kato S, Funahashi K, Shibuya K, Sasaki Y, Urita Y, Hori M, Mizumura S. The metabolic parameters based on volume in PET/CT are associated with clinicopathological N stage of colorectal cancer and can predict prognosis. EJNMMI Res. 2021;11:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Chiu CC, Ho CH, Hung CM, Chao CM, Lai CC, Chen CM, Liao KM, Wang JJ, Wu YC, Shi HY, Lee PH, Lee HM, Yeh LR, Soong TC, Chiang SR, Cheng KC. Correlation of Body Mass Index with Oncologic Outcomes in Colorectal Cancer Patients: A Large Population-Based Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Miyamoto Y, Hiyoshi Y, Tokunaga R, Akiyama T, Daitoku N, Sakamoto Y, Yoshida N, Baba H. Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: A propensity-score analysis. J Surg Oncol. 2020;122:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Tekkis PP, Smith JJ, Heriot AG, Darzi AW, Thompson MR, Stamatakis JD; Association of Coloproctology of Great Britain and Ireland. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum. 2006;49:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |