Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2329
Revised: May 26, 2024
Accepted: June 18, 2024
Published online: July 27, 2024
Processing time: 148 Days and 21.5 Hours
Programmed cell death 1 (PD-1) inhibitors are immune checkpoint inhibitors (ICI) that have demonstrated significant efficacy in treating various advanced malignant tumors. While most patients tolerate treatment well, several adverse drug reactions, such as fatigue, myelosuppression, and ICI-associated colitis, have been reported.
This case involved a 57-year-old male patient with ulcerative colitis complicated by hepatocarcinoma who underwent treatment with tirelizumab (a PD-1 inhibitor) for six months. The treatment led to repeated life-threatening lower gastrointestinal hemorrhage. The patient received infliximab, vedolizumab, and other salvage procedures but ultimately required subtotal colectomy due to uncontrollable massive lower gastrointestinal bleeding. Currently, postoperative gastrointestinal bleeding has stopped, the patient’s stool has turned yellow, and his full blood cell count has returned to normal.
This case highlights the necessity of early identification, timely and adequate treatment of ICI-related colitis, and rapid escalation to achieve the goal of improving prognosis.
Core Tip: Immune checkpoint inhibitors (ICIs), such as ICI-associated colitis, have demonstrated significant efficacy in treating various advanced malignant tumors, but several immune-related adverse drug reactions, such as ICI-associated colitis, have attracted increasing attention. In this article, we present a case of a patient with hepatocarcinoma and ulcerative colitis with ICI-related colitis in which repeated life-threatening lower gastrointestinal hemorrhage occurred during the disease course. Despite comprehensive salvage treatments through multidisciplinary management, the patient eventually required surgical intervention due to persistent gastrointestinal bleeding. This case highlights the necessity of early identification, timely and adequate treatment of ICI-related colitis and rapid escalation to improve patient prognosis.
- Citation: Hong N, Wang B, Zhou HC, Wu ZX, Fang HY, Song GQ, Yu Y. Multidisciplinary management of ulcerative colitis complicated by immune checkpoint inhibitor-associated colitis with life-threatening gastrointestinal hemorrhage: A case report. World J Gastrointest Surg 2024; 16(7): 2329-2336
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2329.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2329
Programmed cell death 1 (PD-1) inhibitors are immune checkpoint inhibitors (ICIs) that bind to the PD-1 receptor on the surface of T cells, blocking the tumor immune escape pathway[1]. It has demonstrated significant efficacy in treating various advanced malignant tumors[2,3]. However, ICIs can induce immune-related adverse events (irAEs), among which gastrointestinal adverse events are the most common. This process mainly involves the colon and is called ICI-associated colitis. The small intestine and upper digestive tract were also involved. Diarrhea is the most common clinical symptom. It is also characterized by abdominal pain, hematochezia, fever, and intestinal perforation[4,5]. Because ICI-related colitis often resembles symptoms of inflammatory bowel disease (IBD), especially ulcerative colitis (UC)[6,7], the diagnosis of ICI-associated colitis is challenging. In this report, we present the case of a patient with UC complicated with liver cancer. After secondary ICI-associated colitis, rare fatal massive hemorrhage in the lower digestive tract occurred repeatedly, and multidisciplinary treatment was successful.
A 57-year-old male patient with UC developed significant abdominal pain and mucopurulent bloody stools after taking a PD-1 inhibitor.
The patient had 5-6 episodes of bloody diarrhea daily approximately 2 months after the use of PD-1 without abdominal pain and underwent right hepatectomy on August 10, 2022. On the ninth day following hepatectomy, his condition worsened, marked by an increase to more than 10 episodes of bloody diarrhea daily, along with new-onset periumbilical and lower abdominal pain, bloating, and nausea, though without vomiting or fever.
He was diagnosed with UC 5 years ago, which was well controlled with both oral and rectal mesalazine. In March 2022, a pathological biopsy in the Department of Liver Surgery confirmed a diagnosis of hepatocellular carcinoma. In preparation for liver surgery, he received comprehensive targeted anticancer therapy for five months, including intravenous tirelizumab (200 mg, administered five times) and daily oral lenvatinib (12 mg).
He had no family history of tumors, autoimmune diseases or genetic disorders.
His temperature was 36.2 °C, his pulse was 84 breaths/minute, his respiration rate was 19 breaths/minute, and his blood pressure was 95/56 mmHg. His abdomen was soft with mild tenderness, no rebound pain, and active bowel sounds at approximately 8 breaths/minute. The bilateral lower limbs showed moderate edema. The surgical incision on the right side of the abdomen appeared dry and clean, with intact stitches. A small amount of yellow fluid was observed in the drainage bag on the right side of the abdomen.
The white blood cell count was 9.37 × 109/L (normal range: 3.5-9.5 × 109/L), the neutrophil ratio was 71.1% (normal range: 40%-75%), the red blood cell count was 2.71 × 1012/L (normal range: 4.3-5.8 × 1012/L), the hemoglobin level was 83 g/L (normal range: 120-160 g/L), the platelet count was 63 × 109/L (normal range: 125-350 × 109/L), the albumin concentration was 26.7 g/L (normal range: 35-55 g/L), the C-reactive protein concentration was 14.2 mg/L (normal range: 0-8 mg/L), the prothrombin time concentration was 18.50 seconds (normal range: 10.5-16 seconds), the D-dimer concentration was 5.13 mg/L (normal range: 0-0.5 mg/L), and the stool routine parameters included white blood cells (+++), red blood cells (++), and a few pus balls; ELISA detection of Mycobacterium tuberculosis revealed reactive pore A20 and pore B13. TBNK cell subsets were detected as follows: CD3-CD16+56+ 2.8% (normal range: 5.6%-30.9%); decreased levels of immunoglobulin complement C3, C4, and IgM; and Th1/2/17 cytokine levels of 170.26 pg/mL (normal range: 0-5.3 pg/mL) and 41.51 pg/mL (normal range: 0-4.91 pg/mL) for interleukin (IL)-6 and IL-10, respectively. Blood tests for cytomegalovirus (CMV) PCR and Epstein-Barr virus PCR were negative, and CMV immunohistochemistry was negative. Parasite ova and Plasmodium were not detected in multiple fecal or blood samples. Blood culture and stool C. difficile culture were negative, and the erythrocyte sedimentation rate and procalcitonin level were within the normal ranges. There was no significant change in fecal calprotectin level before and after treatment.
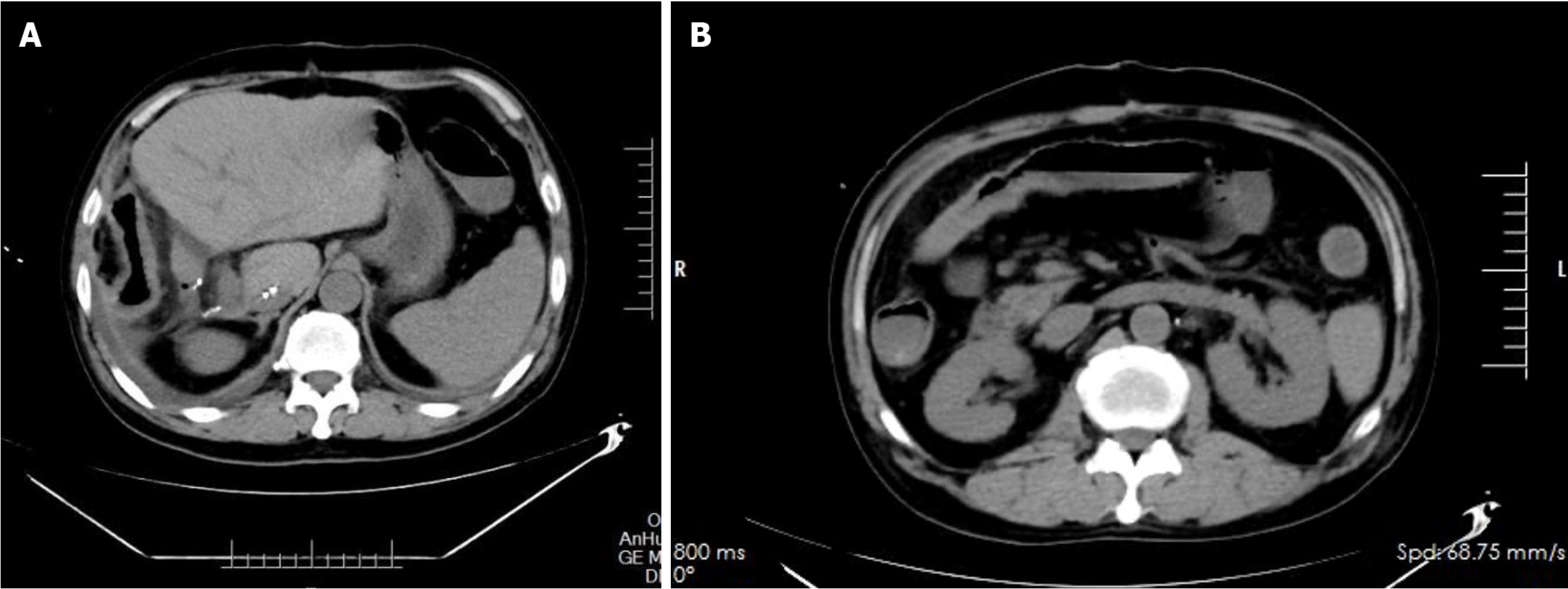
Full abdominal pelvic spiral computed tomography plain scan on August 20, 2022. Figure 1 shows bilateral pleural effusion with adjacent lung tissue atelectasis and pericardial effusion with postoperative liver changes. The walls of the ascending colon, transverse colon, and descending colon were slightly thickened and exhibited uniform edema, while the surrounding fat space was slightly blurred, with evidence of moderately enlarged lymph nodes scattered throughout the region. There was no obvious lesion in the small intestine.
Considering the patient’s medical history, endoscopy and pathology, the final diagnosis was UC accompanied by ICI-associated colitis.
UC accompanied by ICI-associated colitis.
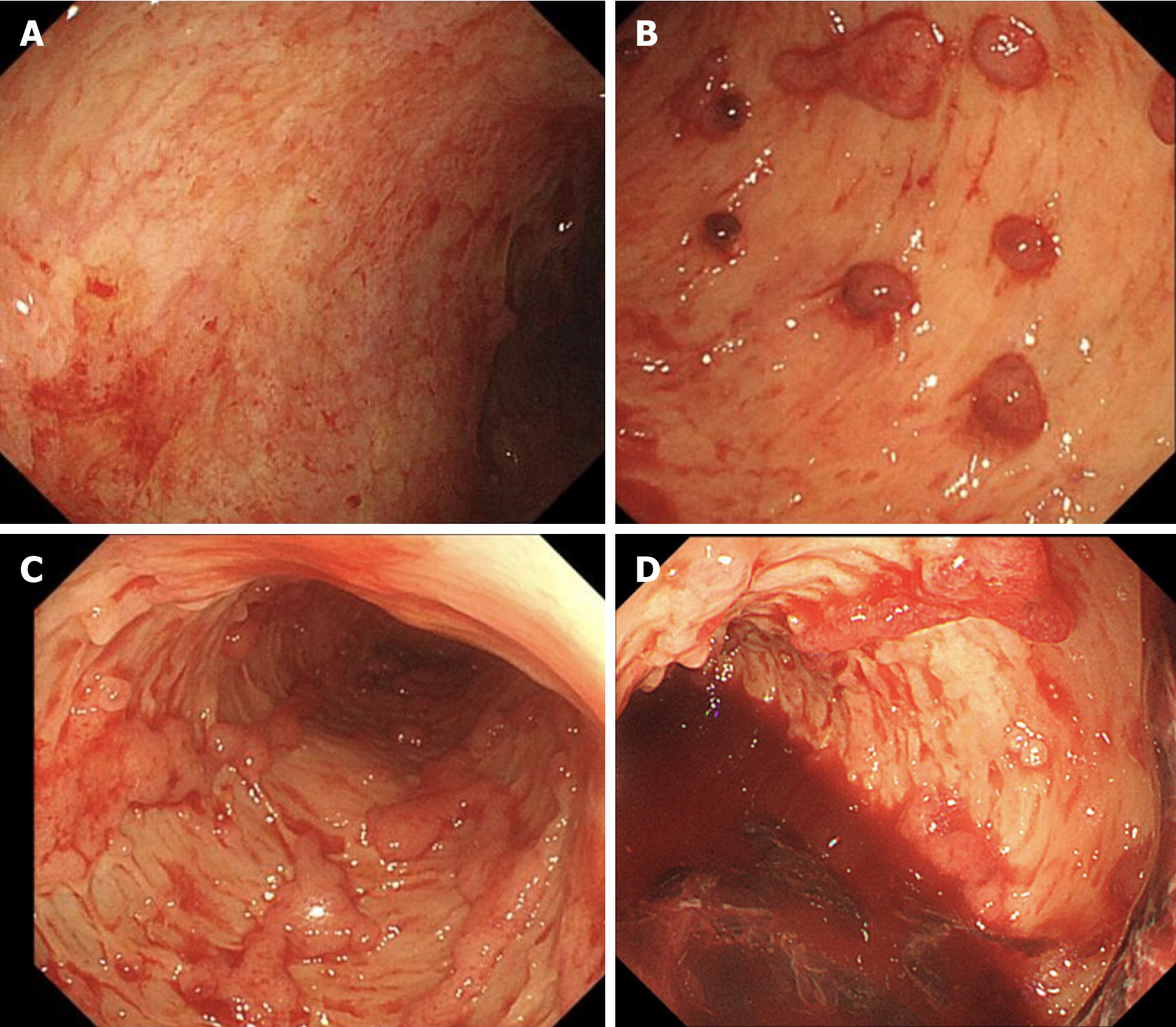
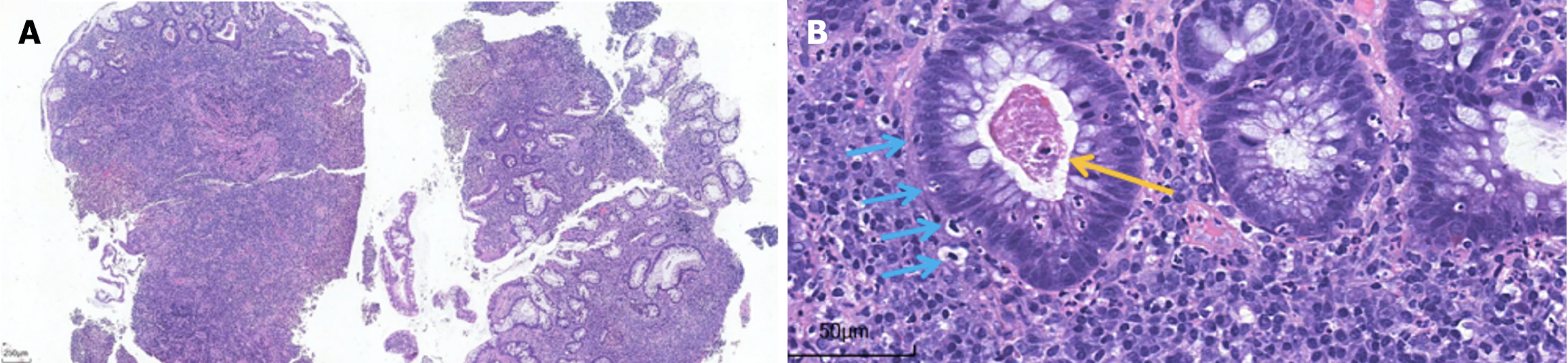
Postoperative management with broad-spectrum antibiotics (cefoperazone sodium and sulbactam sodium, 2 g IV daily) and corticosteroids (methylprednisolone 40 mg IV daily) did not improve the patient’s condition; instead, the frequency of bloody diarrhea intensified to 20 episodes per day. Given the complexity of his condition, he was transferred to the Department of Gastroenterology. Upon transfer, the dose of methylprednisolone was increased to 60 mg IV daily, and somatostatin was administered intravenously with a micropump of 6 mg daily. Despite these treatment adjustments, the bloody diarrhea persisted. To evaluate intestinal inflammation, the first sigmoidoscopy was performed and revealed extensive mucosal exfoliation of the sigmoid colon (Figure 2). Biopsy and pathological examination confirmed the presence of colitis, characterized by surface inflammatory exudation and the loss of mucosal glands (Figure 3).
On August 26, 2022, the patient suddenly experienced massive lower gastrointestinal tract bleeding, resulting in a loss of approximately 4950 mL of blood. Moreover, on August 28, 2022, hematochezia of approximately 2000 mL occurred. Subsequently, the patient's condition deteriorated significantly. The surgical team recommended immediate surgery for hypotension (80/50 mmHg) due to massive bleeding. However, the patient resolutely refused colectomy because of a recent history of right liver surgery. In the critical state (8.26-30), he underwent a massive blood transfusion [B, RH- (+), red blood cell 19 U, fresh plasma 2025 mL, and cryoprecipitate coagulation Factor 18 U] to save his life. To address severe UC accompanied by immune-related colitis induced by a PD-1 inhibitor, the medical team, including American experts who consulted online and multiple disciplines within the hospital, discussed the case of intermittent improvement of hormones in this patient and implemented salvage therapy via 0.5 g (10 mg/kg) intravenous infusion of infliximab. Bloody stools turned into yellow pasty feces, occurring 7 to 8 times per day, and he had a few bloody stools occasionally.
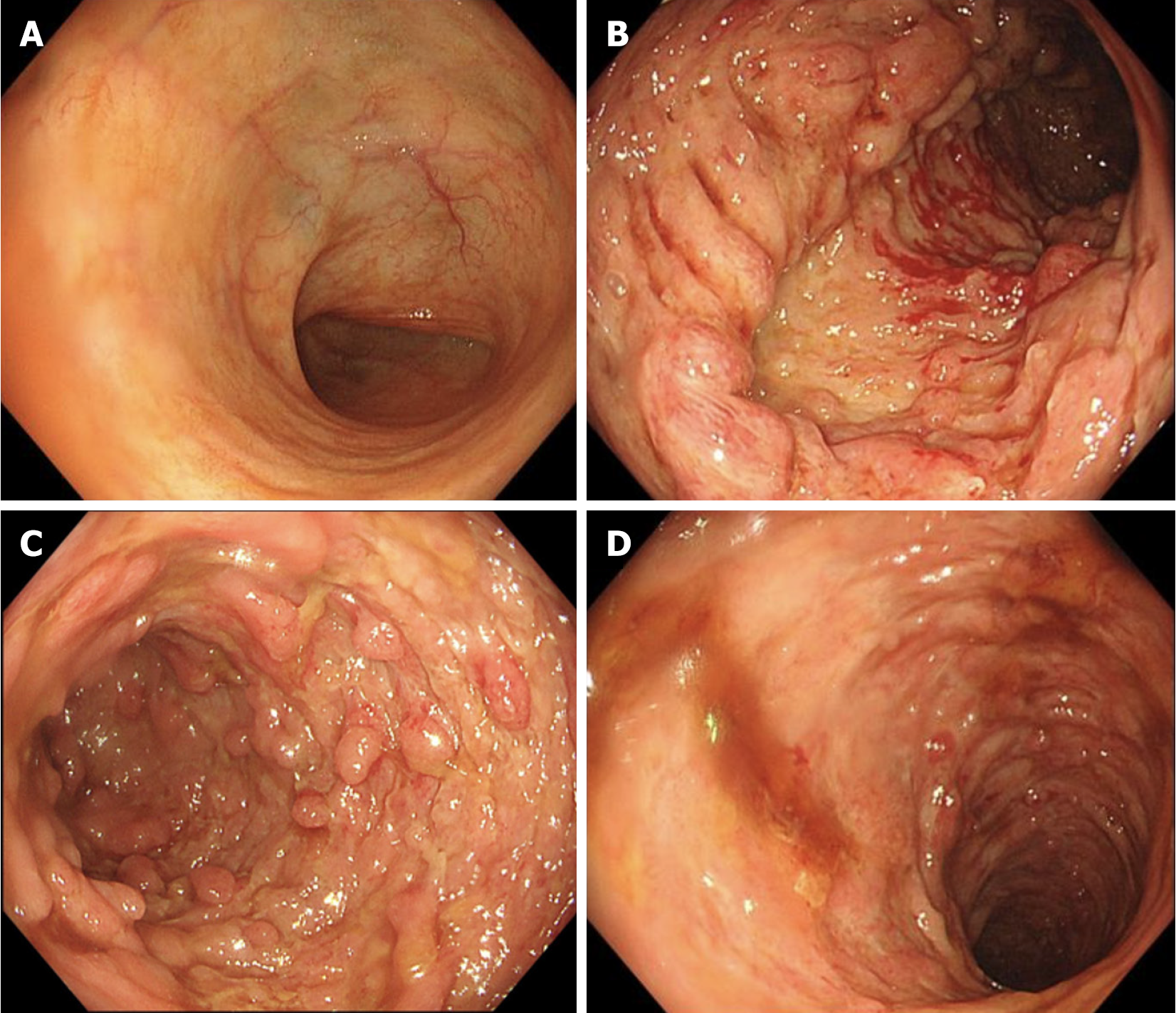
Due to a combination of latent tuberculosis infection and decreased leukocyte and neutropenia, the patient received 300 mg of vedolizumab two weeks and one month after infliximab, respectively, resulting in further improvement, with yellow pasty stool and occasional formed stool approximately 4-5 times a day. Subsequent endoscopic reexamination revealed that the terminal ileum was normal, without blood staining, that the sigmoid lesions were significantly reduced, that the rectal mucosa was hyperemic and edematous, and that no obvious ulcers were observed. However, the ileocecal region, the ascending colon, transverse colon and descending colon exhibited continuous extensive hyperemia, edema, erosion, polypoid hyperplasia, and irregular deep perforated and geographic ulcers, especially in the right half of the colon (Figure 4).
On September 23, 2022, a routine blood test revealed a significant reduction in all three blood cell types (white blood cells 1.66 × 109/L (normal range: 3.5-9.5 × 109/L), red blood cells 2.25 × 109/L (normal range: 4.3-5.8 × 1012/L), and platelets 45 × 109/L (normal range: 125-350 × 109/L)). After excluding patients with malignant hematological diseases through bone marrow aspiration and pathological analysis, immune pancytopenia was considered. The patient received a 300-UG subcutaneous injection of human granulocyte stimulating factor to promote white blood cells and a 12 million U subcutaneous injection of recombinant human IL-11 to promote platelet recovery, along with component transfusion. However, despite a brief increase in white blood cells and platelets, they continued to decline, and the patient experienced hematochezia again, with approximately 100-200 mL each time.
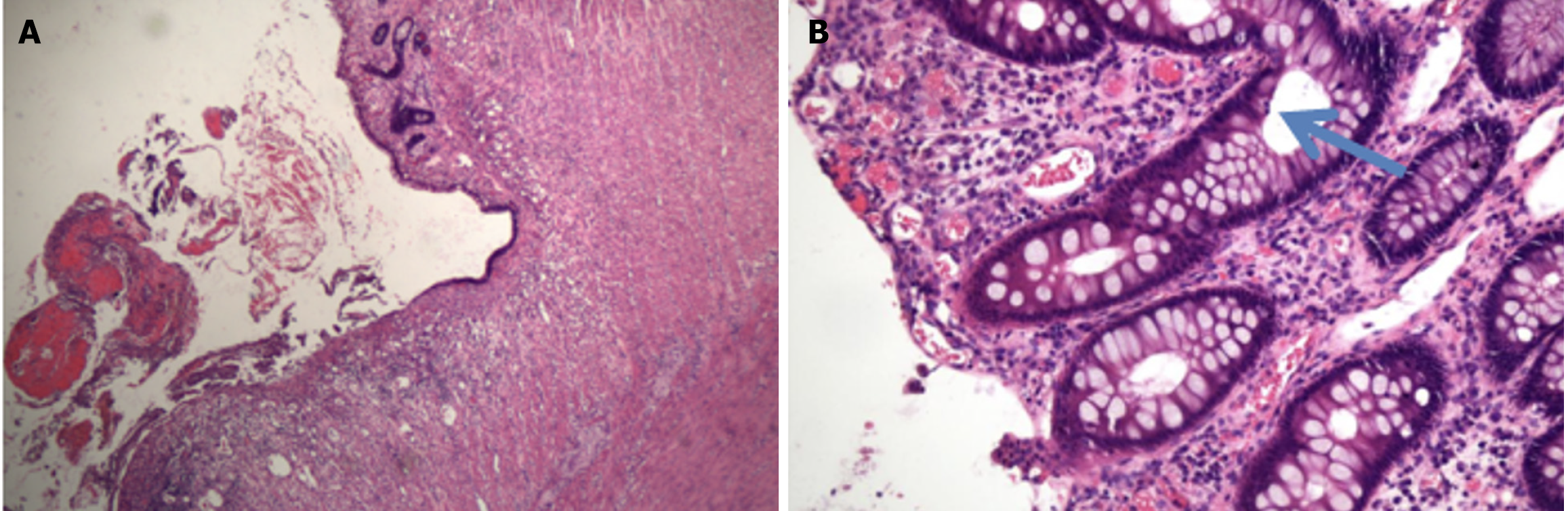
The recurrence and deterioration of the disease prompted the patient to decide to transfer to a hospital in Nanjing for surgery involving subtotal resection of the colon + ileostomy + intestinal adhesion release. Intraoperative exploration of the small intestine revealed no abnormalities, and the whole colon showed chronic inflammatory changes. Postoperative pathology revealed extensive erosion with superficial ulcers and a large number of acute and chronic inflammatory cells infiltrating the mucosal layer and submucosa, including neutrophil or eosinophilic infiltration, cryptinitis, crypt microabscesses, and increased apoptosis, which tends to be UC accompanied by ICI-associated colitis (Figure 5 and Supplementary material).
Currently, postoperative gastrointestinal bleeding has stopped, the patient’s stool has turned yellow, and his full blood cell count has returned to normal.
The use of ICIs has led to significant advancements in cancer treatment, but it has also led to immune-related adverse reactions, with the digestive system being the most commonly affected[8]. ICI-associated colitis typically occurs 6 to 7 weeks after the first use of ICIs and usually achieves remission within 4 months or more after stopping PD-1 inhibitor treatment. Colitis and diarrhea are the main manifestations of ICI-related colitis. The severity of ICI-related colitis is classified into five grades according to the National Cancer Institute (NCI) adverse event evaluation criteria[9]. According to the literature[10], the incidence of colitis caused by PD-1 was 1.4% (95%CI: 1.1%-1.8%), and the incidence of grade 3-4 colitis was 0.9% (95%CI: 0.7%-1.3%). A multicenter retrospective study of IBD patients treated with ICIs showed that cancer patients with IBD had significantly greater rates of gastrointestinal irAEs (41% vs 11%, P < 0.01) and higher grades of diarrhea and colitis than cancer patients without IBD[11]. In this case, the patient had grade 4 colitis according to the NCI grading criteria.
The diagnosis of ICI-associated colitis can be challenging because its clinical symptoms, endoscopic manifestations, and histological features are similar to those of IBD[6,7]. Endoscopic features usually include mucosal edema, erythematous lesions, erosion, ulcers, and other changes in the colon[12]. Endoscopic biopsy has been considered the gold standard criterion for the diagnosis of ICI-related colitis[13]. Microscopic changes in ICI-associated colitis may include increased apoptosis, neutrophil infiltration, crypt microabscesses, erosion, or ulcer formation[14,15]. In this patient, similar changes were observed in postoperative pathology (Figure 5).
Treatment of ICI-associated colitis typically involves glucocorticoids as the first-line therapy for moderate to severe cases. If glucocorticoid therapy is ineffective, infliximab or vedolizumab should be considered, especially for patients with severe endoscopic manifestations or high endoscopic disease activity scores[16]. In this patient, infliximab and vedolizumab were added to the treatment regimen when sufficient hormone therapy failed, resulting in partial improvement of colitis symptoms. However, due to the development of immune pancytopenia and recurrent lower gastrointestinal bleeding, surgery was ultimately performed as a last resort. ICIS-associated colitis may deteriorate to colonic perforation, and colectomy is considered to be the most effective therapy, although the estimated incidence is 1% to 1.5%[17]. In fact, because patients often have extensive colonic lesions, subtotal colectomy is the preferred surgical option[13].
The association between ICI-associated colitis outcome and survival is still unclear[13]. In line with Abu-Sbeih et al[11], we found that patients tend to have more aggressive disease and worsen earlier when the two diseases (UC and ICI-associated colitis) are combined. Although most patients can achieve remission with conservative medical treatment, some patients may require prompt surgery due to serious complications. The experience gained from this case highlights the importance of early diagnosis and prompt surgery to improve clinical outcomes.
Above all, the precise diagnosis and treatment of CIC requires multidisciplinary management and persistent monitoring, and timely adjustment of treatment regimens based on guidelines is essential for effectively managing ICI-associated colitis.
This case highlights the necessity of early identification, timely and adequate treatment of ICI-related colitis, and rapid escalation to improve prognosis. Multidisciplinary management and persistent monitoring are essential for identifying and managing irAEs effectively.
We thank the patient and his wife for their consent to publish their case and everyone involved in the diagnosis and treatment of the patient for their hard work.
| 1. | Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 294] [Reference Citation Analysis (0)] |
| 2. | Sabbatino F, Liguori L, Pepe S, Ferrone S. Immune checkpoint inhibitors for the treatment of melanoma. Expert Opin Biol Ther. 2022;22:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | DeCarli K, Strosberg J, Almhanna K. Immune Checkpoint Inhibitors for Gastrointestinal Malignancies: An Update. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M, Mortier L, Coutzac C, Soularue E, Lanoy E, Kapel N, Planchard D, Chaput N, Robert C, Carbonnel F. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 1451] [Article Influence: 241.8] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, Diab A. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, Zobniw C, Johnson DH, Samdani R, Lum P, Shuttlesworth G, Blechacz B, Bresalier R, Miller E, Thirumurthi S, Richards D, Raju G, Stroehlein J, Diab A. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis. 2018;24:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (4)] |
| 8. | Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Lui RN, Chan SL. Management of Gastrointestinal Side Effects of Immune Checkpoint Inhibitors. Clin Gastroenterol Hepatol. 2021;19:2262-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 11. | Abu-Sbeih H, Faleck DM, Ricciuti B, Mendelsohn RB, Naqash AR, Cohen JV, Sellers MC, Balaji A, Ben-Betzalel G, Hajir I, Zhang J, Awad MM, Leonardi GC, Johnson DB, Pinato DJ, Owen DH, Weiss SA, Lamberti G, Lythgoe MP, Manuzzi L, Arnold C, Qiao W, Naidoo J, Markel G, Powell N, Yeung SJ, Sharon E, Dougan M, Wang Y. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J Clin Oncol. 2020;38:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, Collins M, Chaput N, Robert C, Carbonnel F. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 13. | Tang L, Wang J, Lin N, Zhou Y, He W, Liu J, Ma X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front Immunol. 2021;12:800879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists' perspective. J Clin Pathol. 2018;71:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Star KV, Ho VT, Wang HH, Odze RD. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol. 2013;37:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Kaffenberger BH, Lunning M, McGettigan S, McPherson J, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Pennell N, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wang Y, Weight RM, Johnson-Chilla A, Zuccarino-Catania G, Engh A. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020;18:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 17. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1504] [Article Influence: 188.0] [Reference Citation Analysis (1)] |