Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2296
Revised: May 9, 2024
Accepted: June 4, 2024
Published online: July 27, 2024
Processing time: 129 Days and 21.7 Hours
The Chinese medicine Yangyin Huowei mixture (YYHWM) exhibits good clinical efficacy in the treatment of chronic atrophic gastritis (CAG), but the mechanisms underlying its activity remain unclear.
To investigate the therapeutic effects of YYHWM and its underlying mechanisms in a CAG rat model.
Sprague-Dawley rats were allocated into control, model, vitacoenzyme, and low, medium, and high-dose YYHWM groups. CAG was induced in rats using N-methyl-N′-nitro-N-nitrosoguanidine, ranitidine hydrochloride, hunger and satiety perturbation, and ethanol gavage. Following an 8-wk intervention period, sto
The model group showed gastric mucosal layer disruption and inflammatory cell infiltration. Compared with the blank control group, serum levels of PGI, PGII, and G-17 in the model group were significantly reduced (82.41 ± 3.53 vs 38.52 ± 1.71, 23.06 ± 0.96 vs 11.06 ± 0.70, and 493.09 ± 12.17 vs 225.52 ± 17.44, P < 0.01 for all), whereas those of IL-1β, IL-6, and TNF-α were significantly increased (30.15 ± 3.07 vs 80.98 ± 4.47, 69.05 ± 12.72 vs 110.85 ± 6.68, and 209.24 ± 11.62 vs 313.37 ± 36.77, P < 0.01 for all), and the protein levels of IL-10, JAK1, and STAT3 were higher in gastric mucosal tissues (0.47 ± 0.10 vs 1.11 ± 0.09, 0.49 ± 0.05 vs 0.99 ± 0.07, and 0.24 ± 0.05 vs 1.04 ± 0.14, P < 0.01 for all). Compared with the model group, high-dose YYHWM treatment significantly improved the gastric mucosal tissue damage, increased the levels of PGI, PGII, and G-17 (38.52 ± 1.71 vs 50.41 ± 3.53, 11.06 ± 0.70 vs 15.33 ± 1.24, and 225.52 ± 17.44 vs 329.22 ± 29.11, P < 0.01 for all), decreased the levels of IL-1β, IL-6, and TNF-α (80.98 ± 4.47 vs 61.56 ± 4.02, 110.85 ± 6.68 vs 89.20 ± 8.48, and 313.37 ± 36.77 vs 267.30 ± 9.31, P < 0.01 for all), and evidently decreased the protein levels of IL-10 and STAT3 in gastric mucosal tissues (1.11 ± 0.09 vs 0.19 ± 0.07 and 1.04 ± 0.14 vs 0.55 ± 0.09, P < 0.01 for both).
YYHWM reduces the release of inflammatory factors by inhibiting the IL-10/JAK1/STAT3 pathway, alleviating gastric mucosal damage, and enhancing gastric secretory function, thereby ameliorating CAG development and cancer transformation.
Core Tip: This study aimed to investigate the therapeutic effects of Yangyin Huowei mixture (YYHWM) on chronic atrophic gastritis (CAG) and its potential mechanism. A CAG model was induced through the administration of a composite factor. The study observed the pathological damage to the stomach tissue, gastric secretory function, inflammatory factors, and protein expression levels of the IL-10/JAK1/STAT3 pathway. Ultimately, it was discovered that YYHWM can improve the pathological damage to the stomach tissue and enhance the gastric secretory function of CAG rats, which may be attributed to its suppression of the IL-10/JAK1/STAT3 pathway and reduction of inflammatory factor levels.
- Citation: Xie SS, Zhi Y, Shao CM, Zeng BF. Yangyin Huowei mixture alleviates chronic atrophic gastritis by inhibiting the IL-10/JAK1/STAT3 pathway. World J Gastrointest Surg 2024; 16(7): 2296-2307
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2296.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2296
Chronic atrophic gastritis (CAG) is a precancerous gastric lesion or condition in which the gastric mucosa is subjected to long-term bacterial attack and erosion, resulting in a reduction in the intrinsic glands with or without intestinal glandular metaplasia and/or pseudo-pyloric glandular metaplasia[1]. According to the 2022 Global Cancer Statistics[2], China ranks first in overall cancer incidence and mortality rates, and third in gastric cancer incidence (39.7/10 million) and mortality (28.9/10 million) rates worldwide[3], indicating a high public health risk. Normal gastric mucosal cells often remain as precancerous gastric lesions for long periods before developing into gastric cancer cells. Therefore, timely blocking and reversal of the malignant transformation of CAG are an important strategy for the prevention and treatment of gastric cancer. Moreover, it is crucial to determine the molecular mechanisms of CAG for the development of effective drugs to treat CAG and its associated complications. The interleukin (IL)-10/JAK-1/STAT-3 signaling pathway is closely associated with cellular conductance and is involved in various cellular processes including cell differentiation, survival, proliferation, apoptosis, immunity, inflammation, and pathological processes[4,5].
Treatment of CAG with Western medicine mainly focuses on symptomatic relief. Despite the availability of drugs that offer temporary relief from symptoms, the current treatment methods have certain drawbacks. Acid inhibitors are effective but short-lived, whereas proton pump inhibitors are long-lasting but expensive. Moreover, they have little effect in the blockage and reversal of mucosal atrophy, intestinal metaplasia, or heterotrophic hyperplasia with a risk of cancer[6,7]. Yangyin Huowei mixture (YYHWM) is an herbal formulation widely used for the treatment of CAG based on the traditional Chinese medicine (TCM) concept of nourishing stomach yin. YYHWM is composed of 11 herbs: Rehmannia root, Atractylodes macrocephala, poria, cuttlebone, Curcuma vinegaris, sautéed chickpea, spinosa, dandelion, farnesol, salvia, and roasted licorice. Using network pharmacology, previous studies have confirmed that multiple single drugs in YYHWM regulate the IL-10/JAK1/STAT3 signaling pathway[8]. Previous clinical trials have confirmed the efficacy of YYHWM in treating CAG[9,10]. Therefore, the present study aimed to investigate the therapeutic effects of YYHWM in a rat model of CAG and determine the underlying mechanisms to provide an experimental basis for its clinical application.
Healthy specific pathogen-free (SPF) male Sprague–Dawley rats, aged 4-6 wk and weighing 200 ± 25 g, were obtained from the Laboratory Animal Center of Xinjiang Medical University [Ürümqi, China; Laboratory Animal Production License No. SCXK (Xin) 2018-0002]. The rats were housed at the Laboratory Animal Center of Xinjiang Medical University under constant temperature of (22 ± 2°C), humidity (50%-60%), and alternating 12 h/12 h light/dark cycle. This study was approved by the Experimental Animal Ethics Committee of the Xinjiang Medical University (reference number: IACUC-20210315-03).
YYHWM Chinese medicine-free decoction granules were purchased from Jiangyin Tianjiang Pharmaceutical Co. (Tianjin, China). The following ingredients were purchased: Rehmannia glutinosa (30 g; Production Batch No. 19090481), branch-fried Atractylodis macrocephalae (10 g; Production Batch No. 19120251), poria (10 g; Production Batch No. 20030691), cuttlebone (15 g; Production Batch No. 19110941), vinegar Curcuma longa (9 g; production batch No. 19120951), stir-fried Ji Nei Jin (9 g; Production Batch No. 20040621), Cymbopogon flexuosus (12 g; Production Batch No. 19081041), stir-fried danshiru (6 g; Production Batch No. 19091087), yuanzhi (6 g; Production Batch No. 19121371), Radix et Rhizoma Gastrodiae (6 g; Production Batch No. 19092681), and Radix et Rhizoma Glycyrrhizae (6 g; Production Batch No. 19111701). N-methyl-N′-nitro-N-nitrosoguanidine (MNNG; Shanghai Roan Reagent, Batch No. RH222415), ranitidine hydrochloride (Yunpeng Pharmaceutical Group Co. Ltd., Batch No. E210303), and viomycin capsules (Dezhou Bocheng Pharmaceutical Co. Ltd., Lot No. w20210401) were used in this study. PG-I, PG-II, G-17, IL-1β, IL-6, TNF-α, and IL-10 ELISA kits were purchased from Shanghai Youxuan Biotechnology Co. (E20958, YX-E21185, and YX-E21174, respectively). Antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), IL-10, JAK1, and STAT3 were purchased from CST (Batch No. 5174T, 12163S, 3344T, and 9139T, respectively).
A microtome (Leica, Germany), inverted fluorescence microscope (Nikon, Japan), multifunctional microplate reader (Thermo Fisher Scientific, United States), and JESS ultra-micro multifunctional fully automated protein expression quantitative analysis system (ProteinSimple, United States) were used.
Rats were randomly divided into blank control and modeling groups. A multifactorial composite method was used to replicate a CAG rat model[11]. Special chow was prepared to allow the rats to freely consume 30 mg/g ranitidine hydrochloride prepared using SPF-grade ingredients. Additionally, 100 μg/mL of MNNG dissolved in sterilized water at high temperature away from light was provided to the rats ad libitum. Over 12 wk, the rats were orally administered varying concentrations of ethanol. For the first 9 wk, the rats were administered a 10% ethanol solution equivalent to 5 mL/kg ethanol twice per week. From the 10th to 12th week, the ethanol concentration was increased to 300 mL/L and administered at the same rate and frequency. During the entire modeling period, the rats were fasted for 1 d every other day. After 12 wk, three rats were randomly selected for the pathological examination of their gastric mucosa to assess the model. Rats that successfully underwent modeling were randomly assigned to model, vitacoenzyme, and low-, medium-, and high-dose YYHWM groups. In the low-, medium-, and high-dose YYHWM groups, the combined drug was administered via gavage at a rate of 5.355 g/kg, 10.71 g/kg, and 21.42 g/kg per day, respectively, with a volume of 1 mL/100 g of body weight. In the vitacoenzyme group, the volume of vitacoenzyme administered via gavage at a rate of 1.8 mg/kg was equal to that of the model group. In the blank control and model groups, 9 g/L NaCl was administered via gavage once every 8 wk. On the 2nd day after the end of the experiment, serum and gastric tissues were collected for subsequent testing.
Rat gastric tissue blocks were fixed overnight in 40 mL/L paraformaldehyde, dehydrated, and embedded in paraffin. They were sectioned at a thickness of 5 μm, reheated for 30 min at 60 °C, and treated with xylene, hematoxylin for 5 min, and eosin solution for 5 min. Following dehydration, xylene clearance and slide mounting were performed and images were observed using a microscope.
ELISA was used to measure the levels of PG-I, PG-II, G-17, and the inflammatory factors IL-1β, IL-6, and TNF-α in rat serum. Experiments were performed according to the ELISA kits’ instructions. The results were statistically analyzed after reading them using a microplate reader.
The expression levels of IL-10, JAK1, and STAT3 proteins were quantified by Western blot analysis[12].Gastric mucosal tissue with high-efficiency radioimmunoprecipitation assay lysis buffer was added to a low-temperature high-speed grinder. After grinding, the supernatant was collected via centrifugationat 12000 × g and 4 °C for 10 min. The protein concentration was determined according to the BCA kit’s instructions. Related proteins were detected as described previously using primary antibodies against GAPDH (1:50), IL-10 (1:50), JAK 1 (1:50), and STAT 3 (1:50), as well as HRP-conjugated sheep anti-rabbit/sheep anti-mouse secondary antibody (1:20)[8]. All analyses were conducted using Compass software. Grayscale values were analyzed using ImageJ software.
Statistical data are expressed as the mean ± SD or mean ± SE, and were analyzed using IBM SPSS 23 and GraphPad Prism 9 statistical software. One-way analysis of variance was used to compare the differences among multiple groups. The least significant difference test was used when the variance was uniform, whereas the non-parametric test was used when the variance was not uniform. Differences were considered statistically significant at P < 0.05.
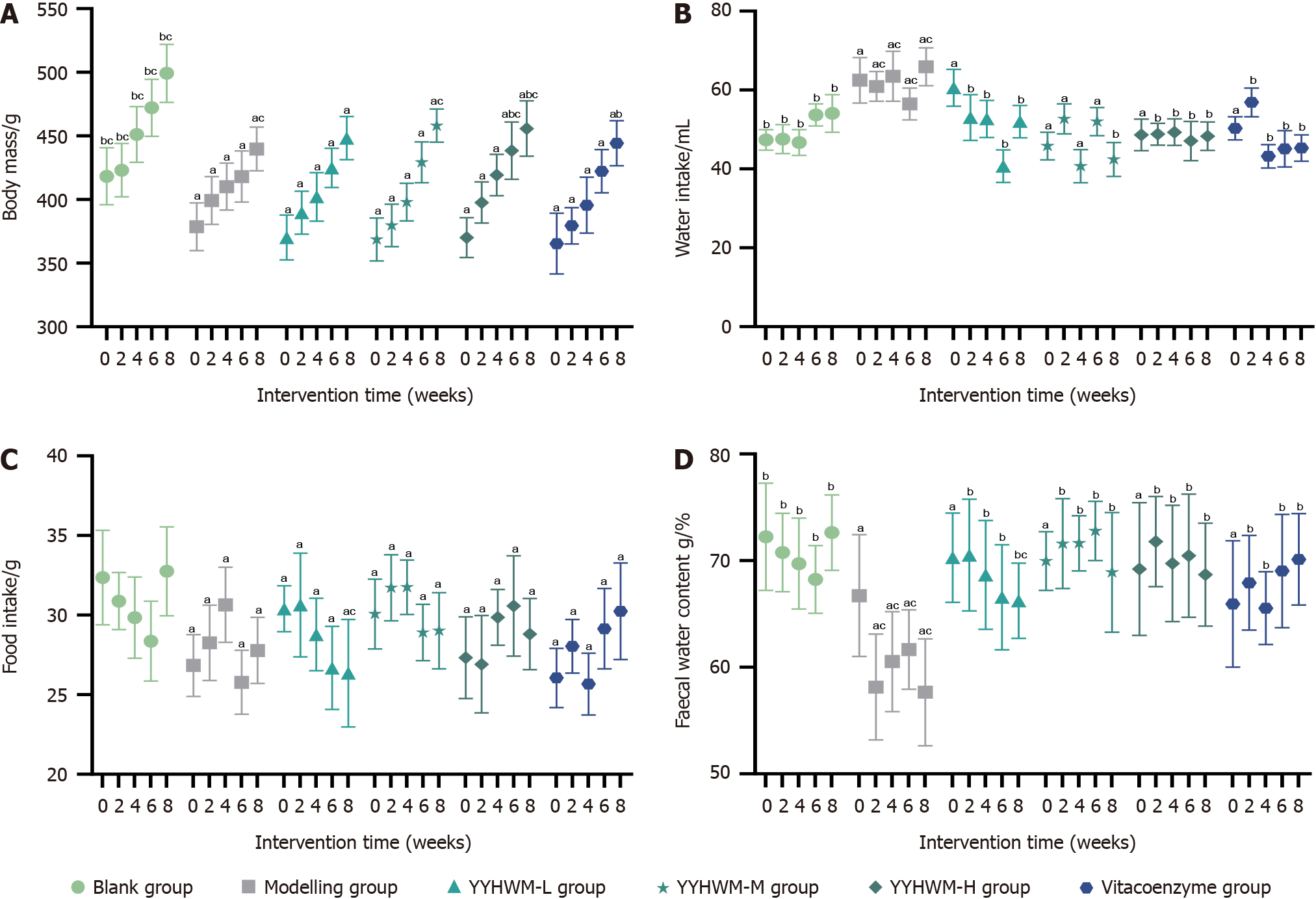
As shown in Table 1 and Figure 1, during the same period, the body weight and food intake of rats in the model, low-, medium-, and high-dose YYHWM, and vitacoenzyme groups were significantly lower (P < 0.05), water intake was significantly higher (P < 0.05), and fecal water content was significantly lower (P < 0.05) than those of the control group, suggesting that the body mass, dietary intake, and fecal water content of the rats in all groups were lower than the normal levels. Compared with the model group, in the 6th wk of the intervention, the body weight of rats in the high-dose YYHWM and vinblastine groups was significantly increased (P < 0.05), water intake was reduced (P < 0.05), and fecal water content was increased (P < 0.05). The body mass of rats in the high-dose YYHWM group was significantly higher at the 6th and 8th week of the intervention than that of rats in the vitacoenzyme group.
| Group | Intervention time | n | Body mass (g) | Water intake (mL) | Food intake (g) | Fecal water content (g/%) |
| Blank control | Week 0 | 10 | 418.32 ± 22.36b,c | 47.38 ± 2.59b | 32.36 ± 2.96 | 72.23 ± 5.03b |
| Week 2 | 10 | 423.16 ± 20.88b,c | 47.55 ± 3.68b | 30.88 ± 1.79 | 70.75 ± 3.67b | |
| Week 4 | 10 | 451.22 ± 21.84b,c | 46.69 ± 3.28b | 29.84 ± 2.55 | 69.71 ± 4.25b | |
| Week 6 | 10 | 472.12 ± 22.36b,c | 53.72 ± 2.80b | 28.36 ± 2.51 | 68.23 ± 3.18b | |
| Week 8 | 10 | 499.24 ± 22.75b,c | 54.07 ± 4.74b | 32.75 ± 2.79 | 72.62 ± 3.53b | |
| Model | Week 0 | 10 | 378.75 ± 18.78a | 62.47 ± 5.79a | 26.84 ± 1.94a | 66.71 ± 5.70a |
| Week 2 | 10 | 399.32 ± 18.68a | 60.92 ± 3.76a,c | 28.26 ± 2.37a | 58.13 ± 4.95a,c | |
| Week 4 | 10 | 410.27 ± 18.35a | 63.50 ± 6.33a,c | 30.65 ± 2.35a | 60.52 ± 4.69a,c | |
| Week 6 | 10 | 418.16 ± 20.07a | 56.47 ± 4.02a,c | 25.78 ± 2.01a | 61.65 ± 3.72a,c | |
| Week 8 | 10 | 439.72 ± 17.20a,c | 65.90 ± 4.79a,c | 27.78 ± 2.08a,c | 57.65 ± 5.01a,c | |
| Low-dose YYHWM | Week 0 | 11 | 370.24 ± 17.58a | 60.58 ± 4.68a | 27.40 ± 1.44a | 65.27 ± 4.18a |
| Week 2 | 11 | 389.76 ± 16.91a | 53.04 ± 5.77b | 30.64 ± 3.26a | 70.51 ± 5.23b | |
| Week 4 | 11 | 402.16 ± 19.02a | 52.69 ± 4.70b | 28.78 ± 2.27a | 68.65 ± 5.09b | |
| Week 6 | 11 | 424.92 ± 15.33a | 40.69 ± 4.13b | 26.68 ± 2.61a | 66.55 ± 4.93b | |
| Week 8 | 11 | 448.38 ± 16.91a | 52.01 ± 4.09b | 26.35 ± 3.38a,c | 66.22 ± 3.52b,c | |
| Medium-dose YYHWM | Week 0 | 11 | 368.71 ± 16.86a | 61.83 ± 3.49a | 27.07 ± 2.19a | 66.94 ± 2.75a |
| Week 2 | 11 | 379.73 ± 16.58a | 52.69 ± 3.82b | 31.72 ± 2.07a | 71.59 ± 4.21b | |
| Week 4 | 11 | 398.18 ± 14.81a | 40.69 ± 4.21b | 31.75 ± 1.71a | 71.62 ± 2.57b | |
| Week 6 | 11 | 429.22 ± 16.05a | 52.01 ± 3.58b | 28.91 ± 1.77a | 72.78 ± 2.78b | |
| Week 8 | 11 | 458.16 ± 13.04a,c | 42.41 ± 4.28b | 29.02 ± 2.39a | 68.89 ± 5.62b | |
| High-dose YYHWM-H | Week 0 | 11 | 370.19 ± 15.67a | 62.63 ± 3.97a | 27.33 ± 2.57a | 67.20 ± 6.22a |
| Week 2 | 11 | 397.72 ± 16.15a | 48.80 ± 2.75b | 26.91 ± 3.06a | 71.78 ± 4.22b | |
| Week 4 | 11 | 419.24 ± 16.24a | 49.32 ± 3.34b | 29.86 ± 1.75a | 69.73 ± 5.45b | |
| Week 6 | 11 | 438.43 ± 22.51a,b,c | 47.09 ± 4.97b | 30.58 ± 3.15a | 70.45 ± 5.77b | |
| Week 8 | 11 | 455.82 ± 21.74a,b,c | 48.29 ± 3.58b | 28.81 ± 2.24a | 68.68 ± 4.82b | |
| Vitacoenzyme | Week 0 | 11 | 365.41 ± 23.80a | 63.30 ± 2.95a | 26.05 ± 1.86a | 65.92 ± 5.92a |
| Week 2 | 11 | 379.43 ± 14.28a | 56.87 ± 3.64b | 28.04 ± 1.68a | 67.91 ± 4.44b | |
| Week 4 | 11 | 395.63 ± 22.03a | 43.21 ± 2.97b | 25.67 ± 1.94a | 65.54 ± 3.40b | |
| Week 6 | 11 | 422.27 ± 16.99a | 45.10 ± 4.62b | 29.15 ± 2.53a | 69.02 ± 5.31b | |
| Week 8 | 11 | 444.35 ± 17.74a,b | 45.27 ± 3.32b | 30.24 ± 3.03a | 70.11 ± 4.27b |
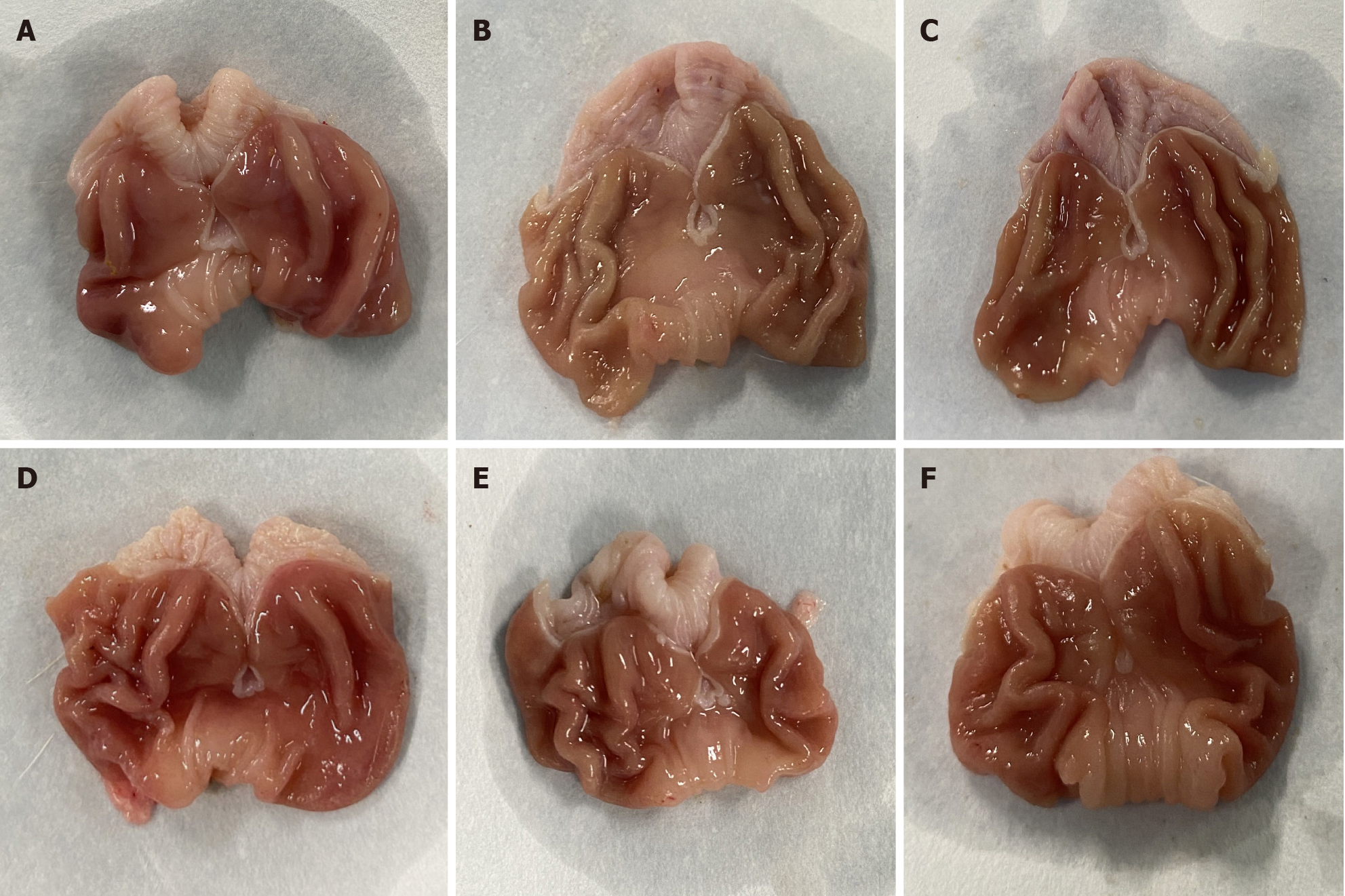
As shown in Figure 2, the gastric mucosa of rats in the blank control group exhibited a bright red color, with thick walls, elasticity, folds, and smoothness. Conversely, the gastric mucosa of rats in the model group displayed a mundane color, with a loss of luster, noticeable thinning of the gastric wall, reduced elasticity, decreased folds in the sinus, and flattened gastric folds. The medium- and high-dose YYHWM and vitacoenzyme groups displayed a gastric mucosal color and luster that tended towards normal, with increased folds and thickness of the gastric wall, as well as restored elasticity.
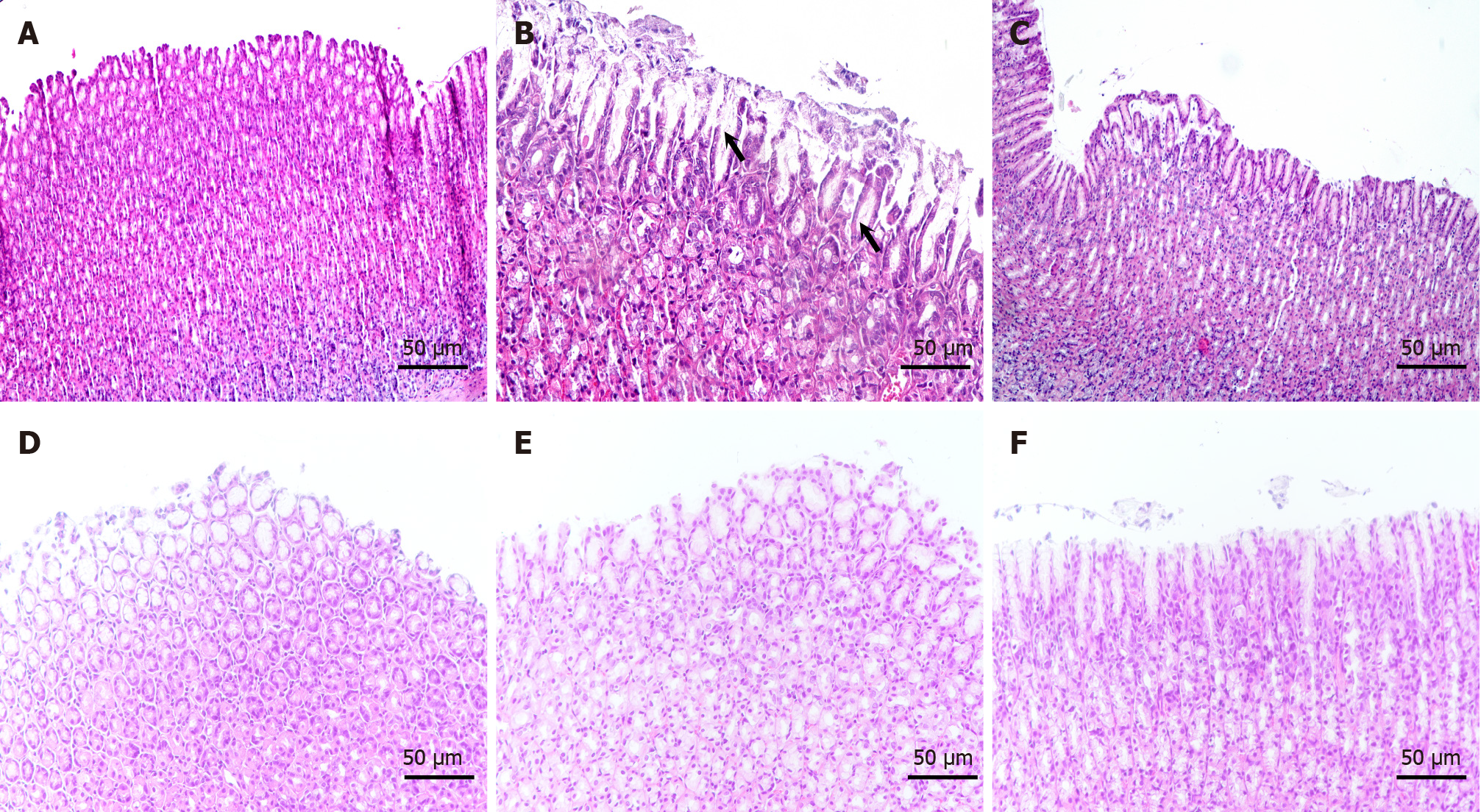
As shown in Figure 3, in the blank group, the thickness of the gastric mucosa and the structure of the intrinsic glands were normal and regular, and the epithelial cells were arranged neatly. In the model group, the gastric mucosa was thinner, the intrinsic gland structure was absent, the mucosa was atrophied, and the gastric mucosal epithelial cells were diminished. Moreover, they were substituted by rapidly proliferating intestinal epithelial cells, resulting in a disturbed cell arrangement. In addition, the submucosa exhibited a diffuse infiltration of chronic inflammatory cells. The gastric mucosa of rats in the group receiving high-dose YYHWM was significantly thickened, leading to a significant increase in intrinsic glands, reduction in gastric mucosal atrophy, and nearly complete disappearance of intestinal epithelial cells. In contrast, the gastric mucosa of rats in the vitacoenzyme group was thickened; however, the intrinsic glands remained relatively disorganized, and the submucosa showed greater infiltration of chronic inflammatory cells.
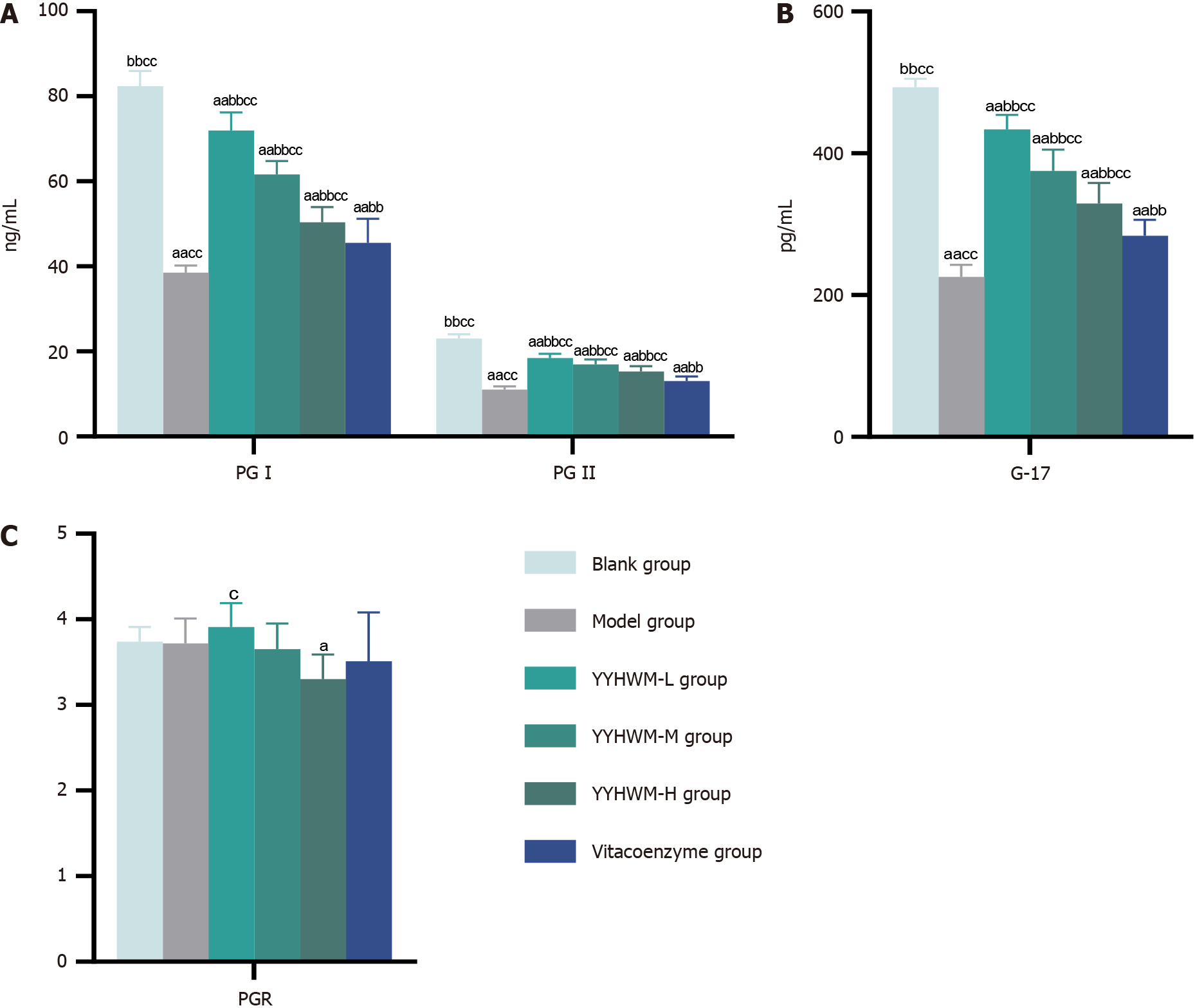
Table 2 and Figure 4 show that the serum levels of PGI, PGII, and gastrin-17 were significantly lower in the model, YYHWM, and vitacoenzyme groups than in the control group (P < 0.01 for all). Moreover, serum levels of PG-I, PG-II, and G-17 were significantly higher in the YYHWM and vitacoenzyme groups than in the model group (P < 0.01 for all). Serum levels of PG-I, PG-II, and G-17 were significantly higher in the YYHWM groups than in the vitacoenzyme group (P < 0.01 for all). However, the differences in serum PGI/PGII ratios between the vitacoenzyme, medium-, and high-dose YYHWM groups were not statistically significant (P > 0.05 for all).
| Group | n | PG-I (ng/mL) | PG-II (ng/mL) | PGR | G-17 (pg/mL) |
| Blank control | 6 | 82.41 ± 3.53d,e | 23.06 ± 0.96d,e | 3.74 ± 0.17 | 493.09 ± 12.17d,e |
| Model | 6 | 38.52 ± 1.71c,e | 11.06 ± 0.70c,e | 3.72 ± 0.29 | 225.52 ± 17.44c,e |
| Low-dose YYHWM | 6 | 71.98 ± 4.26c,d,e | 18.44 ± 1.01c,d,e | 3.91 ± 0.28b | 433.82 ± 20.18c,d,e |
| Medium-dose YYHWM | 6 | 61.64 ± 3.18c,d,e | 16.94 ± 1.19c,d,e | 3.65 ± 0.30 | 375.25 ± 30.03c,d,e |
| High-dose YYHWM | 6 | 50.41 ± 3.53b,c,d | 15.33 ± 1.24b,c,d | 3.30 ± 0.29a | 329.22 ± 29.11b,c,d |
| Vitacoenzyme | 6 | 45.52 ± 5.71c,d | 13.06 ± 1.10c,d | 3.51 ± 0.57 | 283.53 ± 22.66c,d |
| F value | 115.63 | 99.35 | 2.525 | 111.46 | |
| P value | < 0.01 | < 0.01 | 0.051 | < 0.01 | |
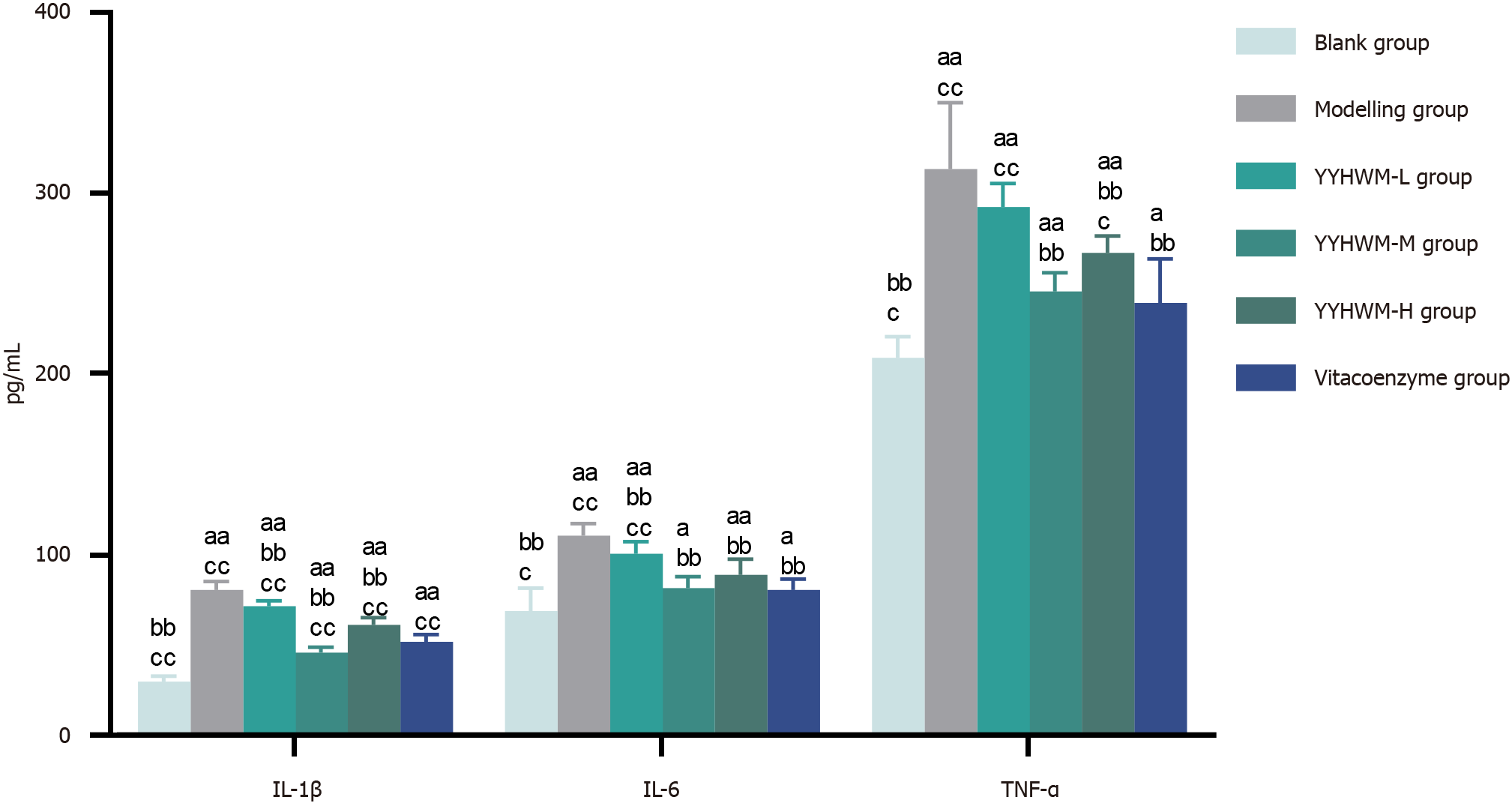
As presented in Table 3 and Figure 5, the levels of IL-1β, IL-6, and TNF-α were significantly higher in the model, YYHWM, and vinpocetine groups than in the blank control group (P < 0.05 or P < 0.01 for all). Serum levels of IL-1β, IL-6, and TNF-α were significantly lower in the medium- and high-dose YYHWM and vinpocetine groups than in the model group (P < 0.01 for all). Compared with the vitacoenzyme group, serum levels of IL-1β were lower in the medium-dose YYHWM group (P < 0.01).
| Group | n | IL-1β | IL-6 | TNF-α |
| Blank control | 6 | 30.15 ± 3.07e,f | 69.05 ± 12.72c,e | 209.24 ± 11.62c,e |
| Model | 6 | 80.98 ± 4.47d,f | 110.85 ± 6.68d,f | 313.37 ± 36.77d,f |
| Low-dose YYHWM | 6 | 71.89 ± 3.03d,e,f | 100.70 ± 6.76d,b,f | 292.47 ± 12.89d,f |
| Medium-dose YYHWM | 6 | 46.08 ± 3.11d,e,f | 81.92 ± 6.14a,e | 245.79 ± 10.29d,e |
| High-dose YYHWM | 6 | 61.56 ± 4.02d,e,f | 89.20 ± 8.48d,e | 267.30 ± 9.31c,d,e |
| Vitacoenzyme | 6 | 52.17 ± 4.12d,e | 80.72 ± 6.04a,e | 239.54 ± 24.15a,e |
| F value | 148.76 | 20.44 | 21.17 | |
| P value | < 0.01 | < 0.01 | < 0.01 | |
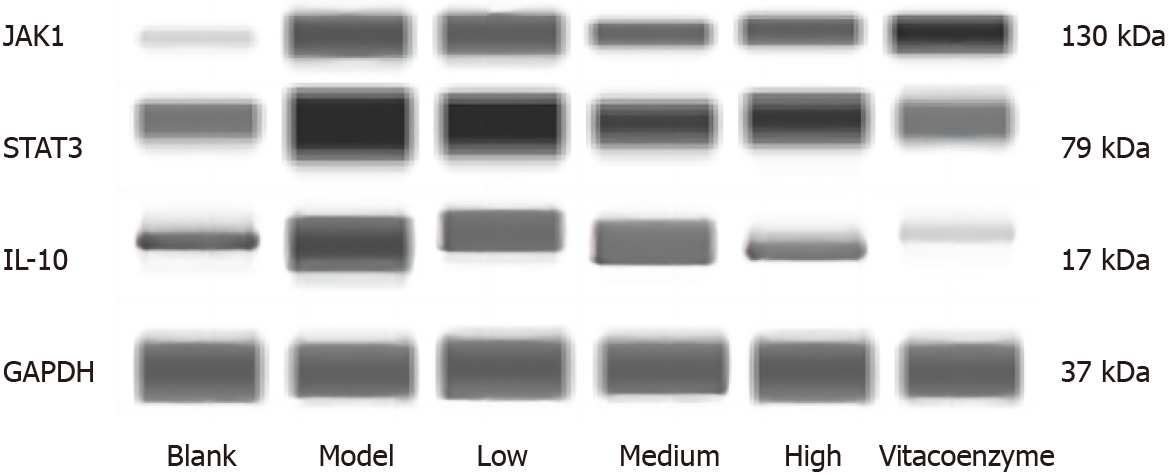
As shown in Table 4 and Figure 6, compared to the blank control group, the protein expression levels of IL-10, JAK1, and STAT3 in the gastric mucosa of rats were significantly higher (P < 0.05 or P < 0.01 for all) in the model group. Compared to the model group, the protein expression levels of IL-10, JAK1, and STAT3 were significantly lower (P < 0.05 or P < 0.01 for all) in all the drug-treated groups. Compared with the vitacoenzyme group, the protein expression levels of IL-10 and STAT3 were significantly reduced in the medium- and high-dose YYHWM groups (P < 0.05 or P < 0.01 for all).
| Group | IL-10/GADPH | JAK1/GADPH | STAT3/GADPH |
| Blank control | 0.47 ± 0.10e | 0.49 ± 0.05e,f | 0.24 ± 0.05e,f |
| Model | 1.11 ± 0.09d,f | 0.99 ± 0.07d,f | 1.04 ± 0.14c,d |
| Low-dose YYHWM | 0.76 ± 0.11a,e | 0.75 ± 0.07d,e,f | 1.07 ± 0.11d,f |
| Medium-dose YYHWM | 0.41 ± 0.12c,e | 0.69 ± 0.07b,d,f | 0.50 ± 0.13e |
| High-dose YYHWM | 0.19 ± 0.07a,e,f | 0.51 ± 0.05d | 0.55 ± 0.09a,e |
| Vitacoenzyme | 0.71 ± 0.08e | 0.38 ± 0.07d | 0.70 ± 0.04b,d |
| F value | 33.39 | 35.70 | 31.08 |
| P value | < 0.01 | < 0.01 | < 0.01 |
In 1995, Correa proposed the following evolution model of gastric mucosal carcinogenesis[13]: CAG, gastrointestinal epithelial hyperplasia, and gastric epithelial dysplasia. Inflammation, cell proliferation, apoptosis, autophagy dysfunction, energy metabolism disorders, and other factors may be involved in gastric mucosal carcinogenesis; however, its exact pathogenesis remains unclear. Conventional treatments for CAG concentrate on managing symptoms, which frequently prove to be inadequate[14]. TCM has garnered increasing interest because of its holistic perspectives and evidence-supported therapeutic approaches. TCM posits that a paucity of gastric yin is a fundamental cause of CAG. Decreased gastric secretions and inadequate gastric nourishment culminate in gastric body atrophy. The combination of nourishing yin and activating the stomach was based on the stomach yin deficiency syndrome. It nourishes the stomach and produces fluids. Studies[9,10] have demonstrated its superior clinical efficacy in treating patients with CAG.
At the end of the study, the rats in each modeling group exhibited yellowing of fur, thinning and dullness, poor nutrition, and weight reduction, indicating successful model creation. After treatment with vinblastine and different doses of YYHWM, the symptoms of rats in each group significantly improved, with high-dose YYHWM demonstrating the most substantial efficacy. The histopathological results demonstrated varying degrees of improvement in gastric damage in CAG rats treated with vitacoenzymes and different doses of YYHWM, with high-dose YYHWM having the most significant effects. ELISA results indicated increased levels of PG-I, PG-II, and gastrin-17 in CAG rats after treatment with vinblastine and different doses of YYHWM, suggesting that drug treatment effectively improved the gastric secretion function of CAG rats.
The pathogenesis of CAG is complex, and in recent years, an increasing number of studies have confirmed that dysregulation of the IL-10/JAK1/STAT3 signaling pathway is closely associated with the development of CAG[15]. IL-10 is a cytokine encoded by the IL-10 gene. In the human body, IL-10 is mainly produced by immune cells[16] and is associated with various cancers such as gastric[15], colon[17], breast[18], pancreatic[19], and cervical[20] cancers. IL-10 can regulate the JAK1/STAT3 signaling pathway[21] and extracellular signal regulation[22] and affects the expression of downstream molecules. In this pathway, activation of upstream JAK1 signaling is the main molecular mechanism for the sustained expression of STAT3[23]. STAT3 has been identified as an oncogenic protein whose hyperactivation enhances epithelial-mesenchymal transition (EMT)[24], tumor angiogenesis[25], extracellular matrix (ECM) degradation[26], and many other processes, thus promoting tumor invasion and metastasis[27]. Furthermore, STAT3 activation induces the activation of signaling molecules related to chronic inflammation, such as NF-κB, cytokines[28], chemokines such as CXCR7 and CXCR12[29], and other mediators, which play a key role in inducing and maintaining a pro-cancer inflammatory envi
This study demonstrated that the serum levels of the inflammatory factors IL-1β, IL-6, and TNF-α increased in model group rats, alongside an increase in the protein expression of IL-10, JAK1, and STAT3 in gastric mucosal tissues, signaling activation of the IL-10/JAK1/STAT3 signaling pathway. Whereas, after the intervention of YYHWM, there was a decrease in serum levels of the inflammatory factors IL-1β, IL-6, and TNF-α and tissue expression of IL-10, JAK1, and STAT3. This suggests that YYHWM inhibits the activation of the IL-10/JAK1/STAT3 signaling pathway and reduces the inflammatory response.
This study has several limitations. First, gastric fluid and gastric acid levels were not investigated. CAG has been demonstrated to reduce gastric acid content owing to gastric mucosal atrophy and decreased gastric acid-secreting cell numbers. Further studies are required to explore the effect of YYHWM on gastric acid content in the gastric fluid. Second, it has not been yet known whether the effects of YYHWM are affected by the activation of the IL-10/JAK1/STAT3 signaling pathway. In the future, it would be beneficial to add this pathway activator to the intervention to observe the effects of YYHWM.
In conclusion, YYHWM mitigates body mass loss and gastric mucosal damage, enhances gastric secretion, and decreases the inflammatory response in CAG rats by inhibiting the IL-10/JAK1/STAT3 signaling pathway. These results provide experimental evidence for the clinical application of YYHWM.
We thank Professor Shi H for her assistance in manuscript revision, Dr. Kong Y for further linguistic revision, and Wu DD for drawing the diagram.
| 1. | Lin XM, Wang L, Xi CH, Wang J, Wang XF, Wang Q, Yuan C. Morphometric features of gastric mucosa in atrophic gastritis: A different pattern between corpus and antrum. Medicine (Baltimore). 2023;102:e33480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11439] [Article Influence: 3813.0] [Reference Citation Analysis (4)] |
| 3. | Yuan MW, Wang HH, Duan RF, Xu KP, Hu SY, Qiao YL, Zhang Y, Zhao F. [Analysis on cancer incidence and mortality attributed to human papillomavirus infection in China, 2016]. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Miguez JSG, Dela Justina V, Bressan AFM, Marchi PGF, Honorio-França AC, Carneiro FS, Clinton Webb R, Tostes RC, Giachini FR, Lima VV. O-Glycosylation with O-linked β-N-acetylglucosamine increases vascular contraction: Possible modulatory role on Interleukin-10 signaling pathway. Life Sci. 2018;209:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kong Y, Zhang Y, Zhao X, Wang G, Liu Q. Carboxymethyl-chitosan attenuates inducible nitric oxide synthase and promotes interleukin-10 production in rat chondrocytes. Exp Ther Med. 2017;14:5641-5646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Russo M, Rodriguez-Castro KI, Franceschi M, Ferronato A, Panozzo MP, Brozzi L, Di Mario F, Crafa P, Brandimarte G, Tursi A. Appropriateness of Proton Pump Inhibitor Prescription Evaluated by Using Serological Markers. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 7. | Di Mario F, Rodriguez-Castro KI, Franceschi M, Landi S, Grillo S, Franzoni L, Russo M, Brandimarte G, Tursi A, Crafa P. Improvement of Symptoms in Patients Affected by Chronic Atrophic Gastritis Using L-Cysteine (Acetium®). Dig Dis. 2023;41:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Shao CM, Xie SS, Zhi Y, Haerheng SAK, Lin BT, Yu YF, Zeng BF. To explore the mechanism of Yangyin Huowei mixture in treating chronic atrophic gastritis based on network pharmacology and experimental verification. Hunan Zhongyiyao Daxue Xuebao. 2023;43:847-856. [DOI] [Full Text] |
| 9. | Qin FH. Clinical effect of Yangyin Huowei mixture in treating chronic atrophic gastritis. Guangming Zhongyi. 2016;31:1418-1419. [DOI] [Full Text] |
| 10. | Li YJ. The effect of Yangyin Huowei mixture on atrophic gastritis. Zhongguo Shiyong Yiyao. 2016;11:190-191. [DOI] [Full Text] |
| 11. | Guo Y, Jia X, Du P, Wang J, Du Y, Li B, Xue Y, Jiang J, Cai Y, Yang Q. Mechanistic insights into the ameliorative effects of Xianglianhuazhuo formula on chronic atrophic gastritis through ferroptosis mediated by YY1/miR-320a/TFRC signal pathway. J Ethnopharmacol. 2024;323:117608. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Olson D, Taylor J, Willis K, Hensley K, Allred S, Zaval M, Farr L, Thurman R, Jain N, Hein R, Ulrich M, Peterson S, Kulukian A. HER2-Selective and Reversible Tyrosine Kinase Inhibitor Tucatinib Potentiates the Activity of T-DM1 in Preclinical Models of HER2-positive Breast Cancer. Cancer Res Commun. 2023;3:1927-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s-1943s. [PubMed] |
| 14. | Qian CS, Lv CY, Li QD, Yanghu QY, Jiang YR, Zheng X, Li Q. Analysis on the treatment of Ye tianshi’s "stomach soup". Zhongyiyao Tongbao. 2022;21:25-26, 32. |
| 15. | Yuan PQ, Wu SV, Wang L, Taché Y. The ghrelin agonist, HM01 activates central vagal and enteric cholinergic neurons and reverses gastric inflammatory and ileus responses in rats. Neurogastroenterol Motil. 2023;35:e14561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | V VR, Ramu A, Chinnappan J, Velmurugan P, Pathak R, Pashameah RA, A Oyouni AA, M Al-Amer O, I Alasseiri M, Hamadi A, A Alanazi M, Sathiamoorthi T. Interleukin-10 as Covid-19 biomarker targeting KSK and its analogues: Integrated network pharmacology. PLoS One. 2023;18:e0282263. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Szczygieł A, Węgierek-Ciura K, Wróblewska A, Mierzejewska J, Rossowska J, Szermer-Olearnik B, Świtalska M, Anger-Góra N, Goszczyński TM, Pajtasz-Piasecka E. Combined therapy with methotrexate nanoconjugate and dendritic cells with downregulated IL-10R expression modulates the tumor microenvironment and enhances the systemic anti-tumor immune response in MC38 murine colon carcinoma. Front Immunol. 2023;14:1155377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Avisar A, Cohen M, Aharon A, Katz R, Bar-Sela G. Positive affect and fatigue as predictors of anti-inflammatory IL-10 concentrations among colorectal cancer patients during adjuvant chemotherapy. J Psychosom Res. 2023;167:111162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Chen L, Qian Y, Guo M, Liu Y, Li J, Wu M, Zhang Y, Wang Y, Peng X, Zhan X. Autologous ex vivo expanded NK cells combined with PD-1 inhibitor improved ascitic fluid immune microenvironment of peritoneal metastatic pancreatic cancer: a case study. Am J Transl Res. 2023;15:316-323. [PubMed] |
| 20. | Li W, An N, Wang M, Liu X, Mei Z. Interleukin-23 receptor defines T helper 1-like regulatory T cells in oral squamous cell carcinoma. Immun Inflamm Dis. 2022;10:e746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 21. | Wang G, Xu B, Shi F, Du M, Li Y, Yu T, Chen L. Protective Effect of Methane-Rich Saline on Acetic Acid-Induced Ulcerative Colitis via Blocking the TLR4/NF-κB/MAPK Pathway and Promoting IL-10/JAK1/STAT3-Mediated Anti-inflammatory Response. Oxid Med Cell Longev. 2019;2019:7850324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Li K, Li J, Wei X, Wang J, Geng M, Ai K, Liang W, Zhang J, Li K, Gao H, Yang J. IL-10 Negatively Controls the Primary T Cell Response of Tilapia by Triggering the JAK1/STAT3/SOCS3 Axis That Suppresses NF-κB and MAPK/ERK Signaling. J Immunol. 2023;210:229-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 23. | Lu R, Zhang L, Wang H, Li M, Feng W, Zheng X. Echinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway. Front Pharmacol. 2022;13:993483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 24. | Alqahtani T, Kumarasamy V, Alghamdi SS, Suliman RS, Bin Saleh K, Alrashed MA, Aldhaeefi M, Sun D. Adefovir Dipivoxil as a Therapeutic Candidate for Medullary Thyroid Carcinoma: Targeting RET and STAT3 Proto-Oncogenes. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Alam MM, Fermin JM, Knackstedt M, Noonan MJ, Powell T, Goodreau L, Daniel EK, Rong X, Moore-Medlin T, Khandelwal AR, Nathan CO. Everolimus downregulates STAT3/HIF-1α/VEGF pathway to inhibit angiogenesis and lymphangiogenesis in TP53 mutant head and neck squamous cell carcinoma (HNSCC). Oncotarget. 2023;14:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 26. | Jalali BM, Likszo P, Lukasik K. STAT3 in porcine endometrium during early pregnancy induces changes in extracellular matrix components and promotes angiogenesis†. Biol Reprod. 2022;107:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Othumpangat S, Noti JD. β-Defensin-1 Regulates Influenza Virus Infection in Human Bronchial Epithelial Cells through the STAT3 Signaling Pathway. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Yin S, He Y, Tang J, Pu J, Jia G, Liu G, Tian G, Chen X, Cai J, Kang B, Che L, Zhao H. Hydroxy-Selenomethionine Mitigated Chronic Heat Stress-Induced Porcine Splenic Damage via Activation of Nrf2/Keap1 Signal and Suppression of NFκb and STAT Signal. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Guo J, Tong CY, Shi JG, Li XJ. C-X-C motif chemokine ligand 12 (CXCL12)/C-X-C motif chemokine receptor 7(CXCR7) regulates epithelial-mesenchymal transition process and promotes the metastasis of esophageal cancer by activating signal transducer and activator of transcription 3 (STAT3) pathway. Bioengineered. 2022;13:7425-7438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |