Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2494
Revised: July 11, 2024
Accepted: July 18, 2024
Published online: August 27, 2024
Processing time: 138 Days and 23.9 Hours
Perianal fistulas pose dual challenges to Crohn's disease (CD) patients. Low patient compliance due to the complexity of existing examination methods plagues the treatment and follow-up management of perianal CD.
To determine the accuracy of endoanal ultrasound (EUS) and shear wave elastography (SWE) for evaluating perianal fistulizing CD (PFCD) activity.
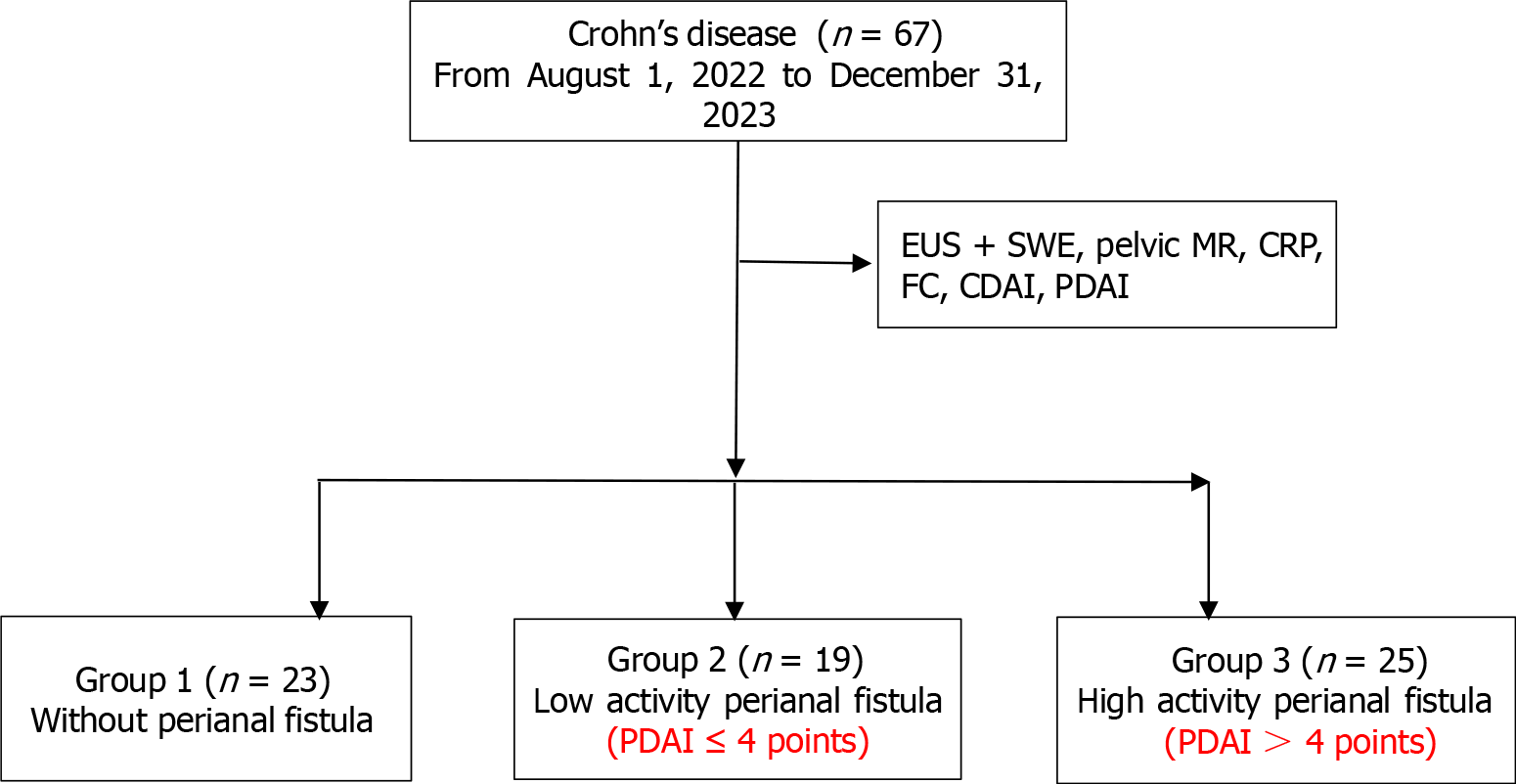
This was a retrospective cohort study. A total of 67 patients from August 2022 to December 2023 diagnosed with CD were divided into three groups: Non-anal fistula group (n = 23), low-activity perianal fistulas [n = 19, perianal disease activity index (PDAI) ≤ 4], high-activity perianal fistulas (n = 25, PDAI > 4) based on the PDAI. All patients underwent assessments including EUS + SWE, pelvic magnetic resonance [pelvic magnetic resonance imaging (MRI)], C-reactive protein, fecal calprotectin, CD activity index, PDAI.
The percentage of fistulas indicated by pelvic MRI and EUS was consistent at 82%, and there was good consistency in the classification of perianal fistulas (Kappa = 0.752, P < 0.001). Significant differences were observed in the blood flow Limberg score (χ2 = 8.903, P < 0.05) and shear wave velocity (t = 2.467, P < 0.05) between group 2 and 3. Shear wave velocity showed a strong negative correlation with magnetic resonance novel index for fistula imaging in CD (Magnifi-CD) score (r = -0.676, P < 0.001), a weak negative correlation with the PDAI score (r = -0.386, P < 0.05), and a weak correlation between the Limberg score and the PDAI score (r = 0.368, P < 0.05).
EUS combined with SWE offers a superior method for detecting and quantitating the activity of perianal fistulas in CD patients. It may be the ideal tool to assess PFCD activity objectively for management strategies.
Core Tip: Perianal fistula in Crohn's disease (CD) is a poor prognostic phenotype with high recurrence. Given the frequent recurrence and need for reintervention, it is crucial to monitor the activity of perianal fistulas in patients with CD and provide appropriate clinical management. Our study found that shear wave velocity is highly accurate to evaluate perianal fistulizing CD (PFCD) activity based on the perianal disease activity index. It can be inferred that endoanal ultrasound + shear wave elastography may be the ideal tool to assess PFCD activity objectively for management strategies.
- Citation: Hong N, Liu WY, Zhang JL, Qian K, Liu J, Ye XJ, Zeng FY, Yu Y, Zhang KG. Assessment of perianal fistulizing Crohn’s disease activity with endoanal ultrasound: A retrospective cohort study. World J Gastrointest Surg 2024; 16(8): 2494-2502
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2494.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2494
Perianal fistula in Crohn's disease (CD) is a poor prognostic phenotype with high recurrence[1]. Perianal fistula in CD is characterized by more complex and multi-branched fistulas, which a comprehensive approach with clinical examination, endoscopic and imaging assessment is required. Globally, the incidence of perianal lesions in CD patients ranges from 20% to 40%, with 26% of patients developing perianal fistulas within 20 years of diagnosis[2,3]. Given the frequent recurrence and need for reintervention, it is crucial to monitor the activity of perianal fistulas in patients with CD and provide appropriate clinical management[4].
Contrast-enhanced pelvic magnetic resonance imaging (MRI) is recommended to assess fistula activity and radiological healing in most clinical studies, however, the associated cost of MRI is expensive, several times more than endoanal ultrasound (EUS). Additionally, the use of enhanced contrast media during the examination may induce the risk of complications such as allergies and kidney damage, the claustrophobic space and long examination time, patients' compliance with MRI examinations is not optimistic. EUS has been recommended by current guidelines as a reliable alternative to MRI with high accuracy[5]. Complete fistula fibrosis is considered to indicate radiological healing[6]. Van Rijn et al[7] revealed that a greater degree of fibrosis predicted a longer period of clinical closure, highlighting the role of fibrosis in the healing process. However, there is currently no definitive standard for differentiating between inflammatory and fibrotic lesions using EUS[8]. Shear wave elastography (SWE) is a widely used noninvasive ultrasonic technology for elastic quantification. It is capable of accurately quantifying the degree of fibrosis[9] and is extensively employed in the diagnosis of various diseases, such as liver cirrhosis[10] and prostate cancer[11].
Currently, there are limited studies on the use of EUS for monitoring the activity of perianal fistulizing CD (PFCD) patients. Recent reports[12,13] support the use of EUS + SWE in monitoring fibrosis in CD-related intestinal stenosis and in differentiating between inflammatory and fibrotic stenosis. However, its performance in assessing the degree of fibrosis and activity in perianal fistulas has not been evaluated. In this study, we aimed to evaluate the efficacy of EUS combined with SWE in assessing perianal fistulas activity in CD.
A total of 67 patients with CD who met the inclusion criteria in the Department of Gastroenterology of The First Affiliated Hospital of University of Science and Technology of China from August 2022 to December 2023 were enrolled and gave informed consent. Enrolled patients were identified from the electronic medical records of the medical center. Demographic, clinical characteristics, biochemical, and imaging data were collected after informed consent was obtained. The inclusion criteria were as follows: (1) According to the ECCO-ESGAR guidelines[14], the patients who were diagnosed with CD and completed EUS + SWE, pelvic MRI, C-reactive protein (CRP), and fecal calprotectin (FC); and (2) Age ≥ 16 years, age ≤ 60 years. The exclusion criteria for patients were as follows: (1) Did not provide written informed consent; (2) Had severe cardiovascular or cerebrovascular disease, mental illness, or anal stenosis; and (3) Were unable to cooperate during EUS.
All patients were evaluated the activity indices of patients with CD and perianal disease by the CD activity index (CDAI) and perianal disease activity index (PDAI). The enrolled patients were divided into three groups based on the presence of perianal fistula detected by pelvic MRI and EUS, as well as the PDAI score. These groups included group 1 without perianal fistulas (23 patients), group 2 with low-activity perianal fistulas (19 patients with PDAI ≤ 4 points), and group 3 with a high-activity perianal fistulas (25 patients with PDAI > 4 points). FC detection in our hospital is a semiquantitative test, with a detection range of 60 to 1000 µg/g. Values greater than 60 µg/g indicated inflammation, while values greater than 1000 µg/g could not be detected. Thus, we established a cut-off value of 500 µg/g calprotein for group. This single-center retrospective cohort study was approved by the Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (USTC, NO 2023-ky-248; Figure 1).
Before the examination, the patient was prepared by emptying their bowels and assuming the left lateral position. A transrectal double-plane probe was then used and protected with a disposable condom before being gently inserted into the anus. The convex array probe was lifted and inserted back and forth within the running area of the fistula. A 360° rotation scan was performed using a double-planar linear array probe. The number of external orifices of the anal fistula and their distance from the anal margin were recorded, along with the number, location, and direction of the subcutaneous segments of the perianal fistula. The orientation of the internal orifices was preliminarily assessed. Ultrasound elastography and acoustic palpation elastic measurement (STQ) were employed to select and fix the elastic imaging area of interest (ROI). When the M-STB index reached a 5-star rating, the elastic value was effectively measured three times consecutively, and the average elastic value (E, measured in kPa) was recorded. Finally, the perianal anatomical structure was thoroughly displayed by scanning the body surface, and blood flow signals of the fistula were visualized using color Doppler flow imaging. Representative sonograms and dynamic images of the fistulas were also obtained if needed. Ultrasound exploration and image analysis were conducted by two experienced doctors. In cases of disagreement, a third senior doctor was consulted. The conversion formula for the shear wave velocity Vs (m/s) and elastic value (kPa) is as follows:
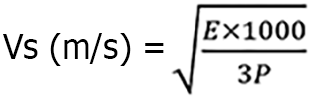
The tissue density, denoted by P, is set at a value of 1540 m/s, which accurately represents the average propagation speed of ultrasound in human soft tissue. This study utilized the improved Limberg classification standard[15]: 0 indicates normal, 1 indicates uncertain, and 2 and 3 indicate activity. Additionally, Limberg level 2 and level 3 were combined for counting.
A Siemens Magnetom Verio 3.0T superconducting MR Imager was utilized to conduct a scan encompassing the area ranging from the anterior superior iliac spine to the subsymphysis pubis. In this study, diffusion-weighted imaging was used with a selected axis position, employing a single self-selected echo plane imaging sequence. The scoring was conducted based on the Magnifri-CD[16] scoring standard. MAGNIFI-CD = 3 × number of fistula tracts + 2 × hyperintensity of primary tract on postcontrast T1-weighted images + 2 × dominant feature + 2 × fistula length + 2 × extension + 1 × inflammatory mass.
The CDAI remains the primary scoring criterion utilized to assess clinical disease activity among patients with CD[17]. The CDAI was measured prior to pelvic floor magnetic resonance imaging or EUS examination. The classification of CDAI is as follows: Inactive disease (< 150), mild disease (150-220), moderate disease (221-450), and severe disease (> 450). The PDAI was used to quantitatively assess the activity of perianal CD[18]. The scoring method included five aspects: Perianal secretions; pain and activity; sexual life; perianal manifestations; and induration. A single score was assigned based on severity, ranging from 0 to 4 points. The maximum PDAI score was 20 points. A total PDAI greater than 4 indicates an active fistula or a localized inflammatory response with 87% accuracy[19,20].
Normally distributed data are expressed as the mean ± SD, and the independent sample t test was used for comparing groups. For nonnormally distributed measurement data, the median M (P25, P75) was used. The Kruskal-Wallis method and H test were used for intergroup comparisons, and the Dunn–Bonferroni method was used for further within-group comparisons. The classified data are presented as a percentage of cases, and the Pearson χ2 test or Fisher’s exact probability method was used for group comparisons. Missing values for CRP were processed using mean interpolation, and the interpolated variable was named CRP (new). The kappa consistency test was used for paired data. A Kappa consistency standard[21] of 0.81-1.00 represented strong consistency, 0.61-0.80 represented substantial consistency, 0.41-0.60 represented moderate consistency, 0.21-0.40 represented fair consistency, and < 0.20 represented poor consistency. Spearman correlation analysis was used for correlating nonnormally distributed quantitative or rank variables. Statistical analysis was performed using SPSS 26.0 software, and P < 0.05 was considered to indicate statistical significance.
There were no significant differences in age, sex ratio, educational level, disease behavior, or drug therapy among the three groups. However, there was a statistically significant difference in perianal fistula duration among the three groups (H = 21.475, P < 0.001). Concerning the perianal fistula duration, there were statistically significant differences between group 1 and 3 (P < 0.01), as well as between group 1 and 2 (P < 0.001). When considering the history of anal fistula surgery, there were statistically significant differences among the three groups (χ2 = 13.727, P < 0.01). Specifically, there were differences between group 1 and 3, as well as between group 1 and 2 (P < 0.05). However, there were no significant differences between group 2 and 3 in terms of the perianal fistula duration or the history of anal fistula surgery. Furthermore, there were significant differences in rectal involvement among the three groups (χ2 = 8.869, P < 0.05). Specifically, there were differences between group 1 and 3 (P < 0.05), while there were no significant differences between group 1 and 2 or between group 2 and 3 (P > 0.05; Table 1).
| Variables | Group 1 (n = 23) | Group 2 (n = 19) | Group 3 (n = 25) | Statistics | P value |
| Age [M (P25, P75), years] | 34.0 (24.0, 43.0) | 30.0 (22.0, 38.0) | 28.0 (20.5, 37) | H = 2.194 | 0.334 |
| Sex, n (%) | |||||
| Male | 13 (56.5) | 12 (63.2) | 18 (72.0) | χ2 = 1.260 | 0.532 |
| Female | 10 (43.5) | 7 (36.8) | 7 (28.0) | ||
| Perianal fistula duration [M (P25, P75), years] | 0 (0, 0), (1) | 3.0 (1.0, 4.0) (2) | 2.0 (0.1, 4.0) (2) | H = 21.475 | < 0.0011 |
| Surgical history of perianal fistula, n (%) | |||||
| Yes | 2 (8.7) (1) | 12 (63.2) (2) | 10 (40.0) (2) | χ2 = 13.727 | 0.0011 |
| No | 21 (91.3) | 7 (36.8) | 15 (60.0) | ||
| Rectal involvement, n (%) | |||||
| Yes | 5 (21.7) (1) | 5 (26.3) (1), (2) | 15 (60.0) (2) | χ2 = 8.869 | 0.0121 |
| No | 18 (78.3) | 14 (73.7) | 10 (40.0) | ||
| Disease behavior, n (%) | |||||
| B1 | 14 (60.9) | 12 (63.2) | 15 (60.0) | χ2 = 0.047 | 0.977 |
| B2/B3/B2 + B3 | 9 (39.1) | 7 (36.8) | 10 (40.0) | ||
| Drug therapy, n (%) | |||||
| No | 6 (26.1) | 6 (31.6) | 15 (60.0) | χ2 = 7.312 | 0.115 |
| Biologics | 12 (52.2) | 11 (57.9) | 7 (28.0) | ||
| Others | 5 (21.7) | 2 (10.5) | 3 (12.0) | ||
| Literacy level, n (%) | |||||
| Junior high school education or below | 7 (30.4) | 3 (15.8) | 7 (28.0) | χ2 = 7.956 | 0.093 |
| High school education | 13 (56.5) | 11 (57.9) | 7 (28.0) | ||
| Bachelor degree or above | 3 (13.0) | 5 (26.3) | 11 (44.0) |
Due to a missing CRP value in group 1 and 2 respectively, an interpolation method was used to process the missing data. The interpolated variable was named CRP (new). There were no significant differences in the serum CRP or CRP (new) levels among the three groups. There were significant differences in FC among the three groups (χ2 = 24.060, P < 0.001). Significant differences were observed between group 1 and 3 (P < 0.05), as well as between group 2 and 3 (P < 0.05). However, there was no significant difference between group 1 and 2 (P > 0.05; Table 2).
| Variables | Group 1 (n = 23) | Group 2 (n = 19) | Group 3 (n = 25) | Value | P value |
| CRP [M (P25, P75), mg/L] | 5.17 (2.20, 14.6) | 4.84 (3.28, 23.29) | 13.5 (4.02,27.75) | H = 3.832 | 0.147 |
| CRP (new) [M (P25, P75), mg/L]1 | 5.9 (2.3, 13.9) | 6.03 (3.3, 17.935) | 13.5 (4.02,27.75) | H = 3.532 | 0.171 |
| Fecal calprotectin2 [n (%), ug/g] | |||||
| < 60 | 3 (13.6) (1) | 2 (11.8) (1) | 16 (64.0) (2) | χ2 = 24.060 | < 0.001a |
| 60-500 | 13 (59.1) (1) | 13 (76.5) (1) | 3 (12.0) (2) | ||
| > 500 | 6 (27.3) (1) | 2 (11.8) (1) | 6 (24.0) (2) |
There were statistically significant differences in the CDAI among the three groups (H = 12.893, P < 0.01). Group 3 had higher scores than group 2 (P < 0.05) and group 1 (P < 0.01). Furthermore, there were statistically significant differences in the PDAI among the three groups (H = 49.006, P < 0.001). Group 3 had higher scores than group 2 (P < 0.001) and group 1 (P < 0.001; Table 3).
The numbers of patients with one, two, and three anal fistulas found by both magnetic resonance imaging (MR) and EUS methods were 33, 2, and 1, respectively. In 7 patients, more fistulas were detected by MR than by EUS and in 1 patient, more fistulas were detected by EUS than by MR. The combined number of fistulas detected by both methods indicated 82% agreement. Based on the American Gastroenterological Association guidelines, anal fistulas are categorized as either simple or complex. MR revealed 14 patients with simple anal fistulas and 30 patients with complex anal fistulas. On the other hand, EUS revealed 17 patients with simple anal fistulas and 27 patients with complex anal fistulas. Thirteen patients were diagnosed with simple anal fistulas, and 26 patients were diagnosed with complex anal fistulas consistently by both methods. The results of the anal fistula classification, whether simple or complex, demonstrated significant consistency (Κ = 0.752, P < 0.001; Table 4).
| EUS diagnosis | MR diagnosis | Kappa | P value | |
| Simple fistulas | Complex fistulas | |||
| Simple fistulas | 13 | 4 | 0.752 | 0.000 |
| Complex fistulas | 1 | 26 | ||
The shear wave velocity of group 2 was 2.70 ± 0.52 m/s, while that of group 3 was 2.30 ± 0.57 m/s. These values were consistent with a normal distribution and homogeneous variance. A statistically significant difference in shear-wave velocity was observed between the two groups (t = 2.467, P < 0.05). In group 2, there were 11 patients with grade 0 blood flow, 5 patients with grade 1, 5 patients with grade 2, and no patients with grade 3. Conversely, group 3 had 4 cases of grade 0 blood flow, 8 cases of grade 1, 15 cases of grade 2, and 1 case of grade 3 blood flow. According to the Pearson χ2 test, there were statistically significant differences in the blood flow Limberg grades between the two groups (χ2 = 8.903, P < 0.05; Table 5).
| Variables | Group 2 (n = 19) | Group 3 (n = 25) | Value | P value |
| Shear wave velocity, m/S | 2.70 ± 0.52 m/S | 2.30 ± 0.57 | t = 2.467 | 0.018 |
| Limberg grade | ||||
| Level 0 | 11 | 4 | χ2 = 8.903 | 0.012 |
| Level 1 | 5 | 8 | ||
| Level 2 or above | 5 | 16 |
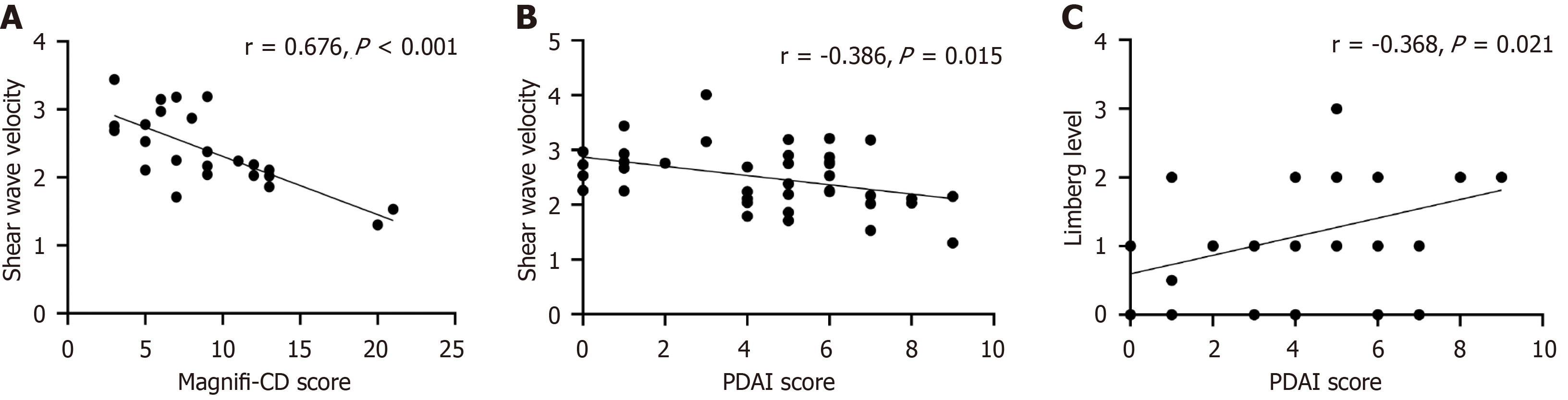
In group 2 and group 3, a total of 24 patients underwent enhanced pelvic MRI, and the Magnifi-CD score was calculated to compare the correlations. The SWV was 2.25 (2.03, 2.85) m/s, while the Magnifi-CD score was 8.50 (5.25, 12.00). Spearman correlation analysis revealed a strong negative correlation between the SWV and the Magnifi-CD score (rs = -0.676, P < 0.001; Figure 2A). Further correlation analysis was conducted in 39 patients to examine the relationship between the SWV and factors such as the PDAI, Limberg classification and serum CRP levels. The SWV was 2.53 (2.11, 2.87) m/s, the PDAI was 5.00 (2.00, 6.00), and the blood flow was 1.00 (0.00, 2.00), and this study revealed that a weak negative correlation between the SWV and the PDAI score (rs = -0.386, P < 0.05; Figure 2B) ,whereas the correlation between Limberg classification and PDAI score was weak (rs = 0.368, P < 0.05; Figure 2C) .Based on the scatter diagram and correlation analysis, no significant correlation was found between SWV and factors such as the CDAI score, CRP level, or Limberg classification. Furthermore, there was no correlation between the number or type of fistula and the PDAI score.
Our study found that shear wave velocity was highly accurate to evaluate PFCD activity based on the PDAI. Based on these observations, it can be inferred that the elastic modulus and shear-wave velocity may serve as indicators of the degree of fibrosis in anal fistulas. Furthermore, the activity of anal fistulas can also be effectively identified.
The MAGNIFI-CD[16] serves as an indicator for assessing the severity of anal fistulas associated with CD through enhanced MR. It was also verified to be accurate in predicting long-term clinical closure and seem valuable in follow-up of perianal CD[6]. In our study, we utilized the MAGNIFI-CD score as a benchmark and discovered a significant negative correlation between shear wave velocity and the MAGNIFI-CD score (r = -0.676, P < 0.001). This finding contributes to further bolstering the potential of SWE as a valuable tool for effectively monitoring the activity and extent of CD anal fistulas.
The Limberg grade is a recognized semiquantitative evaluation standard for assessing blood flow in the intestinal wall. It serves as an indicator of CD disease activity and correlates with the endoanal score[22]. Previous studies[13] have demonstrated significant differences in the Limberg grading scores of intestinal wall blood flow among different levels of inflammation, with Limberg III serving as the threshold for distinguishing mild/moderate inflammation from severe inflammation. In this study, EUS was utilized to measure the blood flow in fistulas, and the modified Limberg grading system was used[15]. The findings revealed that the Limberg grade for the high-activity anal fistula group was significantly greater than that for the low-activity anal fistula group, and the difference between the two groups was statistically significant. These results indicate that the blood flow of the fistula can provide insight into the activity of the fistula to a certain extent.
This study, for the first time, employed EUS + SWE to evaluate the elasticity of different active perianal fistulas in CD patients. This study revealed that the elastic modulus of the high-activity anal fistula group was significantly lower than that of the low-activity anal fistula group. According to the conversion formula, the shear wave velocity of the high active anal fistula group was also significantly lower than that of the low active anal fistula group. Multiple studies have shown that both techniques have similar accuracy in diagnosing PFCD[4]. Notably, our study also revealed strong agreement between EUS and MRI in determining the number of fistulas, as well as high consistency in classifying anal fistulas as either simple or complex (Kappa = 0.752, P < 0.001).
However, EUS lacks clear criteria to differentiate perianal fistula activity. According to Losco et al[19], active anal fistulas exhibit a significantly lower gray tone on intracavitary ultrasound than inactive anal fistulas. But this approach may heavily rely on the sonographer's experience. It is crucial to develop more objective and user-friendly methods to assess the activity of anal fistulas. SWE is a new technology that has emerged widely in the field of ultrasound in recent years. This study employed to select and fix the elastic imaging area of interest (ROI), when the M-STB index reached a 5-star rating, the elastic value was effectively measured three times consecutively, and the average elastic value (E, measured in kPa) was recorded. SWE allows for the quantification of fibrosis levels and offers several notable advantages, such as being noninvasive, repeatable, objective, and easy[11].
The PDAI has been widely used to accurately assess the activity of CD anal fistulas in various clinical trials[19,20]. In this particular study, shear wave velocity exhibited a weak negative correlation with the PDAI score (r = -0.386, P < 0.05), while vascular grade showed a weak positive correlation with the PDAI score (r = 0.368, P < 0.05). In other clinical trials showed, the poor correlation between MAGNIFI-CD and PDAI, CDAI, and other clinical indicators might be attributed to the minimal radiological changes posttreatment unless complete healing of the fistula occurs, the significant time interval between symptom relief and notable radiological improvement, and the subjective nature of clinical symptoms[23]. Nonetheless, radiological healing or complete fibrosis of fistulas is recognized as a crucial prognostic measure that has been associated with improved long-term outcomes in patients with PFCD[6,24]. Despite the weak correlation, the significant associations between shear wave velocity, vascular grade, and the PDAI suggest that these factors can provide insight into the trend of anal fistula activity.
In this study, it was found that patients with high activity anal fistulas had significantly greater CDAI scores and FC levels than did those with low activity anal fistulas or without anal fistulas. Currently, it remains unclear whether the CDAI directly correlates with the activity of anal fistula. It is suspected that most patients with highly active anal fistulas also have active intestinal lesions simultaneously. FC has been showed as a marker of mucosal inflammation in CD[25]. However, a retrospective study in the Netherlands[26] revealed that FC can be used to distinguish between active perianal fistulas in patients with CD and ordinary anal fistulas, even in the absence of intestinal ulcers. However, it should be noted that the association between active anal fistula and FC has not been proven to be causal[3].
This study has several limitations that need to be acknowledged. First, importantly, this was a single-center prospective study, which inherently has certain limitations in terms of its design and patient numbers. To ensure the accuracy and validity of the results, strict inclusion and exclusion criteria were utilized. Second, this study primarily assessed the activity of anal fistulas. As the majority of patients had mild anal fistula lesions and did not meet the criteria for anesthesia surgical exploration, the traditional surgical gold standard was not employed. Instead, the PDAI, which is more appropriate for evaluating the activity of CD anal fistulas, was utilized as the benchmark for diagnosing anal fistula activity.
In summary, EUS combined with SWE offers a superior method for evaluating and quantitating the activity of perianal fistulas in CD patients. It may be the ideal tool to assess PFCD activity objectively for management strategies. However, importantly, our findings require confirmation and validation through larger-scale, multicenter studies.
| 1. | Chidi VN, Schwartz DA. Imaging of perianal fistulizing Crohn's disease. Expert Rev Gastroenterol Hepatol. 2015;9:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 708] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | Kotze PG, Shen B, Lightner A, Yamamoto T, Spinelli A, Ghosh S, Panaccione R. Modern management of perianal fistulas in Crohn's disease: future directions. Gut. 2018;67:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, de Buck van Overstraeten A, Burke JP, Buskens CJ, Colombo F, Dias JA, Eliakim R, Elosua T, Gecim IE, Kolacek S, Kierkus J, Kolho KL, Lefevre JH, Millan M, Panis Y, Pinkney T, Russell RK, Shwaartz C, Vaizey C, Yassin N, D'Hoore A. ECCO-ESCP Consensus on Surgery for Crohn's Disease. J Crohns Colitis. 2018;12:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, Levesque BG, D'Haens G; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Chambaz M, Verdalle-Cazes M, Desprez C, Thomassin L, Charpentier C, Grigioni S, Armengol-Debeir L, Bridoux V, Savoye G, Savoye-Collet C. Deep remission on magnetic resonance imaging impacts outcomes of perianal fistulizing Crohn's disease. Dig Liver Dis. 2019;51:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | van Rijn KL, Meima-van Praag EM, Bossuyt PM, D'Haens GR, Gecse KB, Horsthuis K, Snijder HJ, Tielbeek JAW, Buskens CJ, Stoker J. Fibrosis and MAGNIFI-CD Activity Index at Magnetic Resonance Imaging to Predict Treatment Outcome in Perianal Fistulizing Crohn's Disease Patients. J Crohns Colitis. 2022;16:708-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Halligan S, Stoker J. Imaging of fistula in ano. Radiology. 2006;239:18-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2020;214:W20-W26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 11. | Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Shear-Wave Elastography for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-Analysis. AJR Am J Roentgenol. 2017;209:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Ślósarz D, Poniewierka E, Neubauer K, Kempiński R. Ultrasound Elastography in the Assessment of the Intestinal Changes in Inflammatory Bowel Disease-Systematic Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Chen YJ, Mao R, Li XH, Cao QH, Chen ZH, Liu BX, Chen SL, Chen BL, He Y, Zeng ZR, Ben-Horin S, Rimola J, Rieder F, Xie XY, Chen MH. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn's Disease. Inflamm Bowel Dis. 2018;24:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1148] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 15. | Novak KL, Nylund K, Maaser C, Petersen F, Kucharzik T, Lu C, Allocca M, Maconi G, de Voogd F, Christensen B, Vaughan R, Palmela C, Carter D, Wilkens R. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A Reliability and Inter-rater Variability Study on Intestinal Ultrasonography in Crohn's Disease. J Crohns Colitis. 2021;15:609-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Hindryckx P, Jairath V, Zou G, Feagan BG, Sandborn WJ, Stoker J, Khanna R, Stitt L, van Viegen T, Shackelton LM, Taylor SA, Santillan C, Mearadji B, D'Haens G, Richard MP, Panes J, Rimola J. Development and Validation of a Magnetic Resonance Index for Assessing Fistulas in Patients With Crohn's Disease. Gastroenterology. 2019;157:1233-1244.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Kim ES, Park KS, Cho KB, Kim KO, Jang BI, Kim EY, Jung JT, Jeon SW, Jung MK, Lee HS, Yang CH, Lee YK; Daegu–Gyeongbuk Gastrointestinal Study Group (DGSG). Development of a Web-based, self-reporting symptom diary for Crohn's Disease, and its correlation with the Crohn's Disease Activity Index. J Crohns Colitis. 2017;11:1449-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27-32. [PubMed] |
| 19. | Losco A, Viganò C, Conte D, Cesana BM, Basilisco G. Assessing the activity of perianal Crohn's disease: comparison of clinical indices and computer-assisted anal ultrasound. Inflamm Bowel Dis. 2009;15:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Schwartz DA, Ghazi LJ, Regueiro M. Guidelines for medical treatment of Crohn's perianal fistulas: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2015;21:737-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 22. | Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, Dubinsky M, Seidman EG. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Vuyyuru SK, Solitano V, Singh S, Hanzel J, Macdonald JK, Danese S, Peyrin Biroulet L, Ma C, Jairath V. Scoring Indices for Perianal Fistulising Crohn's Disease: A Systematic Review. J Crohns Colitis. 2024;18:836-850. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Papamichael K, Cheifetz AS. Defining and predicting deep remission in patients with perianal fistulizing Crohn's disease on anti-tumor necrosis factor therapy. World J Gastroenterol. 2017;23:6197-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J Crohns Colitis. 2013;7:e641-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Stevens TW, D'Haens GR, Duijvestein M, Bemelman WA, Buskens CJ, Gecse KB. Diagnostic accuracy of faecal calprotectin in patients with active perianal fistulas. United European Gastroenterol J. 2019;7:496-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |