Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2167
Revised: May 31, 2024
Accepted: June 20, 2024
Published online: July 27, 2024
Processing time: 117 Days and 8.5 Hours
In recent years, pure laparoscopic radical surgery for Bismuth-Corlette type III and IV hilar cholangiocarcinoma (HCCA) has been preliminarily explored and applied, but the surgical strategy and safety are still worthy of further improve
To summarize and share the application experience of the emerging strategy of “hepatic hilum area dissection priority, liver posterior separation first” in pure laparoscopic radical resection for patients with HCCA of Bismuth-Corlette types III and IV.
The clinical data and surgical videos of 6 patients with HCCA of Bismuth-Corlette types III and IV who underwent pure laparoscopic radical resection in our department from December 2021 to December 2023 were retrospectively analyzed.
Among the 6 patients, 4 were males and 2 were females. The average age was 62.2 ± 11.0 years, and the median body mass index was 20.7 (19.2-24.1) kg/m2. The preoperative median total bilirubin was 57.7 (16.0-155.7) μmol/L. One patient had Bismuth-Corlette type IIIa, 4 patients had Bismuth-Corlette type IIIb, and 1 patient had Bismuth-Corlette type IV. All patients successfully underwent pure laparoscopic radical resection following the strategy of “hepatic hilum area dissection priority, liver posterior separation first”. The operation time was 358.3 ± 85.0 minutes, and the intraoperative blood loss volume was 195.0 ± 108.4 mL. None of the patients received blood transfusions during the perioperative period. The median length of stay was 8.3 (7.0-10.0) days. Mild bile leakage occurred in 2 patients, and all patients were discharged without serious surgery-related complications.
The emerging strategy of “hepatic hilum area dissection priority, liver posterior separation first” is safe and feasible in pure laparoscopic radical surgery for patients with HCCA of Bismuth-Corlette types III and IV. This strategy is helpful for promoting the modularization and process of pure laparoscopic radical surgery for complicated HCCA, shortens the learning curve, and is worthy of further clinical application.
Core Tip: In recent years, pure laparoscopic radical surgery for Bismuth-Corlette types III and IV hilar cholangiocarcinoma (HCCA) has been preliminarily explored and applied, while the surgical strategy and safety remain worthy of further improvement. We summarized the application experience of the strategy of “hepatic hilum area dissection priority, liver posterior separation first” in pure laparoscopic radical resection for patients with HCCA of Bismuth-Corlette types III and IV. All the 6 patients successfully received pure laparoscopic radical resection with this strategy. None of the patients had blood transfusion during perioperative period. All patients were discharged without serious surgery-related complications. The strategy of “hepatic hilum area dissection priority, liver posterior separation first” is safe and feasible in the pure laparoscopic radical surgery for Bismuth-Corlette types III and IV HCCA.
- Citation: Hu XS, Wang Y, Pan HT, Zhu C, Chen SL, Zhou S, Liu HC, Pang Q, Jin H. “Hepatic hilum area priority, liver posterior first”: An optimized strategy in laparoscopic resection for type III-IV hilar cholangiocarcinoma. World J Gastrointest Surg 2024; 16(7): 2167-2174
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2167.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2167
Hilar cholangiocarcinoma (HCCA) is a malignant bile duct epithelial tumor originating from the common hepatic duct, left and right hepatic ducts and conjunctures and accounts for approximately 50%-70% of cholangiocarcinomas[1]. HCCA is difficult to diagnose in the early stage, and patients’ prognosis is poor. Surgical resection remains the only curative treatment option for HCCA[2]. Due to the complex anatomical structure of the hepatic hilum, the variation rate of the bile duct and blood vessels is high. In particular, for Bismuth-Corlette type III and IV patients, the tumor invaded the hepatic duct, left and right hepatic ducts and secondary bile duct. Patients often exhibit hepatic artery (HA), portal vein, intrahepatic and extrahepatic bile duct invasion and intrahepatic duct variation. As a result, the surgical resection rate is low, and the incidence rates of postoperative complications and mortality are relatively high[3]. At present, patients with HCCA of Bismuth-Corlette types III and IV usually undergo radical operations via open surgery. In recent years, with the development of the concept and technology of the laparoscopic technique, pure laparoscopic radical surgery for Bismuth-Corlette type III and IV HCCA has also been preliminarily explored and applied, but the surgical strategy and safety are still worthy of further improvement and attention[4,5].
In recent years, on the basis of long-term clinical practice, our team summarized the surgical strategy of “hepatic hilum area dissection priority, liver posterior separation first” for the pure laparoscopic radical surgical procedure for patients with Bismuth-Corlette type III and IV HCCA. To date, this strategy has achieved good surgical results in clinical application, and the relevant clinical data and surgical video analyses are summarized.
The clinical data and surgical videos of 6 Bismuth-Corlette type III and IV HCCA patients who underwent laparoscopic radical surgery in the Department of Hepatopancreatobiliary Surgery of Anhui No. 2 Provincial People’s Hospital from December 2021 to December 2023 was retrospectively analyzed. The operation was performed by the same team, and all patients underwent pure laparoscopy. The inclusion criteria were as follows: (1) ≥ 18 years of age; (2) Diagnosed with Bismuth-Corlette type III or IV HCCA, and preoperative evaluation preliminarily suggested that radical surgery was feasible; (3) Eastern Cooperative Oncology Group score 0-2; (4) No obvious surgical contraindications, such as abnormal laboratory indicators and obvious dysfunction of vital organs; and (5) No other active malignant tumors.
The use of relevant clinical data in this study was in line with the requirements of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Second People’s Hospital of Anhui Province (approval number: 2022-011). Written informed consent was preoperatively obtained from the patients.
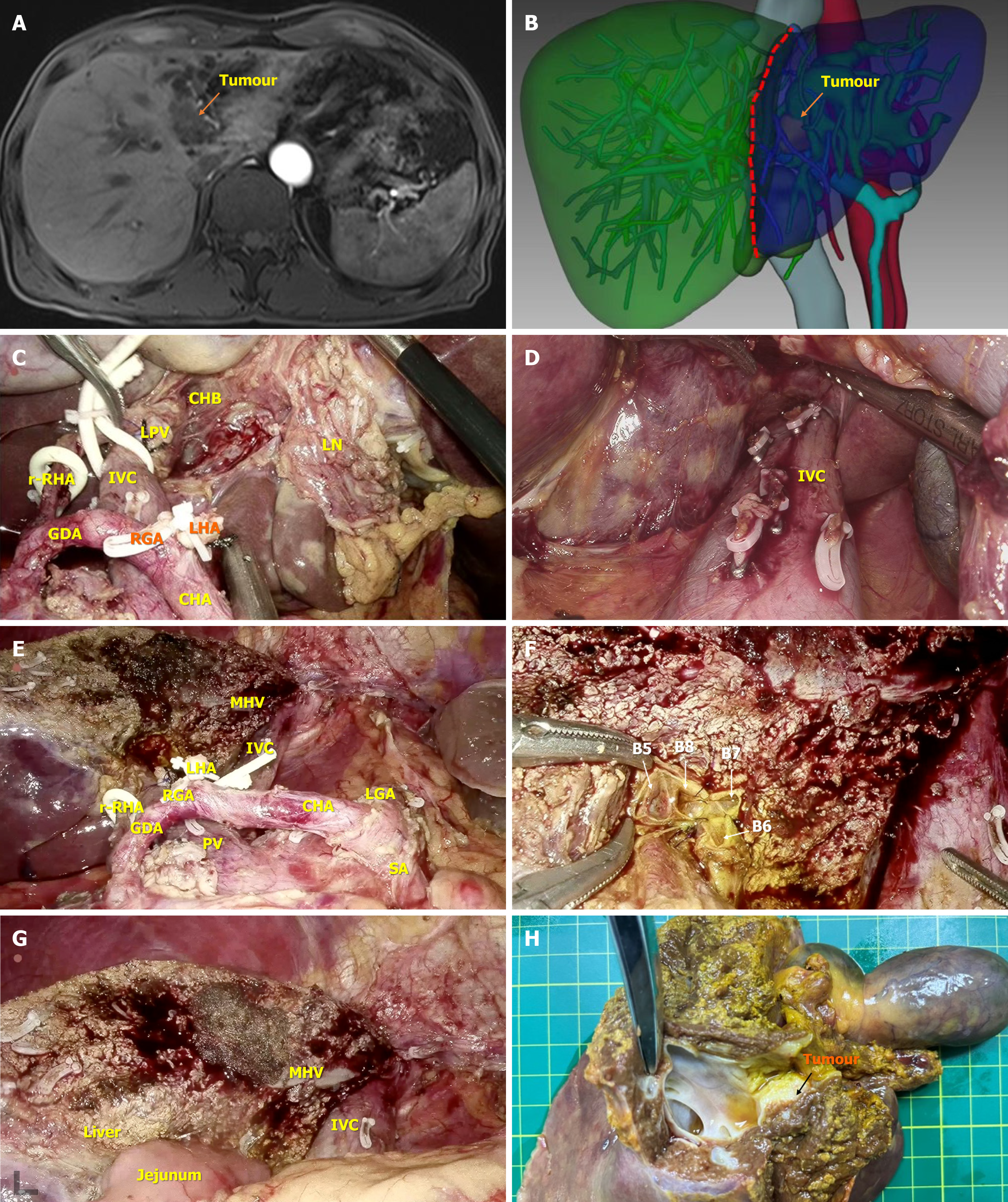
After the improvement of the preoperative preparation, general anesthesia was used, and the patient was placed in a supine, split-legged position. The trocar layout was similar to that reported in a previous study (Figure 1)[6]. Routine abdominal exploration was performed to exclude abdominal and hepatic metastasis. Kocher incision separation was performed at the upper right of the pancreatic head, and the No. 13a lymph node behind the pancreatic head was removed for rapid frozen pathological examination. If the tested lymph node was negative, the hepatic hilar area was cleared. Provided that it was positive, the Kocher incision was further expanded, and rapid frozen pathological examinations of the No. 16a2 and 16b1 lymph nodes were performed. If the No. 16 lymph node was still positive, further surgery was stopped. The clearance process of the hepatic hilar area was carried out according to the “five steps four quadrants” modularized en bloc dissection technique reported in our recent study[7]. The surgical procedures were implemented as follows (Figure 2).
The No. 8a lymph node was dissected and separated through the upper border of the pancreas, behind which the common HA was exposed and suspended, and then the inferior portal vein (PV) was exposed. Subsequently, according to the HA/PV axis, the hepatic hilar region was divided into four zones: Upper, lower, left, and right.
The hepatic hilum was separated along the 12-point position of the HA/PV surface. The No. 12a and 8p lymph nodes as well as the surrounding tissues were pushed to the left and right zones but were not severed via the combination of blunt and sharp dissection. Ultimately, the full course of the HA/PV axis was exposed. If the main stem of the HA or PV was invaded by the tumor, resectability was re-evaluated. In patients with both HA and PV invasion, termination of surgery was recommended. The operation was continued if the lateral HA and PV were not invaded or vascular reconstruction was feasible after the evaluation.
The lower left zone was separated toward the abdominal trunk along the HA/PV axis. The No. 8P lymph node was cleared, after which the left zone of the HA/PV axis was cleared in a counterclockwise direction along the right margin of the left gastric artery, until the left edge of the PV was completely exposed. The dissected tissues in the left zone were pushed behind the PV.
The No. 12b lymph node and surrounding tissues in the right zone of the HA/PV axis were pushed to the middle and distal bile duct using a combination of blunt and sharp dissection until the right margin of the PV was completely exposed and the right gastric artery was severed. Subsequently, the common bile duct was severed at the upper border of the pancreas, and the distal bile duct incision margin was frozen for pathological examination until the result was negative. Then, the distal bile duct stump could be closed by absorption suture. Hepatopancreatoduodenectomy should be considered if the pancreatic biliary freezing test is positive.
The PV was separated from the No. 12p lymph node and surrounding tissues. Below the PV, the aforementioned left zone clearance tissue together with the HA/PV axis clearance tissue were removed from the right posterior side. After the dissociation of the gallbladder, the posterior and upper tissues in the right region were partially separated from the hepatic portal and eventually attached to the hepatic duct and the gallbladder, and the en bloc clearance process in the hepatic hilar region was completed.
Combined with the preoperative examinations, the invasiveness of the cancer in the retained hepatic duct was further investigated. The lateral PV, HA and hepatic duct could be severed in turn provided that the hepatic duct was not significantly invaded. The incision margin of the stump of the reserved bile duct was sent for pathological examination and tissue was resected until the pathology was negative (if there were multiple bile ducts on the reserved side, they were examined separately).
The retrohepatic and inferior vena cava space was separated through the combined left-right approach, and the short hepatic vein was successively disconnected until the top, left and right sides of the retrohepatic inferior vena cava were completely exposed.
The perihepatic straplines were dissociated, and the liver parenchyma was severed successively from the foot to the head side with an ultrasonic knife along the middle of the hepatic ischemic line and the middle hepatic vein. The encountered large blood vessels and bile ducts were severed by vascular clamp or suture. After hepatectomy, absorbable suture materials were used to collage the remaining hepatic duct stump for hepatojejunostomy (Roux-en-Y).
The jejunum and part of the mesentery were severed at approximately 20 cm from the distal end of the Troidel ligament. The distal jejunum was lifted to the stump of the hepatic duct for hepatojejunostomy, and the proximal jejunum was anastomosed approximately 45 cm from the distal end of the anastomosis. After reconstruction of the digestive tract, the mesenteric hiatus was closed, and the abdominal cavity was rinsed. After confirming that there was no intraperitoneal bleeding or biliary leakage, routine drainage was performed next to the hepatic wound and bilioenteric anastomosis, and a horizontal incision was made in the subnavel area to remove the whole surgical specimen.
Sex, age, body mass index (BMI), Bismuth-Corlette type, preoperative total bilirubin (TB) and other laboratory indicators, preoperative management measures, and relevant clinical data such as operation time, intraoperative blood loss, tumor-node-metastasis stage, and perioperative complications were recorded.
SPSS 25.0 statistical software was used to record, process and analyze the data. The measurement data are expressed as the mean ± SD or median (range), as appropriate. Count data are expressed as frequencies.
A total of 6 patients were enrolled, 4 of whom were males and 2 of whom were females. The mean age was 62.2 ± 11.0 years, and the median BMI was 20.7 (19.2-24.1) kg/m2. All patients underwent preoperative thin-layer enhanced abdominal computed tomography/magnetic resonance imaging, magnetic resonance cholangiopancreatography, three-dimensional visual reconstruction and related laboratory examinations. In addition, Bismuth-Corlette III or IV HCCA patients were diagnosed before surgery, and the feasibility of radical surgery was initially assessed. One patient had Bismuth-Corlette type IIIa, 4 had Bismuth-Corlette type IIIb, and 1 had Bismuth-Corlette type IV. All 6 patients were admitted with significantly elevated serum TB levels. Therefore, preoperative percutaneous transhepatic cholangiodrainage was used to relieve jaundice, and biliary recycling was also applied in all patients. When the serum TB concentration decreased to a satisfactory level (< 85 μmol/L), laparoscopic radical surgery for HCCA was performed. The median preoperative TB was 20.6 (11.9-70) μmol/L. The preoperative baseline data are shown in Table 1.
| Patient ID | Gender | Age (year) | BMI (kg/m2) | Diagnosis | Bismuth classification | Preoperative PTCD (yes/no) | Preoperative TB (μmol/L) |
| 1 | Male | 58 | 19.2 | HCCA | IIIb | Yes | 22.1 |
| 2 | Male | 66 | 23.1 | HCCA | IIIa | Yes | 43.5 |
| 3 | Male | 71 | 24.1 | HCCA | IV | Yes | 70.0 |
| 4 | Male | 78 | 20.2 | HCCA | IIIb | Yes | 11.9 |
| 5 | Female | 55 | 21.0 | HCCA | IIIb | Yes | 19.1 |
| 6 | Female | 50 | 20.4 | HCCA | IIIb | Yes | 17.2 |
All patients successfully completed HCCA radical surgery by using the strategy of “hepatic hilum area dissection priority, liver posterior separation first”. The operative time was 358.3 ± 85.0 minutes, and the volume of intraoperative blood loss was 195.0 ± 108.4 mL. None of the patients received intraoperative blood transfusions. Postoperative bile leakage occurred in 2 patients who were thoroughly cured by prolonging the extraction time of the abdominal drainage tube. The postoperative length of hospital stay was 8.3 (7.0-10.0) days. All patients were successfully discharged without serious surgery-related complications. The intraoperative and postoperative baseline data are shown in Table 2.
| Patient ID | Surgical procedure | Operation time (minutes) | Blood loss (mL) | Complication (yes/no) | Biliary margin negative (yes/no) | Tumor size (cm) | Vascular invasion (yes/no) | TNM stage | Hospital stay (days) |
| 1 | LHR + CLR + BA | 280 | 100 | Yes, BL | Yes | 2.1 | No | T2N0M0 | 8 |
| 2 | RHR + CLR + BA | 310 | 200 | No | Yes | 1.8 | No | T2N0M0 | 9 |
| 3 | CHR + CLR + BA | 520 | 400 | Yes, BL | Yes | 3.3 | No | T2N0M0 | 10 |
| 4 | LHR + CLR + BA | 360 | 120 | No | Yes | 2.0 | No | T2N1M0 | 7 |
| 5 | LHR + CLR + BA | 320 | 150 | No | Yes | 1.1 | No | T1N0M0 | 8 |
| 6 | LHR + CLR + BA | 360 | 200 | No | Yes | 3.8 | Yes | T3N2M0 | 8 |
Radical surgery for HCCA has always been a difficult problem in the field of hepatobiliary and pancreatic surgery. As complicated anatomical structures are involved in HCCA, surgeons are must be aware of the vasculature adjacent to the tumor during the operation. Therefore, surgery for HCCA is difficult, and the risk of postoperative complications is high[8]. In particular, due to wider tumor involvement and more complicated anatomical structures, radical surgery is more difficult in patients with HCCA of Bismuth-Corlette types III and IV[5]. The main characteristics of the types of tumors are as follows: (1) There is a high risk of local aggression: Bismuth-Corlette types III and IV HCCA are usually highly invasive and often invade surrounding vital blood vessels and tissues, which significantly increases the difficulty of the operation[9]; (2) A high proportion of lymph node metastasis: Patients with HCCA of Bismuth-Corlette types III and IV have a relatively high risk of lymph node metastasis, which may lead to a high risk of postoperative recurrence and metastasis. Therefore, thorough lymph node dissection is essential for these patients[10]. It is particularly important to optimize the technology used for modular and en bloc clearance of the hepatic hilar region[11,12]; (3) A high rate of surgical margin positivity: Due to the particularity of the tumor location and invasiveness, the rate of surgical margin positivity is relatively high in Bismuth-Corlette type III and IV HCCA patients, which further increases the risk of postoperative recurrence[13,14]; and (4) A high risk of intraoperative bleeding: Due to the extensive vascular network in the hepatic hilar area, complex anatomical variation factors, and the requirement for extensive hepatectomy, the risk of intraoperative bleeding is high[15,16]. Therefore, strict intraoperative vascular protection and control are essential in Bismuth-Corlette type III and IV HCCA patients.
In recent years, with the continuous development of laparoscopic surgery concepts and technology, pure laparoscopic radical surgery for HCCA has gradually attracted the attention of surgeons. Laparoscopic technology has the advantages of a surgical field amplification effect and unique perspective, which can make surgical operation more accurate, especially when operating on the posterior side of various tissues and organs[17]. However, for patients with HCCA of Bismuth-Corlette types III and IV, there are still some challenges and difficulties in the selection of radical laparoscopic surgery strategies and surgical safety[5,18]. Extensive experience in laparoscopic surgery and sufficient technical reserve are the basic requirements for surgeons[19]. Therefore, to date, this type of operation has only been explored and studied in a few experienced high-level medical centers.
In view of the above surgical difficulties, our team has summarized surgical strategies for “hepatic hilum area dissection priority, liver posterior separation first” for long-term clinical practice. The clinical application of this surgical strategy resulted in satisfactory curative effects in 6 patients. All 6 patients successfully completed the operation, and compared with previous reports of similar operations, the application of this strategy could shorten the operation time, reduce intraoperative blood loss, and shorten the postoperative hospital duration[20]. In addition, none of the patients experienced serious perioperative complications. Postoperative follow-up further revealed that the operative effect was satisfactory, and the quality of life significantly improved. Therefore, this surgical strategy is safe and feasible in surgical practice and has important clinical significance for optimizing surgical procedures and improving treatment efficacy and quality of life.
Briefly, there are several advantages of this strategy. First, this technical strategy focuses on priority exploration and dissection of the hepatic hilar region, which is favorable for accurately evaluating the resectability of tumors during surgery and preventing unnecessary surgical trauma. In addition, the priority clearance of the hepatic hilar region is favorable for the distinction and protection of vital blood vessels in the hilar region, which is conducive to the disconnection or blocking of hepatic blood vessels during subsequent hepatectomy[21]. Therefore, this strategy reduces the risk of intraoperative bleeding and complications and improves the safety of surgery[21]. Second, the strategy is preferred for en bloc clearance in the hepatic hilar region, which improves the effectiveness of radical surgery, prevents tumor residue and reduces postoperative recurrence risk[22]. In addition, the adoption of the strategy of posterior liver separation can fully exploit the advantages of laparoscopy, and surgeons can address the short hepatic vein behind the liver in advance, which is not easy to reveal from a conventional perspective and is prone to accidental bleeding. The release of the space behind the liver is conducive to subsequent extended hepatectomy combined with caudate lobectomy. In addition, it reduces intraoperative bleeding and improves the radical excision rate and safety of the operation[23]. Finally, the implementation of this strategy helps to modularize and streamline the entire surgical procedure to shorten the surgical time, reduce intraoperative bleeding, and improve the surgical completion rate. Therefore, laparoscopic radical surgery is expected to become the preferred strategy for patients with HCCA of Bismuth-Corlette types III and IV.
Although this study has yielded some profound findings, there are still several shortcomings. First, the sample size was relatively small, and it is necessary to further expand the sample size and carry out multicenter clinical research. Second, the applicability and effectiveness of this strategy for patients with different pathological types, surgeons with different levels of experience and patients at different medical centers should be further explored. Third, the follow-up period was too short to assess long-term outcomes. Long-term follow-up and assessment of postoperative quality of life are still needed to fully understand the long-term effects of this strategy.
In conclusion, the emerging strategy proposed by our team, “hepatic hilum area dissection priority, liver posterior separation first”, is safe and feasible for the pure laparoscopic radical surgery of patients with HCCA of Bismuth-Corlette types III and IV. This strategy is helpful for promoting the modularization and process of pure laparoscopic radical surgery for complicated HCCA and for shortening the learning curve and is worthy of further clinical application.
| 1. | Soares KC, Jarnagin WR. The Landmark Series: Hilar Cholangiocarcinoma. Ann Surg Oncol. 2021;28:4158-4170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (36)] |
| 2. | Anderson B, Doyle MBM. Surgical Considerations of Hilar Cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28:601-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (36)] |
| 3. | Ruzzenente A, Bagante F, Olthof PB, Aldrighetti L, Alikhanov R, Cescon M, Koerkamp BG, Jarnagin WR, Nadalin S, Pratschke J, Schmelzle M, Sparrelid E, Lang H, Iacono C, van Gulik TM, Guglielmi A; Perihilar Cholangiocarcinoma Collaboration Group. Surgery for Bismuth-Corlette Type 4 Perihilar Cholangiocarcinoma: Results from a Western Multicenter Collaborative Group. Ann Surg Oncol. 2021;28:7719-7729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (16)] |
| 4. | Wang W, Fei Y, Liu J, Yu T, Tang J, Wei F. Laparoscopic surgery and robotic surgery for hilar cholangiocarcinoma: an updated systematic review. ANZ J Surg. 2021;91:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (35)] |
| 5. | Cipriani F, Ratti F, Fiorentini G, Reineke R, Aldrighetti L. Systematic review of perioperative and oncologic outcomes of minimally-invasive surgery for hilar cholangiocarcinoma. Updates Surg. 2021;73:359-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (35)] |
| 6. | Xiong F, Peng F, Li X, Chen Y. Preliminary comparison of total laparoscopic and open radical resection for hepatic hilar cholangiocarcinoma a single-center cohort study. Asian J Surg. 2023;46:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (35)] |
| 7. | Hu XS, Wang Y, Pan HT, Zhu C, Chen SL, Liu HC, Pang Q, Jin H. "Five steps four quadrants" modularized en bloc dissection technique for accessing hepatic hilum lymph nodes in laparoscopic pancreaticoduodenectomy. World J Gastrointest Surg. 2024;16:503-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (36)] |
| 8. | Liu QQ, Shi XD, Ye YF, Tang QB, Lin HM, Yu XH, Zhang R, Liu C. Real-world experience of postoperative adjuvant chemoimmunotherapy in patients with perihilar cholangiocarcinoma at high risk of recurrence. Cancer Immunol Immunother. 2023;72:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (36)] |
| 9. | Guedj N. Pathology of Cholangiocarcinomas. Curr Oncol. 2022;30:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (35)] |
| 10. | Li J, Zhou MH, Ma WJ, Li FY, Deng YL. Extended lymphadenectomy in hilar cholangiocarcinoma: What it will bring? World J Gastroenterol. 2020;26:3318-3325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (36)] |
| 11. | Jena SS, Mehta NN, Nundy S. Surgical management of hilar cholangiocarcinoma: Controversies and recommendations. Ann Hepatobiliary Pancreat Surg. 2023;27:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Nagino M, Clavien PA. Demise of "Hilar En Bloc Resection by No-touch Technique" as Surgery for Perihilar Cholangiocarcinoma: Dissociation Between Theory and Practice. Ann Surg. 2021;274:e385-e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hu HJ, Jin YW, Shrestha A, Ma WJ, Wang JK, Liu F, Zhu YY, Zhou RX, Regmi P, Cheng NS, Li FY. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: A single institution experience in China. Cancer Med. 2019;8:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Yin Y, Tao J, Xian Y, Hu J, Li Y, Li Q, Xiong Y, He Y, He K, Li J. Survival analysis of laparoscopic surgery and open surgery for hilar cholangiocarcinoma: a retrospective cohort study. World J Surg Oncol. 2024;22:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Dou C, Wu W, Liu J, Jin L, Hu Z, Zhang C. Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc. 2020;34:4382-4387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Rebelo A, Ukkat J, Klose J, Ronellenfitsch U, Kleeff J. Surgery With Arterial Resection for Hilar Cholangiocarcinoma: Protocol for a Systematic Review and Meta-analysis. JMIR Res Protoc. 2021;10:e31212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Guadagni S, Comandatore A, Furbetta N, Di Franco G, Carpenito C, Bechini B, Vagelli F, Ramacciotti N, Palmeri M, Di Candio G, Morelli L. Robotic Hepatectomy plus Biliary Reconstruction for Bismuth Type III and Type IV Hilar Cholangiocarcinoma: State of the Art and Literature Review. J Pers Med. 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Feng F, Cao X, Liu X, Qin J, Zhang S, Li Q, Liu J. Laparoscopic resection for Bismuth type III and IV hilar cholangiocarcinoma: How to improve the radicality without direct palpation. J Surg Oncol. 2019;120:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Hu HJ, Wu ZR, Jin YW, Ma WJ, Yang Q, Wang JK, Liu F, Li FY. Minimally invasive surgery for hilar cholangiocarcinoma: state of art and future perspectives. ANZ J Surg. 2019;89:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Xu Y, Zhang Y. Current status of laparoscopic radical hilar cholangiocarcinoma in Mainland China. Biosci Trends. 2020;14:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kamachi H, Kamiyama T, Tsuruga Y, Orimo T, Wakayama K, Shimada S, Kakisaka T, Yokoo H, Yamashita K, Taketomi A. Transparenchymal glissonean approach: a novel surgical technique for advanced perihilar bile duct cancer. Langenbecks Arch Surg. 2018;403:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, Seehofer D. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Monden K, Sadamori H, Hioki M, Takakura N. Laparoscopic Left Hepatectomy with Resection of the Spiegel Lobe Using the Modified Caudate Lobe-First Approach. Ann Surg Oncol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |