Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2461
Revised: June 17, 2024
Accepted: July 8, 2024
Published online: August 27, 2024
Processing time: 179 Days and 19.5 Hours
Gastric cancer is associated with significant undernutrition responsible for an increase in morbidity and mortality after gastrectomy.
To evaluate the impact of enteral nutrition by jejunostomy feeding in patients undergoing gastrectomy for cancer.
Between 2003 and 2017, all patients undergoing gastrectomy for cancer treatment were included retrospectively. A group with jejunostomy (J + group) and a group without jejunostomy (J - group) were compared.
Of the 172 patients included, 60 received jejunostomy. Preoperatively, the two groups were comparable with respect to the nutritional parameters studied (body mass index, albumin, etc.). In the postoperative period, the J + group lost less weight and albumin: 5.74 ± 8.4 vs 9.86 ± 7.5 kg (P = 0.07) and 7.2 ± 5.6 vs 14.7 ± 12.7 g/L (P = 0.16), respectively. Overall morbidity was 25% in the J + group and 36.6% in the J - group (P = 0.12). The J + group had fewer respiratory, infectious, and grade 3 complications: 0% vs 5.4% (P = 0.09), 1.2% vs 9.3% (P = 0.03), and 0% vs 4.7% (P = 0.05), respectively. The 30-day mortality was 6.7% in the J + group and 6.3% in the J - group (P = 0.91).
Jejunostomy feeding after gastrectomy improves nutritional characteristics and decreases postoperative morbidity. A prospective study could confirm our results.
Core Tip: Jejunostomy feeding in patients undergoing gastrectomy for cancer significantly improves postoperative nutritional status and reduces complications. A study of 172 patients showed that those with jejunostomy had less weight loss, better albumin levels, and fewer respiratory, infectious, and grade 3 complications compared to those without jejunostomy. Overall morbidity was lower, though 30-day mortality rates were similar between the groups.
- Citation: Jaquet R, Rivkine E, De Souza N, Roudié J. Benefits of jejunostomy feeding in patients who underwent gastrectomy for cancer treatment. World J Gastrointest Surg 2024; 16(8): 2461-2473
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2461.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2461
Gastric cancer is a serious pathology responsible for significant mortality. Worldwide, it is the fifth most common cancer but the third leading cause of cancer deaths, with a fatality rate of 75%[1]. According to the National Cancer Institute, gastric cancer is more common in the Caribbean arc, especially in Martinique, affecting 1.58 times more men and 2.31 times more women than elsewhere in the world. Gastrectomy, the only potentially curative treatment, is a source of significant morbidity, which, according to the literature, varies from 11% to 46%[2]. The poor nutritional status of patients with gastric cancer, secondary to various factors such as impaired gastric mechanics or decreased dietary intake secon
In Martinique, it seems that patients are particularly fragile and malnourished, with a consultation time that is often delayed. Gastric cancers appear to be frequently diagnosed at an advanced or even symptomatic stage (high occlusive syndrome, haemorrhagic tumours). Recently, the fragility of these patients has motivated the surgeons of our department to introduce jejunostomy feeding. Our objective was therefore to evaluate the impact of enteral feeding by jejunostomy in patients undergoing gastrectomy for cancer treatment.
We conducted a monocentric retrospective study in the University Hospital of Fort de France in Martinique between 1 January 2003 and 30 April 2017, seeking to compare different postoperative results in all patients who had undergone gastrectomy for cancer treatment. The inclusion criterion was any adult patient (≥ 18 years) who had been diagnosed with gastric cancer and who had undergone gastrectomy. There were no exclusion criteria. Two groups were compared: A jejunostomy group (J + group) and a group without jejunostomy (J - group). The evaluation of morbimortality was the primary endpoint. Secondary criteria were length of stay and survival time.
Jejunostomy tube placement according to Fontan[5]: The technique according to Fontan was chosen with direct insertion of the tube into the jejunal lumen without an intraparietal path. The insertion orifice of the tube was fixed directly to the parietal peritoneum without prior burying.
We had the authorization of the National Commission for Informatics and Liberties for the recovery of the names of patients corresponding to our search criteria (No. 2065145 v 0). The operative procedures were found thanks to the coding of the Common Classification of Medical Acts (CCAM), and the diagnoses were found using the 10th version of the International Classification of Diseases (ICD10) motivated by the Program of Medicalization of Information Systems. The Department of Medical Information of the University Hospital of Fort de France was contacted to find patients corresponding to the CCAM and ICD10 codes.
All demographic, histological, nutritional, anaesthetic, operative, postoperative, and length-of-stay data were collected. The complications were graded according to the Dindo-Clavien classification[6] (grade 1: Any deviation of normal postoperative consequences without the need for intervention, except the administration of antiemetics, antipyretics, analgesics, diuretics, or electrolytes; grade 2: A complication requiring pharmacological treatment, different from that used for a grade 1 complication; grade 3: A complication requiring surgical, endoscopic, or radiological treatment; grade 4: A life-threatening complication requiring hospitalization in an intensive care unit; grade 5: Death). Vital status data, dates of the latest news, and death dates were mostly present. The vital missing data could be retrieved by contacting the Martinican Association for Epidemiological Research in Cancer. Nutritional data - weight, body mass index (BMI), albuminemia, prealbuminemia, prognostic nutritional index (PNI)[7], PNI status < 46[8], and nutritional status defined by ANAES (undernutrition and severe undernutrition)[9] - were recorded preoperatively at least one month before the operation and up to two months postoperatively. When two values of the same variable were available preoperatively, the value closest to the date of intervention was used. In the postoperative period, the value closest to two months after the intervention date was used to have a clearer picture of the patient’s nutritional status, whether the patient was enterally fed or not.
All data acquisition was carried out in an Excel spreadsheet (Microsoft, Redmond, WA, United States). Categorical variables were expressed as number of cases and percentages, while continuous variables were expressed as mean and standard deviation. Categorical variables were compared by Pearson’s χ2 test for all multinomial and binomial variables except when one of the expected contingency table counts was less than 5, in which case Fisher's exact test was used. Continuous variables were compared using Student's t-test after systematic verification of the homogeneity of the variances by a Levene test. Overall and disease-free survival times were analysed by the Kaplan-Meier method, and survival curves of the two groups were compared by the Mantel-Cox test (or log-rank test). Survival times were expressed by their median value with a 95%CI, and their dispersion was expressed by the interquartile range. All tests were bilateral, and P < 0.05 was considered significant. Analyses were conducted on the entire cohort, with subgroup analysis for partial and total gastrectomies. A multivariate analysis adjusted for age, sex, dementia, prior Helicobacter pylori infection, history of stroke, heart failure, atrial fibrillation and severe preoperative undernutrition was performed. Statistical analysis was performed using SPSS, version 20.0, for Mac (Armonk, NY, United States).
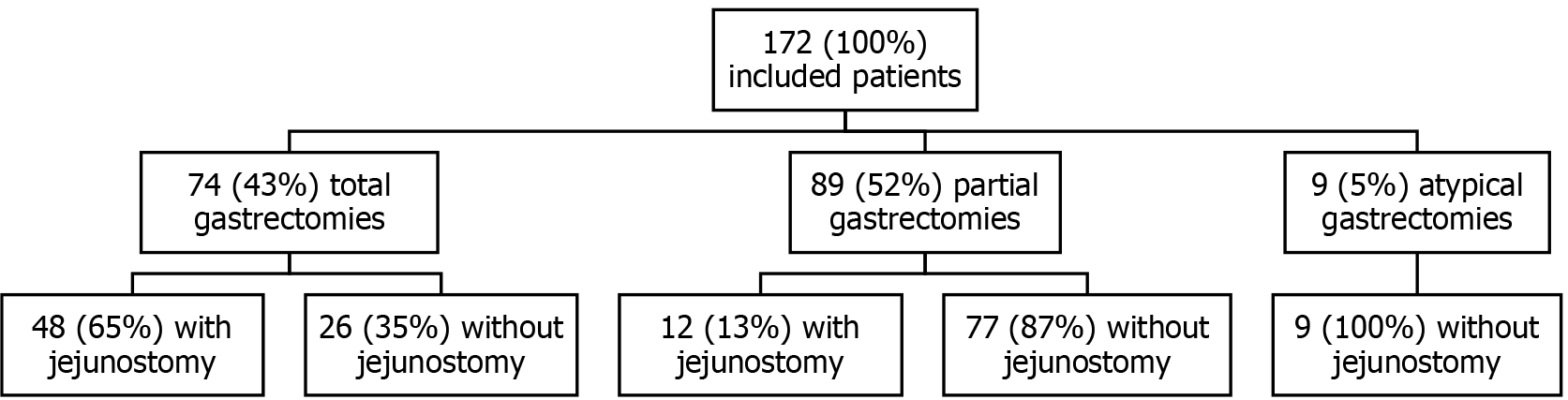
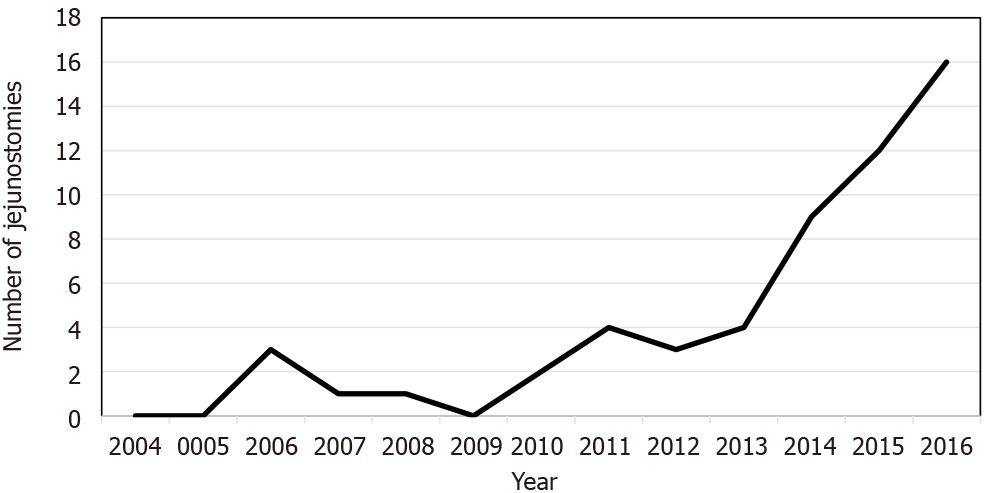
A total of 172 patients underwent gastrectomy for cancer treatment between 1 January 2003 and 30 April 2017. Of these, 74 (43%) had a total gastrectomy, 89 (52%) had a partial gastrectomy, and 9 (5%) had an atypical gastrectomy. Forty-eight (65%) of the patients with a total gastrectomy had jejunostomy, while 12 (13%) of the patients with a partial gastrectomy had jejunostomy. No patients with an atypical gastrectomy received jejunostomy (Figure 1). The baseline characteristics of the two groups were comparable except for the male sex, which was more frequently represented in the J + group than in the J - group: 43 (71.7%) vs 57 (50.9%) (P = 0.01; Table 1). The preoperative nutritional status of both groups was similar, although the BMI values of the J + group patients tended to be lower: 22.7 ± 4.6 vs 24.2 ± 4.8 kg/m² (P = 0.09; Table 2). Tumours in the J + group were more advanced than those in the J - group, especially T4 tumours: 19 (31.7%) vs 15 (13.4%) (P = 0.036; Table 3). Over time, the number of patients who received jejunostomy increased, especially from 2010 to 2011 (Figure 2).
| Variables | J + (n = 60) | J - (n = 112) | P value |
| Age (years, mean ± SD) | 69.4 ± 12.6 | 70.2 ± 11.2 | 0.65 |
| Male | 43 (71.7) | 57 (50.9) | 0.01a |
| H. pylori infection | 11 (18.3) | 13 (11.6) | 0.25 |
| Smoking | 6 (10.2) | 12 (10.8) | 1 |
| Medical background | 40 (66.7) | 77 (69.4) | 0.73 |
| Dementia | 5 (8.3) | 4 (3.6) | 0.28 |
| Stroke | 6 (10) | 7 (6.2) | 0.38 |
| AF | 7 (11.7) | 8 (7.2) | 0.4 |
| CAD | 4 (6.7) | 7 (6.2) | 1 |
| Heart failure | 6 (10) | 5 (4.5) | 0.2 |
| LEAD | 4 (6.7) | 7 (6.2) | 1 |
| Diabetes | 15 (25) | 28 (25) | 1 |
| Other cancer | 12 (20) | 18 (16.1) | 0.53 |
| Abdominal surgery | 18 (30) | 46 (41.1) | 0.19 |
| AC/APA | 21 (35.6) | 26 (23.9) | 0.11 |
| Corticoids/IT | 1 (1.7) | 2 (1.8) | 1 |
| WHO score | |||
| 0 | 18 (38.3) | 37 (38.9) | 0.58 |
| 1 | 20 (42.6) | 43 (45.3) | |
| 2 | 5 (10.5) | 12 (12.6) | |
| 3 | 4 (8.5) | 3 (3.2) |
| Variables | J + (n = 60) | J - (n = 112) | P value |
| Weight (kg, mean ± SD) | 64 ± 12.9 | 66.7 ± 14.3 | 0.23 |
| BMI (kg/m2, mean ± SD) | 22.7 ± 4.6 | 24.2 ± 4.8 | 0.09 |
| Weight loss (kg, mean ± SD) | 12.2 ± 6.4 | 10.1 ± 4.3 | 0.15 |
| Proportion weight loss (%, mean ± SD) | 15.9 ± 8.5 | 13.9 ± 5.5 | 0.21 |
| Duration of weight loss (months, mean ± SD) | 3.9 ± 5.2 | 4.7 ± 4.3 | 0.52 |
| Preoperatory albuminemia (g/L, mean ± SD) | 32.3 ± 6.9 | 32.6 ± 8.6 | 0.87 |
| Preoperatory prealbuminemia (g/L, mean ± SD) | 0.19 ± 0.06 | 0.17 ± 0.05 | 0.3 |
| PNI (mean ± SD) | 41.2 ± 8.8 | 39.8 ± 12 | 0.74 |
| PNI < 46 | 18 (54.5) | 13 (81.2) | 0.22 |
| Undernutrition | 21 (36.2) | 34 (35.7) | 1 |
| Severe undernutrition | 16 (28.1) | 17 (17.9) | 0.55 |
| Variables | J + (n = 60) | J - (n = 112) | P value |
| Adenocarcinoma | 47 (79.7) | 87 (78.4) | 1 |
| Grade of differentiation | |||
| Good | 5 (10.9) | 7 (9) | 0.76 |
| Moderate | 18 (39.1) | 38 (48.7) | 0.35 |
| Poor | 20 (43.5) | 31 (39.7) | 0.71 |
| Contingent of independent cells | 23 (38.3) | 33 (30.3) | 0.31 |
| GIST | 9 (15.5) | 22 (20.2) | 0.53 |
| Low risk | 1 (11.1) | 8 (40) | 0.2 |
| Medium risk | 1 (11.1) | 3 (15) | 1 |
| High risk | 7 (77.8) | 9 (45) | 0.13 |
| Tumour ≤ 3 cm | 10 (16.7) | 20 (18.9) | 0.84 |
| T | |||
| 0 | 0 (0) | 3 (2.7) | 0.036a |
| 1 | 5 (8.3) | 12 (10.7) | |
| 2 | 6 (10) | 21 (18.8) | |
| 3 | 23 (38.8) | 39 (34.8) | |
| 4 | 19 (31.7) | 15 (13.4) | |
| N0 | 18 (32.1) | 31 (34.4) | 0.86 |
| M0 | 50 (90.9) | 79 (84.9) | 0.45 |
| Clear margins | 45 (75) | 92 (84.4) | 0.15 |
| Vascular invasion | 25 (43.1) | 48 (44) | 1 |
| Perineural invasion | 27 (46.6) | 39 (36.1) | 0.24 |
Concerning morbidity and mortality, there was no difference in 30-day mortality between the groups: 6.7% vs 6.3% for the J + and J - groups, respectively (P = 1). In total, 56 patients presented at least one complication, with a total of 84 complications recorded. The overall complication rate was 25% in the J + group, compared to 36.6% in the J - group, with no statistically significant difference (P = 0.12; Table 4). Regarding the specific complications, the rate of anastomotic fistula was comparable: 2 (3.3%) vs 6 (5.4%) for the J + and J - groups, respectively (P = 0.71). Patients in the J + group had fewer infectious complications than those in the J - group: 2 (1.2%) vs 16 (9.3%) (P = 0.03). They also tended to present fewer respiratory complications: 0 (0%) vs 6 (5.4%) (P = 0.09; Table 4). There were no differences in complications according to the Dindo-Clavien classification for grades 1, 2, 4, and 5 between the groups. However, patients in the J + group had fewer grade 3 complications: 0 (0%) vs 8 (4.7%) (P = 0.05). Regarding high-grade complications (≥ 4), we observed two grade 4 complications all in J - group patients: One case of respiratory distress due to hypoxemic pneumonia caused by Klebsiella pneumoniae infection and one case of massive pulmonary embolism. Additionally, there were 11 grade 5 complications, including 4 in patients in group J +, including four cases of septic shock due to hypoxemic pneumonia, one case of aspiration pneumonia in a J + patient, one case of bilateral massive pulmonary embolism in a J + patient, one case of duodenal fistula leading to septic shock, one case of cardiorespiratory arrest, one case of hemorrhage on postoperative day 2, one case of extensive small bowel necrosis in a J + patient on postoperative day 11, and one death of unknown cause (J + patient).
| J + (n = 60) | J - (n = 112) | P value | |
| Global complications | 15 (25) | 41 (36.6) | 0.12 |
| Thromboembolic | 2 (1.2) | 4 (2.3) | 1 |
| Respiratory (other than aspiration pneumonia and thromboembolic) | 0 (0) | 6 (5.4) | 0.09 |
| Aspiration pneumonia | 2 (3.3) | 0 (0) | 0.12 |
| Haemorrhagic | 1 (1.7) | 6 (5.4) | 0.42 |
| Infectious | 2 (1.2) | 16 (9.3) | 0.03a |
| Deep collection | 2 (1.2) | 3 (1.7) | 1 |
| Ileus | 4 (2.3) | 10 (5.8) | 0.77 |
| Occlusion | 0 (0) | 3 (1.7) | 0.55 |
| Fistula | 2 (3.3) | 6 (5.4) | 0.71 |
| Others | 3 (1.7) | 9 (5.2) | 0.54 |
| Renutrition syndrome | 1 (0.6) | 0 (0) | 0.34 |
| Probe obstruction | 1 (0.6) | 0 (0) | 0.34 |
| Abscess around the probe | 1 (0.6) | 0 (0) | 0.34 |
Among the secondary outcomes, the lengths of stay varied between groups. The length of stay was longer for the J + group: On average 20.49 ± 9.32 vs 16.45 ± 9.47 days (P = 0.01). These longer durations were at the expense of conventional hospitalization: 17.75 ± 8.50 vs 14.06 ± 7.71 days (P = 0.01), with no significant difference between the groups regarding the postoperative length of stay (i.e., between the day of the intervention and the day of discharge) (Table 5). Concerning the postoperative nutritional characteristics, these worsened in both groups, but this worsening was less among patients in the J + group: A tendency towards less weight loss, 5.74 ± 8.4 vs 9.86 ± 7.5 kg (P = 0.07), and a smaller decrease in albumin, 7.2 ± 5.6 vs 14.7 ± 12.7 g/L (P = 0.15; Table 6).
| Variables | J + | J - | P value |
| Weight (kg, mean ± SD) | 58.86 ± 11.8 | 56 ± 9.8 | 0.34 |
| Weight loss (kg, mean ± SD) | 5.74 ± 8.4 | 9.86 ± 7.5 | 0.07 |
| Proportion weight loss (%, mean ± SD) | 10.25 ± 8 | 14.19 ± 9.5 | 0.12 |
| Albuminemia (g/L, mean ± SD) | 31.4 ± 5.5 | 32.3 ± 10.9 | 0.8 |
| Loss of albuminemia (g/L, mean ± SD) | 7.2 ± 5.6 | 14.7 ± 12.7 | 0.15 |
| Proportion albuminemia loss (%, mean ± SD) | 20 ± 15.6 | 40 ± 33.1 | 0.16 |
In subgroup analysis, whether in the partial (Table 7) or total (Table 8) gastrectomy cohort, there was no significant difference on morbidity between the groups with or without jejunostomy. In multivariate analysis (Table 9), in the entire cohort, a history of dementia was a significant predictor of overall complications: Odds ratio (OR) = 6.42, 95%CI: 1.19-52.4, P = 0.04, and infectious complications: OR = 9.75, 95%CI: 1.03-88.8, P = 0.04. Additionally, among partial gastrectomies, a prior Helicobacter pylori infection increased the risk of other complications: OR = 6.41, 95%CI: 0.85-43.91, P = 0.04.
| J + (n = 12) | J - (n = 77) | P value | |
| Global complications | 4 (33.3) | 30 (38.9) | 0.96 |
| Thromboembolic | 0 (0) | 2 (2.6) | 1 |
| Respiratory (other than aspiration pneumonia and thromboembolic) | 0 (0) | 6 (7.8) | 0.7 |
| Aspiration pneumonia | 1 (8.3) | 0 (0) | 0.28 |
| Haemorrhagic | 0 (0) | 3 (3.9) | 1 |
| Infectious | 0 (0) | 10 (12.9) | 0.4 |
| Deep collection | 0 (0) | 2 (2.6) | 1 |
| Ileus | 1 (8.3) | 8 (10.4) | 1 |
| Occlusion | 0 (0) | 2 (2.6) | 1 |
| Fistula | 1 (6.5) | 5 (8.3) | 1 |
| Others | 1 (8.3) | 7 (9.1) | 1 |
| Renutrition syndrome | 0 (0) | 0 (0) | 1 |
| Probe obstruction | 0 (0) | 0 (0) | 1 |
| Abscess around the probe | 1 (8.3) | 0 (0) | 0.28 |
| J + (n = 48) | J - (n = 26) | P value | |
| Global complications | 11 (22.9) | 7 (26.9) | 0.92 |
| Thromboembolic | 2 (4.2) | 1 (3.8) | 1 |
| Respiratory (other than aspiration pneumonia and thromboembolic) | 0 (0) | 0 (0) | 1 |
| Aspiration pneumonia | 1 (2.1) | 0 (0) | 1 |
| Haemorrhagic | 1 (2.1) | 1 (3.8) | 1 |
| Infectious | 2 (4.2) | 4 (15.4) | 0.21 |
| Deep collection | 2 (4.2) | 1 (3.8) | 1 |
| Ileus | 3 (6.3) | 1 (3.8) | 1 |
| Occlusion | 0 (0) | 0 (0) | 1 |
| Fistula | 1 (2.1) | 1 (3.8) | 1 |
| Others | 2 (4.2) | 2 (7.7) | 0.92 |
| Renutrition syndrome | 1 (2.1) | 0 (0) | 1 |
| Probe obstruction | 1 (2.1) | 0 (0) | 1 |
| Abscess around the probe | 0 (0) | 0 (0) | 1 |
| Type of cohort | Variables | Age | Sex (M) | Dementia | H. pylori infection | Sroke | Heart failure | Atrial fibrillation | Severe undernutrition | ||||||||||||||||
| Estimates | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | |
| Entire cohort | Global complications | 1 | 0.97-1.03 | 0.51 | 0.79 | 0.41-1.55 | 0.49 | 6.42 | 1.19-52.4 | 0.04a | 1.74 | 0.67-4.45 | 0.25 | 0.26 | 0.03-1.26 | 0.14 | 0.66 | 0.12-2.88 | 0.61 | 0.6 | 0.13-2.16 | 0.47 | 1.28 | 0.61-2.64 | 0.52 |
| Thromboembolic | 0.95 | 0.88-1.02 | 0.18 | 1.37 | 0.24-10.54 | 0.73 | - | - | - | 0.78 | 0.03-6.63 | 0.85 | - | - | - | 8.36 | 0.33-110.9 | 0.12 | - | - | - | 0.51 | 0.03-3.54 | 0.56 | |
| Respiratory | 1.05 | 0.96-1.16 | 0.33 | 0.36 | 0.04-2.1 | 0.27 | 7 | 0.21-113 | 0.19 | 4.15 | 0.42-32.3 | 0.17 | - | - | - | 1.47 | 0.05-16.94 | 0.78 | 1.94 | 0.09-16.88 | 0.59 | 0.2 | 0-2.05 | 0.27 | |
| Aspiration pneumonia | 1.17 | 0.93-1.77 | 0.28 | 2.13 | 0.05-603 | 0.7 | 12.79 | 0.12-863 | 0.21 | 2.34 | 0.01-102 | 0.66 | - | - | - | 12.1 | 0.11-760 | 0.21 | - | - | - | 0.31 | 0-17.5 | 0.61 | |
| Haemorrhagic | 0.99 | 0.93-1.06 | 0.8 | 0.51 | 0.1-2.41 | 0.39 | - | - | - | 1.34 | 0.07-9 | 0.8 | - | - | - | - | - | - | - | - | - | 1.11 | 0.15-5.5 | 0.91 | |
| Infectious | 0.99 | 0.95-1.04 | 0.69 | 0.53 | 0.19-1.48 | 0.23 | 9.75 | 1.03-88.8 | 0.04a | 0.66 | 0.09-2.9 | 0.63 | - | - | - | 0.66 | 0.03-4.97 | 0.73 | 1.72 | 0.23-8.36 | 0.54 | 0.64 | 0.16-2.03 | 0.48 | |
| Deep collection | 1 | 0.92-1.09 | 0.92 | 0.13 | 0-1.03 | 0.09 | - | - | - | - | - | - | - | - | - | 13.84 | 0.4-488 | 0.11 | - | - | - | 3.21 | 0.44-26.99 | 0.24 | |
| Ileus | 1.03 | 0.98-1.09 | 0.22 | 1.05 | 0.33-3.47 | 0.94 | - | - | - | 1.36 | 0.19-5.94 | 0.71 | - | - | - | 0.87 | 0.04-6.01 | 0.91 | 1.68 | 0.22-8.16 | 0.56 | 2.06 | 0.62-6.48 | 0.22 | |
| Occlusion | 0.98 | 0.87-1.1 | 0.22 | 0.4 | 0.02-4.84 | 0.94 | - | - | - | 2.77 | 0.1-40.5 | 0.71 | 9.62 | 0.33-215.5 | 0.99 | - | - | - | - | - | - | - | - | - | |
| Fistula | 1.01 | 0.95-1.08 | 0.81 | 0.21 | 0.03-1 | 0.07 | 0.92 | 0.02-14.57 | 0.96 | 1.53 | 0.14-9.89 | 0.68 | 2.1 | 0.07-23.7 | 0.59 | 2.38 | 0.09-27.2 | 0.52 | - | - | - | 2.52 | 0.49-12.9 | 0.25 | |
| Others | 1.01 | 0.95-1.07 | 0.79 | 1.36 | 0.39-5.42 | 0.64 | 2.53 | 0.22-20.3 | 0.41 | 2.11 | 0.39-8.61 | 0.33 | 1.6 | 0.16-10.13 | 0.65 | - | - | - | - | - | - | 1.03 | 0.23-3.82 | 0.97 | |
| Partial gastrectomies | Global complications | 1.02 | 0.98-1.07 | 0.34 | 0.8 | 0.29-2.17 | 0.66 | - | - | - | 4.41 | 0.93-25.3 | 0.07 | 0.53 | 0.03-4.15 | 0.59 | 1.11 | 0.13-7.9 | 0.92 | 0.61 | 0.08-3.3 | 0.59 | 0.86 | 0.29-2.4 | 0.77 |
| Thromboembolic | 0.98 | 0.82-1.22 | 0.83 | 0.57 | 0.01-20.3 | 0.74 | - | - | - | - | - | - | - | - | - | 39.9 | 0.88-540 | 0.06 | - | - | - | 0.98 | 0.02-32.7 | 0.99 | |
| Respiratory | 1.03 | 0.93-1.15 | 0.63 | 0.38 | 0.04-2.8 | 0.37 | 4.27 | 0.12-72.3 | 0.33 | 7.56 | 0.61-96.3 | 0.09 | - | - | - | 2.39 | 0.07-46.7 | 0.57 | 3.92 | 0.16-49.8 | 0.31 | 0.09 | 0-1.18 | 0.14 | |
| Aspiration pneumonia | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Haemorrhagic | 1.01 | 0.91-1.14 | 0.86 | 2.03 | 0.17-47.1 | 0.58 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.03 | 0.17-47.1 | 0.99 | |
| Infectious | 1.1 | 0.98-1.17 | 0.17 | 0.8 | 0.17-3.57 | 0.77 | 11.3 | 0.91-271.8 | 0.07 | 0.39 | 0.01-4.33 | 0.53 | - | - | - | 0.98 | 0.03-11.3 | 0.99 | 1.43 | 0.06-12.1 | 0.77 | 0.56 | 0.08-2.88 | 0.515 | |
| Deep collection | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Ileus | 1.03 | 0.97-1.12 | 0.34 | 1.36 | 0.3-6.53 | 0.69 | - | - | - | 1.1 | 0.05-8.21 | 0.94 | - | - | - | 1.33 | 0.06-11.7 | 0.82 | 1.11 | 0.05-9.6 | 0.93 | 2.27 | 0.51-10.4 | 0.27 | |
| Occlusion | 0.98 | 0.82-1.22 | 0.85 | 0.49 | 0.01-16.38 | 0.67 | - | - | - | - | - | - | 17.8 | 0.52-929 | 0.09 | - | - | - | - | - | - | - | - | - | |
| Fistula | 1.02 | 0.94-1.16 | 0.63 | 0.07 | 0-0.74 | 0.07 | 0.29 | 0-12.11 | 0.56 | 6.96 | 0.39-137.9 | 0.17 | 16.35 | 0.42-702 | 0.1 | 2.07 | 0.04-45.9 | 0.66 | - | - | - | 4.51 | 0.53-54.47 | 0.18 | |
| Others | 1.01 | 0.93-1.1 | 0.8 | 1.72 | 0.29-13.95 | 0.57 | 2.81 | 0.16-31.48 | 0.43 | 6.41 | 0.85-43.91 | 0.05 | 3.53 | 0.28-31.38 | 0.27 | - | - | - | - | - | - | 0.67 | 0.07-4 | 0.68 | |
| Total gastrectomies | Global complications | 0.98 | 0.93-1.03 | 0.38 | 0.73 | 0.22-2.52 | 0.61 | - | - | - | 0.73 | 0.1-3.62 | 0.72 | - | - | - | - | - | - | 1.01 | 0.05-8.95 | 0.99 | 2.3 | 0.62-8.4 | 0.2 |
| Thromboembolic | 0.93 | 0.82-1.02 | 0.15 | 2.5 | 0.16-85.16 | 0.54 | - | - | - | 2.98 | 0.11-49.1 | 0.44 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Respiratory | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Aspiration pneumonia | 1.11 | 0.91-1.63 | 0.46 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Haemorrhagic | 1 | 0.89-1.16 | 0.95 | 0.42 | 0.01-11.99 | 0.57 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.17 | 0.12-86.87 | 0.43 | |
| Infectious | 0.93 | 0.84-1.00 | 0.08 | 0.28 | 0.03-1.77 | 0.19 | - | - | - | 0.84 | 0.03-8.17 | 0.89 | - | - | - | - | - | - | 10.75 | 0.33-309 | 0.14 | 0.85 | 0.04-8.44 | 0.9 | |
| Deep collection | 1 | 0.9-1.12 | 0.96 | 0.18 | 0.01-2.4 | 0.21 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 7.53 | 0.61-184.88 | 0.13 | |
| Ileus | 1.04 | 0.94-1.19 | 0.47 | 1.85 | 0.19-46.88 | 0.63 | - | - | - | - | - | - | - | - | - | - | - | - | 4.92 | 0.17-87.66 | 0.27 | 1.68 | 0.07-20.5 | 0.69 | |
| Occlusion | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Fistula | 0.93 | 0.82-1.04 | 0.24 | 0.64 | 0.02-20.36 | 0.78 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Others | 1.02 | 0.94-1.13 | 0.62 | 0.38 | 0.04-3.54 | 0.37 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.04 | 0.05-9.63 | 0.97 | |
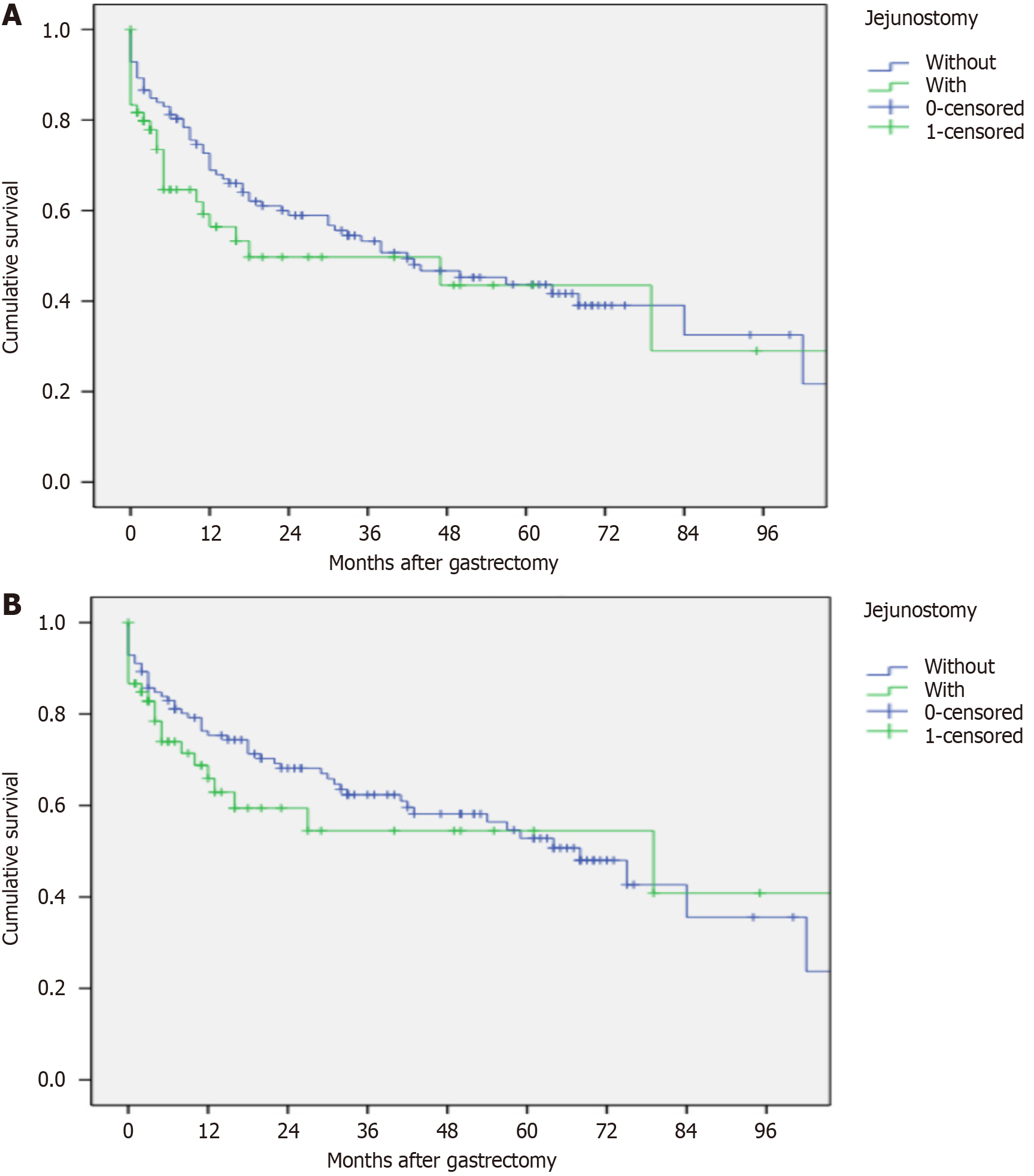
The median recurrence-free survival time of the cohort was 38 months with 95%CI: 20.1-55.8. There was no difference in median survival time between the J + and J - groups: 18 months with 95%CI: 0-59.7 vs 42 months with 95%CI: 20.3-63.6, respectively (P = 0.22; Figure 3A). The median overall survival time of the cohort was 68 months with 95%CI: 41.2-94.7. There was no difference in overall survival between the J + and J - groups: 79 months with 95%CI: 0-168.1 vs 68 months with 95%CI: 50.1-85.8, respectively (P = 0.36; Figure 3B).
Malnutrition (pre- and postoperative) is a recognized factor in increasing morbidity in the management of patients undergoing high-risk surgery[4]. We conducted a retrospective study seeking to compare morbidity and mortality in patients undergoing gastrectomy for cancer treatment. Overall, our groups were initially comparable, both in terms of clinical characteristics and in terms of nutritional characteristics. Histologically, the tumours in the J + group were a little more advanced. We demonstrated a significant positive impact of postoperative enteral nutrition via jejunostomy on some complications. In fact, enterally fed patients had fewer infectious complications, 2 (1.2%) vs 16 (9.3%) (P = 0.03); tended to have fewer respiratory complications, 0 (0%) vs 6 (5.4%) (P = 0.09); and had fewer grade 3 complications according to the Dindo-Clavien classification, 0 (0%) vs 8 (4.7%) (P = 0.05). Furthermore, the postoperative nutritional status, although worse for both groups, was better in patients fed via jejunostomy, both regarding weight loss, 5.74 ± 8.4 vs 9.86 ± 7.5 kg (P = 0.07), and regarding albumin decrease, 7.2 ± 5.6 vs 14.7 ± 12.7 g/L (P = 0.15). This lower morbidity in the group refed by jejunostomy led to an increase in the overall hospitalization length of stay, which in reality depended on the conventional hospitalization length of stay (in the digestive surgery department). This is more likely a reflection of the number of patients with a total gastrectomy than of enteral nutrition. Indeed, the majority of patients with jejunostomy underwent more often a total gastrectomy, 48 (65%), than a partial gastrectomy, 12 (13%). Moreover, for some patients, preoperative hospitalization was necessary to refeed them with parenteral nutrition before surgery. Feeding jejunostomy is crucial for the nutritional management of gastric cancer patients due to the high prevalence of malnutrition in this population. It allows direct enteral feeding, thus preventing post-surgery malnutrition complications, which significantly improves patients' quality of life and clinical outcomes. Early implementation of feeding jejunostomy can prevent nutritional deterioration and optimize recovery following gastrectomy[9].
We did not demonstrate any difference in survival (recurrence-free and overall survival) in our study. It is noteworthy that our findings align with those of a previous study in terms of survival outcomes and nutritional status following the implantation of a jejunostomy[10]. In this recent retrospective study including 125 patients who received preoperative chemoradiotherapy followed by surgery for esophageal cancer, the implementation of a feeding jejunostomy was associated with less weight loss during neoadjuvant treatment: 8 Lbs vs 13 Lbs (P = 0.003) between feeding jejunostomy tube (FJT) placement and no FJT patients, but was not associated with reduced toxicity due to neoadjuvant chemoradiotherapy or improved survival.
In the literature, few studies have compared the presence and absence of enteral feeding via jejunostomy in patients undergoing gastrectomy for cancer treatment. To date, the largest study is a retrospective study by Sun et al[11], which included 2980 patients, with the primary endpoint being the complication rate at 30 days[11]. Their results are similar to ours, with no difference in complication rate between the groups: 38.8% vs 36.1% (P = 0.32). Unlike our study, their study observed differences in nutritional levels, with greater recent weight loss among patients with jejunostomy: 21% vs 14.8% (P < 0.01). We used numerous variables to assess nutritional status: Weight, albuminemia, BMI, prealbuminemia, and PNI, which are all highly predictive markers of nutritional status[7,12,13]. The absence of differences in preoperative nutritional status between the groups allowed comparisons of interpretable postoperative results to be made.
We did not find any difference between the groups regarding the fistula rate, which was 3.3% in the J + group, compared to 5.4% in the J - group (P = 0.71). However, in the meta-analysis by Lewis et al[14], comparing early enteral feeding to fasting in patients undergoing gastrointestinal resection for cancer treatment, patients fed early enterally had fistula rates ranging from 2% to 7%, compared to 1% to 25% in fasting patients. In this meta-analysis, 7 out of 11 trials showed a reduced risk of anastomotic leakage in patients fed early enterally, with a relative risk in favour of enteral feeding (hazard ratio = 0.53; 95%CI: 0.26-1.08; P = 0.08). Of these 7 trials, 2 used jejunostomy feeding as a means of refeeding[15,16].
Our results showed a reduction in infectious complications in patients refed by jejunostomy: 1.2% vs 9.3% (P = 0.03). In one study, early enteral feeding was found to be a protective factor against overall infectious risk (relative risk = 0.72; 95%CI: 0.54-0.98; P = 0.036)[14]. This protective factor was found for parietal abscesses and the occurrence of pneumonia. Other trials have demonstrated that the preoperative nutritional status is correlated with postoperative infectious complications and that nutritional support helps reduce this excess morbidity in patients operated on for cancer of the gastrointestinal tract[17,18]. Another multicentre trial including 837 patients found contradictory results[19] with the meta-analysis by Lewis et al[14]. The authors revealed more infectious complications in the jejunostomy group (36% vs 19%; P < 0.01), whether surgical site infections (14% vs 6%; P < 0.01) or intra-abdominal infections (11% vs 4%; P < 0.01). However, this study presented numerous confounding biases: Patients with jejunostomy had a greater incidence of weight loss, a lower BMI, more extensive resections, and more advanced tumor node metastasis classification.
Interestingly, our study found a trend towards fewer respiratory complications, which in Lewis et al’s meta-analysis[14] translated into less pneumonia. Parallel to this observation, the postoperative nutritional status of patients refed by jejunostomy tended to be better than that of patients without jejunostomy. It is very likely that there is a beneficial nutritional effect on the diaphragmatic muscle, providing better postoperative ventilation. Indeed, stomach cancer is one of the most common causes of sarcopenia11[20]. However, this sarcopenia, a consequence of protein-energy malnutrition, is an independent factor in surgical complications after total gastrectomy[21]. It is therefore not surprising that by improving nutritional status, we reduce sarcopenia, including diaphragmatic sarcopenia, thus reducing ventilatory disorders and the resulting complications. In the literature, this improvement in nutritional status was found in a pilot study whose objective was to evaluate the impact of enteral nutrition by jejunostomy at home 6 weeks after esopha
Our study must be interpreted in light of its strengths and limitations. Indeed, we conducted a retrospective study prone to confounding biases. Misclassification biases may have arisen, particularly related to missing data, which could lead to over- or underestimation of patients' nutritional status. While all patients had at least one variable to define their nutritional status, overestimation may have occurred. Our study is a comprehensive cohort study that included all patients over 15 consecutive years, enriched notably by the higher incidence of stomach cancer in the Caribbean region. Prospective randomized trials are needed to confirm our findings.
A jejunostomy feeding tube placed intraoperatively after gastrectomy for cancer treatment helps reduce infectious and respiratory morbidity, as well as grade 3 complications, and significantly improves the postoperative nutritional status of patients.
| 1. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Baiocchi GL, Giacopuzzi S, Marrelli D, Reim D, Piessen G, Matos da Costa P, Reynolds JV, Meyer HJ, Morgagni P, Gockel I, Lara Santos L, Jensen LS, Murphy T, Preston SR, Ter-Ovanesov M, Fumagalli Romario U, Degiuli M, Kielan W, Mönig S, Kołodziejczyk P, Polkowski W, Hardwick R, Pera M, Johansson J, Schneider PM, de Steur WO, Gisbertz SS, Hartgrink H, van Sandick JW, Portolani N, Hölscher AH, Botticini M, Roviello F, Mariette C, Allum W, De Manzoni G. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer. 2019;22:172-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Di Costanzo J. [Role of preoperative nutritional status on postoperative morbidity]. Ann Fr Anesth Reanim. 1995;14 Suppl 2:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Chambrier C, Sztark F; Société Francophone de nutrition clinique et métabolisme (SFNEP); Société française d’anesthésie et réanimation (SFAR). French clinical guidelines on perioperative nutrition. Update of the 1994 consensus conference on perioperative artificial nutrition for elective surgery in adults. J Visc Surg. 2012;149:e325-e336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Abdel-Lah Mohamed A, Abdel-Lah Fernández O, Sánchez Fernández J, Pina Arroyo J, Gómez Alonso A. [Surgical access routes in enteral nutrition]. Cir Esp. 2006;79:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8618] [Article Influence: 538.6] [Reference Citation Analysis (0)] |
| 7. | Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 981] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537-10544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 9. | Carrillo Lozano E, Osés Zárate V, Campos Del Portillo R. Nutritional management of gastric cancer. Endocrinol Diabetes Nutr (Engl Ed). 2021;68:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Jain R, Shaikh T, Yee JL, Au C, Denlinger CS, Handorf E, Meyer JE, Dotan E. Impact of Clinical Markers of Nutritional Status and Feeding Jejunostomy Use on Outcomes in Esophageal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Sun Z, Shenoi MM, Nussbaum DP, Keenan JE, Gulack BC, Tyler DS, Speicher PJ, Blazer DG 3rd. Feeding jejunostomy tube placement during resection of gastric cancers. J Surg Res. 2016;200:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | O'Gorman P, McMillan DC, McArdle CS. Longitudinal study of weight, appetite, performance status, and inflammation in advanced gastrointestinal cancer. Nutr Cancer. 1999;35:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus "nil by mouth" after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 502] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 15. | Watters JM, Kirkpatrick SM, Norris SB, Shamji FM, Wells GA. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg. 1997;226:369-77; discussion 377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, Shike M, Brennan MF. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567-77; discussion 577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 238] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroenterol. 2012;18:673-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Patel SH, Kooby DA, Staley CA 3rd, Maithel SK. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol. 2013;107:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kim EY, Kim K, Kim YS, Ahn HK, Jeong YM, Kim JH, Choi WJ. Prevalence of and Factors Associated with Sarcopenia in Korean Cancer Survivors: Based on Data Obtained by the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2011. Nutr Cancer. 2017;69:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Chen FF, Zhang FY, Zhou XY, Shen X, Yu Z, Zhuang CL. Role of frailty and nutritional status in predicting complications following total gastrectomy with D2 lymphadenectomy in patients with gastric cancer: a prospective study. Langenbecks Arch Surg. 2016;401:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Bowrey DJ, Baker M, Halliday V, Thomas AL, Pulikottil-Jacob R, Smith K, Morris T, Ring A. A randomised controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: report on a pilot and feasibility study. Trials. 2015;16:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Li K, Zeng Z, Zhang Z, Ye X, Yu J, Kang W. Comparisons of nutritional status and complications between patients with and without postoperative feeding jejunostomy tube in gastric cancer: a retrospective study. J Gastrointest Oncol. 2023;14:97-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |