Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2157
Revised: May 11, 2024
Accepted: June 14, 2024
Published online: July 27, 2024
Processing time: 103 Days and 0.1 Hours
Gastrointestinal symptoms are common in patients with uremia undergoing he
To assess the occurrence and factors influencing gastrointestinal symptoms in patients with uremia undergoing hemodialysis.
We retrospectively selected 98 patients with uremia who underwent regular hemo
Gastrointestinal symptoms included indigestion, constipation, reflux, diarrhea, abdominal pain, and eating disorders, and the total average GSRS score was 1.35 ± 0.47. This study showed that age, number of tablets, dialysis time, glucocorticoid, parathyroid hormone (PTH), combined diabetes mellitus and C-reactive protein (CRP) were independent risk factors for gastrointestinal symptoms in patients with uremia undergoing hemodialysis, whereas body mass index (BMI), hemoglobin (Hb), and urea clearance index were independent protective factors
Gastrointestinal symptoms are mostly mild in patients with uremia undergoing hemodialysis, most commonly including dyspepsia, eating disorders, and gastroesophageal reflux. The independent influencing factors mainly include the BMI, age, number of pills taken, dialysis time, urea clearance index, Hb, use of glucocorticoids, and thyroid hormone level. PTH, CRP, and diabetes are clinically related factors influencing the occurrence of gas
Core Tip: Gastrointestinal symptoms in hemodialysis patients with uremia seriously affects patients' quality of life. This study showed that the independent influencing factors related to the occurrence of gastrointestinal symptoms were mainly body mass index, age, number of tablets, dialysis time, urea clearance index, hemoglobin, use of glucocorticoid, parathyroid hormone, C-reactive protein, and diabetes mellitus. In clinical practice, variable factors can be controlled for prophylaxis.
- Citation: Yuan D, Wang XQ, Shao F, Zhou JJ, Li ZX. Study on the occurrence and influencing factors of gastrointestinal symptoms in hemodialysis patients with uremia. World J Gastrointest Surg 2024; 16(7): 2157-2166
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2157.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2157
The global incidence of chronic kidney disease (CKD) is as high as 13.4%, causing a significant economic burden[1]. With a delay in CKD management, the disease progresses and eventually develops into end-stage renal disease, also called uremia, requiring kidney transplantation or dialysis alternative therapies to maintain life[2]. The pathogenesis of uremia mainly involves the accumulation of urinary toxins such as urea, creatinine, parathyroid hormone (PTH), electrolytes, and body fluid, and hormonal imbalance caused by kidney injury resulting from many diseases in the body[3]. Uremia is a serious stage of kidney disease, with patients showing clinical manifestations of anorexia, vomiting, nausea, weight loss, fatigue, mental state changes, muscle spasms, and other symptoms[4], inducing serious harm to the physical and mental health of patients.
Hemodialysis is the main treatment for uremia in clinical practice[5,6]. Although it can delay the development of ure
Studies have shown that uremic toxin stimulation, anemia, drugs, depression, and anxiety can lead to gastrointestinal dysfunction[11]; however, the factors affecting the occurrence of gastrointestinal symptoms in hemodialysis patients and the correlation with other complications need further confirmation[8,12]. This study aimed to understand the occurrence and factors influencing gastrointestinal symptoms in hemodialysis patients with uremia and provide evidence for clinical prevention in advance.
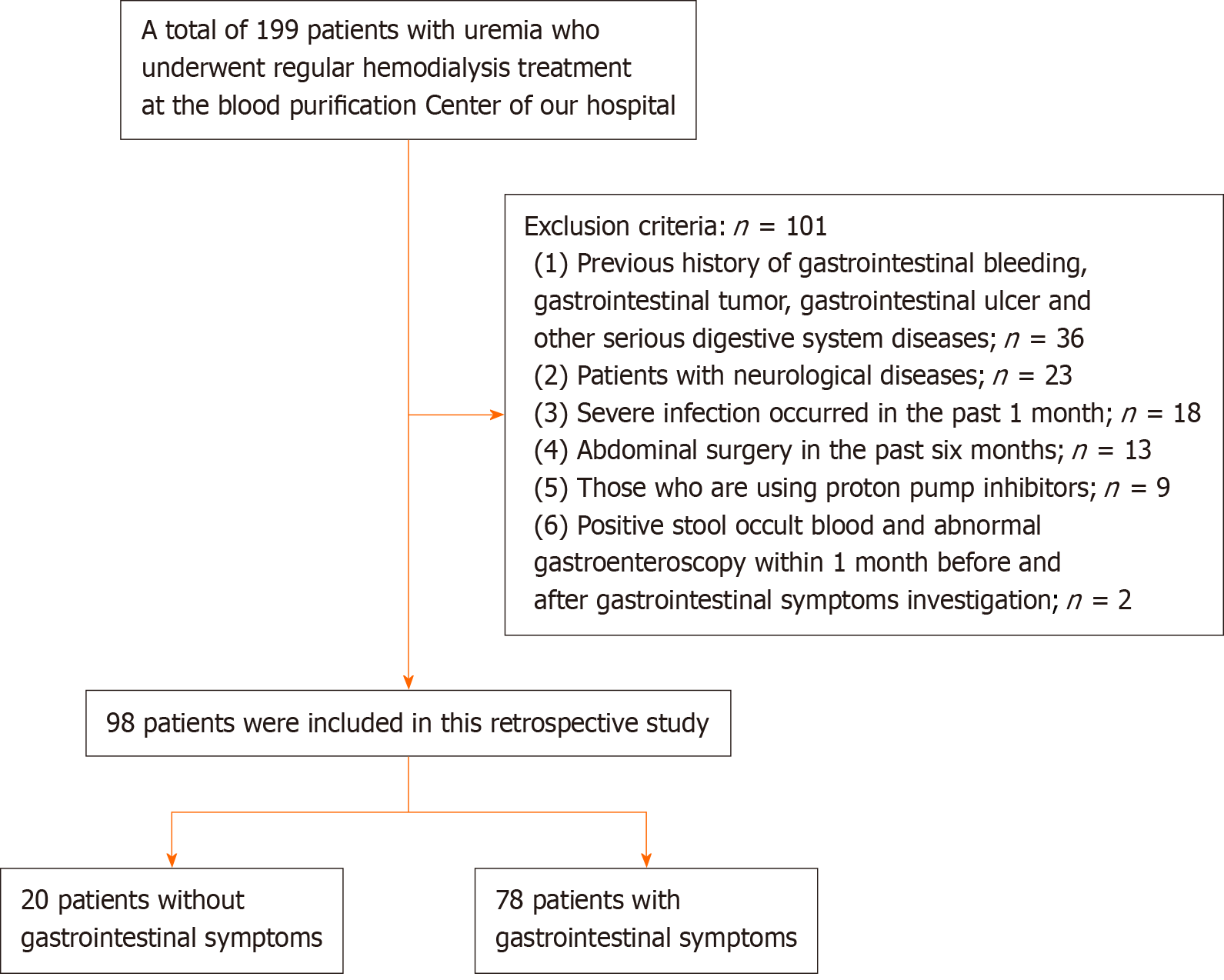
We included 98 patients with uremia who underwent regular hemodialysis treatment in the blood purification center of our hospital from December 2022 to December 2023. The Gastrointestinal Symptom Grading Scale (GSRS)[13] score was selected when evaluating the gastrointestinal symptoms of the patients, and the patients were divided into two groups [with (78 cases) and without (20 cases) gastrointestinal symptoms], according to whether they had gastrointestinal symptoms. The daily drug-taking and general data of patients were collected, and the total GSRS score and scores of various dimensions, general data, and disease-related conditions of patients were compared and analyzed. Univariate and multivariate analyses were performed.
Inclusion criteria[14] were as follows: (1) Age 18-80 years; (2) Clear consciousness, ability to answer questions correctly; and (3) Hemodialysis ≥ 3 months.
Exclusion criteria were as follows: (1) Previous history of gastrointestinal bleeding, gastrointestinal tumor, gastroin
Clinical data such as patients' basic condition and blood biochemical test laboratory indexes in the past 2 wk were collected and comprehensively analyzed.
(1) General data collection: Basic information included complicated diabetes mellitus, dialysis time, glucocorticoid use, number of tablets, body mass index (BMI), age, type of kidney injury, sex, dialysis treatment method, etc; (2) Laboratory examination[15]: Fasting venous blood of patients was collected before hemodialysis. Serum albumin (ALB), total cho
All statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, United States). Using a Pearson normality test, data was first checked for normality. Statistical analysis was carried out with two-tailed t-tests when data passed a normality test. When the data did not fit normal distribution, a non-parametric test was chosen instead. Mea
Figure 1 shows a flowchart illustrating the patient selection process. Among the 98 patients, 78 had gastrointestinal symptoms, with an incidence rate of 79.59% (78/98). Among the 78 patients with gastrointestinal symptoms, 36 were male and 42 were female, with an average age of 59.84 ± 9.56 years and average BMI of 18.84 ± 2.23 kg/m2. Twenty-seven patients received glucocorticoids and forty-six patients had diabetes mellitus, and the duration of hemodialysis was 28.68 ± 8.93 mo. Fifty-three patients were treated with simple hemodialysis, and seventeen patients underwent hemodialysis combined with peritoneal dialysis. Thirty-three patients had a renal injury, and thirty-seven patients had primary renal injury. The number of tablets taken per day was 6.36 ± 2.02. Among the 20 patients without gastrointestinal symptoms, 12 were male, and 8 were female, with an average age of 45.76 ± 6.52 years and a BMI of 22.16 ± 3.68 kg/m2. Four patients were treated with glucocorticoids, and nine patients had diabetes. The duration of hemodialysis was 19.67 ± 6.64 months. Twelve patients were treated with simple hemodialysis, and eight patients underwent hemodialysis combined with peritoneal dialysis. Nine patients had a renal injury, and eleven had primary renal injury. The number of tablets taken per day was 4.03 ± 1.85.
The results showed significant differences in age, BMI, glucocorticoid use, diabetes mellitus, hemodialysis time, and the number of tablets taken per day between the two groups (Table 1).
| Variables | Having gastrointestinal symptoms group, n = 78 | No gastrointestinal symptoms group, n = 20 | t/χ2 | P value |
| Sex | 2.602 | 0.199 | ||
| Male | 36 | 12 | ||
| Female | 42 | 8 | ||
| Age in year | 59.84 ± 9.56 | 45.76 ± 6.52 | 5.654 | < 0.001 |
| BMI in kg/m2 | 18.84 ± 2.23 | 22.16 ± 3.68 | 6.943 | < 0.001 |
| Using glucocorticoids | ||||
| Yes | 27 | 4 | 4.695 | 0.013 |
| No | 43 | 16 | ||
| Combined diabetes | 4.579 | 0.017 | ||
| Yes | 46 | 9 | ||
| No | 24 | 11 | ||
| Dialysis time in month | 28.68 ± 8.93 | 21.67 ± 6.64 | 2.453 | 0.036 |
| Dialysis treatment method | 1.624 | 0.203 | ||
| Alone | 53 | 12 | ||
| Union | 17 | 8 | ||
| Type of renal injury | 1.203 | 0.138 | ||
| Secondary renal injury | 33 | 9 | ||
| Primary disease | 37 | 11 | ||
| Number of tablets taken as tablets/day | 6.36 ± 2.02 | 4.03 ± 1.85 | 7.984 | < 0.001 |
The laboratory examination indexes of the two groups were PTH, Hb, urea clearance index, CRP, ALB, TC, TG, BUN, and SCr. As shown in Table 2, PTH, Hb, urea clearance index, and CRP were significantly different between the two groups (P < 0.05).
| Variable | Having gastrointestinal symptoms group, n = 78 | No gastrointestinal symptoms group, n = 20 | t value | P value |
| PTH in pg/mL | 168.76 ± 19.15 | 103.57 ± 9.83 | 15.978 | < 0.001 |
| Hb in g/L | 87.03 ± 10.95 | 118.26 ± 16.79 | 9.606 | < 0.001 |
| Urea clearance index in kt/v | 0.69 ± 0.23 | 0.93 ± 0.34 | 3.29 | 0.012 |
| CRP in mg/L | 24.23 ± 3.02 | 13.46 ± 2.89 | 15.674 | < 0.001 |
| ALB in g/L | 35.96 ± 4.17 | 37.93 ± 3.62 | 0.521 | 0.893 |
| TC in mmol/L | 5.57 ± 1.06 | 6.08 ± 1.59 | 1.478 | 0.117 |
| TG in mmol/L | 1.21 ± 0.53 | 1.52 ± 0.67 | 1.323 | 0.193 |
| BUN in mmol/L | 7.49 ± 0.62 | 7.38 ± 0.55 | 0.723 | 0.472 |
| SCr in μg/L | 95.45 ± 6.78 | 96.87 ± 6.03 | 0.664 | 0.509 |
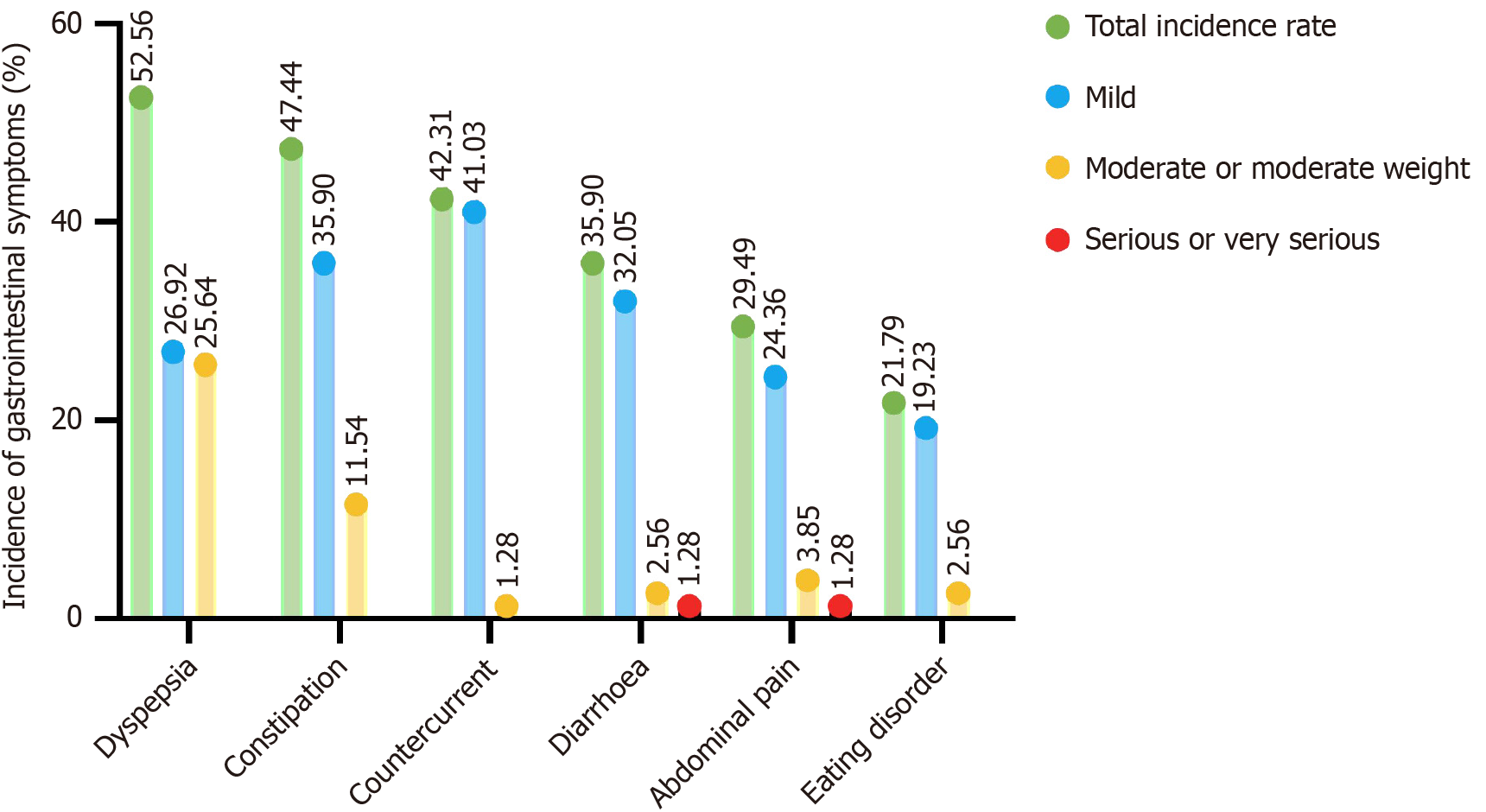
Among the 78 patients with gastrointestinal symptoms, 41, 37, 33, 28, 23, and 17 patients had dyspepsia, constipation, reflux, diarrhea, abdominal pain, and eating disorders, respectively. The incidence rates from high to low were indigestion (52.56%), constipation (47.44%), reflux (42.31%), diarrhea (35.90%), abdominal pain (29.49%), and eating disorders (21.79%; Table 3 and Figure 2).
| Variable | n | Incidence, % |
| Dyspepsia | 41 | 52.56 |
| Mild | 21 | 26.92 |
| Moderate or moderate weight | 20 | 25.64 |
| Constipation | 37 | 47.44 |
| Mild | 28 | 35.90 |
| Moderate or moderate weight | 9 | 11.54 |
| Countercurrent | 33 | 42.31 |
| Mild | 32 | 41.03 |
| Moderate or moderate weight | 1 | 1.28 |
| Diarrhea | 28 | 35.90 |
| Mild | 25 | 32.05 |
| Moderate or moderate weight | 2 | 2.56 |
| Serious or very serious | 1 | 1.28 |
| Abdominal pain | 23 | 29.49 |
| Mild | 19 | 24.36 |
| Moderate or moderate weight | 3 | 3.85 |
| Serious or very serious | 1 | 1.28 |
| Eating disorder | 17 | 21.79 |
| Mild | 15 | 19.23 |
| Moderate or moderate weight | 2 | 2.56 |
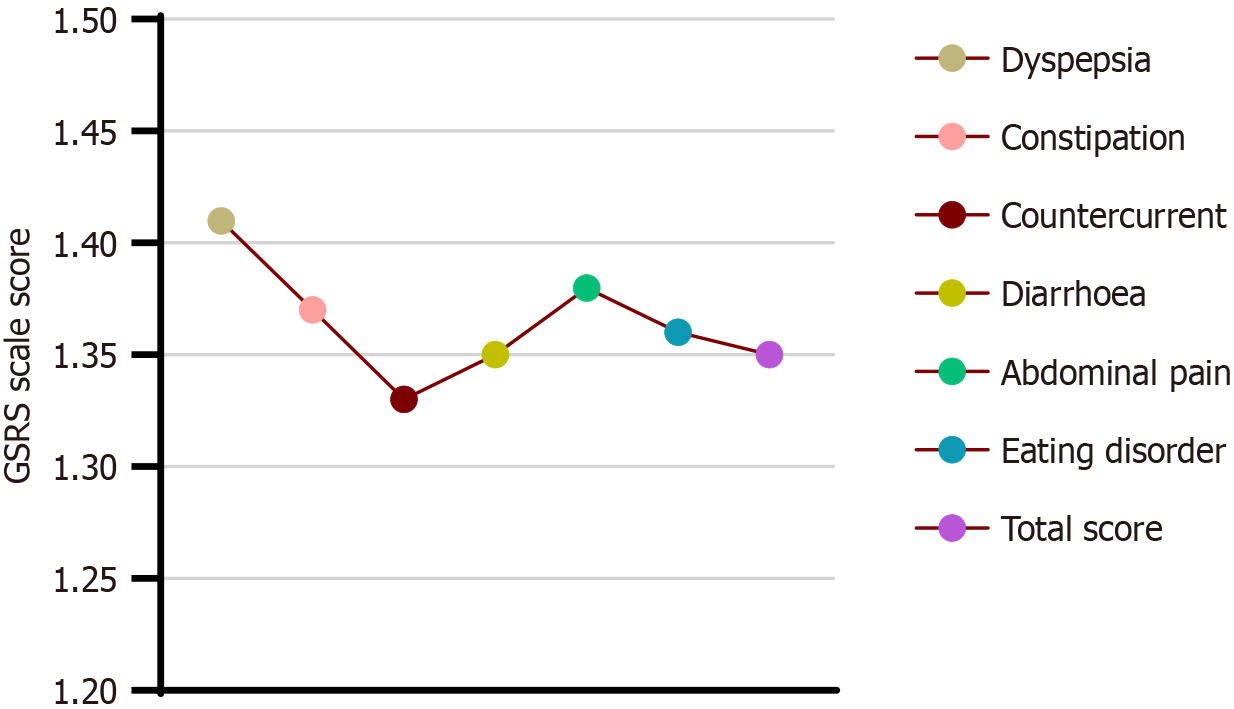
The total average GSRS scale score was 1.35 ± 0.47 points, including 1.41 ± 0.65, 1.37 ± 0.46, 1.33 ± 0.42, 1.35 ± 0.44, 1.38 ± 0.69, and 1.36 ± 0.60 points for digestive disorders, constipation, reflux, diarrhea, abdominal pain, and eating disorders, respectively (Table 4 and Figure 3).
| Variable | Average score of entries, points |
| Dyspepsia | 1.41 ± 0.65 |
| Constipation | 1.37 ± 0.46 |
| Countercurrent | 1.33 ± 0.42 |
| Diarrhea | 1.35 ± 0.44 |
| Abdominal pain | 1.38 ± 0.69 |
| Eating disorder | 1.36 ± 0.60 |
| Total score | 1.35 ± 0.47 |
According to the results in Tables 1 and 2, 10 indexes, including age, BMI, glucocorticoid use, diabetes mellitus, he
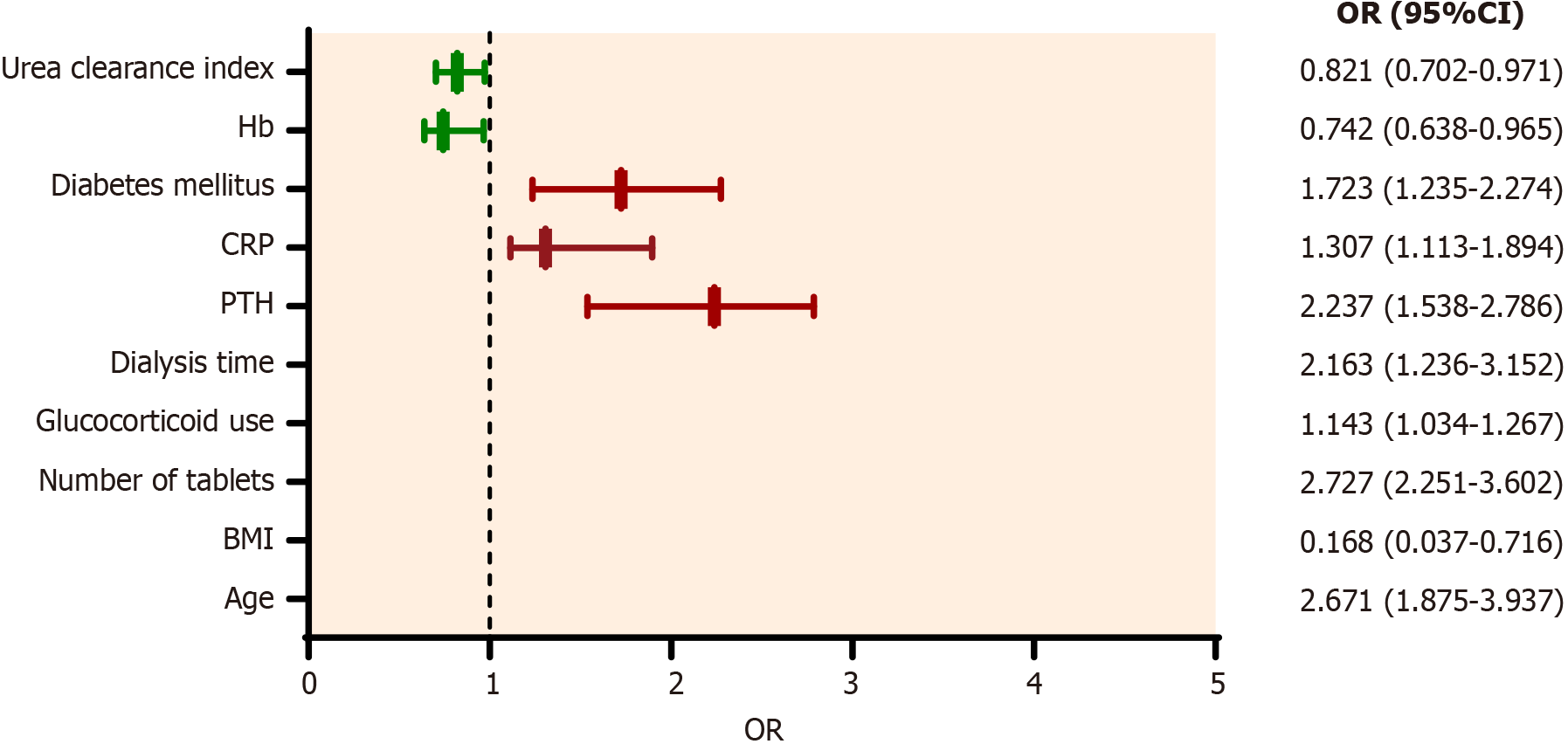
To further determine the independent factors influencing gastrointestinal symptoms in patients with uremia undergoing hemodialysis, 10 factors obtained by univariate analysis were taken as independent variables, and multivariate logistic regression analysis was performed. Our results showed that age, number of tablets, glucocorticoid use, dialysis time, PTH, CRP, and diabetes mellitus were independent risk factors for gastrointestinal symptoms in patients with uremia undergoing hemodialysis. At the same time, BMI, Hb, and urea clearance index were independent protective factors (Table 5 and Figure 4).
| Variable | P value | OR | 95%CI |
| Age | < 0.001 | 2.671 | 1.875-3.937 |
| BMI | 0.018 | 0.168 | 0.037-0.716 |
| Number of tablets | 0.016 | 2.727 | 2.251-3.602 |
| Glucocorticoid | 0.032 | 1.143 | 1.034-1.267 |
| Dialysis time | < 0.001 | 2.163 | 1.236-3.152 |
| PTH | 0.036 | 2.237 | 1.538-2.786 |
| CRP | 0.041 | 1.307 | 1.113-1.894 |
| Diabetes mellitus | 0.002 | 1.723 | 1.235-2.274 |
| Hb | 0.023 | 0.742 | 0.638-0.965 |
| Urea clearance index | 0.039 | 0.821 | 0.702-0.971 |
Uremia is also known as end-stage renal failure[18]. The clinical pathogenesis of uremia involves the retention of toxic metabolites in the body, resulting in blood poisoning and renal function deterioration syndrome, with digestive system symptoms such as nausea, vomiting, abdominal distension, and diarrhea[19]. Digestive system symptoms are often the earliest manifestation in patients with uremia. In this study, the incidence of gastrointestinal symptoms in patients with uremia undergoing hemodialysis was 79.59%, with dyspepsia (52.56%) accounting for the highest incidence, followed by constipation and reflux and relatively few symptoms of abdominal pain and diarrhea. Despite the high incidence of gastrointestinal symptoms in this study, the GSRS score was generally low, and most patients had mild symptoms, consistent with the results of Drüeke and Ikizler[6]. The reason for the high incidence of gastrointestinal symptoms may be that in patients with uremia undergoing hemodialysis, due to the decrease of prostaglandin synthesis that protects the gastric mucosa, gastric mucosal resistance decreases with the increase of urea nitrogen. Anemia or metabolic disorders puts the gastric mucosa in a state of chronic ischemia and hypoxia for a long time, which leads to the occurrence of gas
Several studies have reported age as a risk factor for gastrointestinal symptoms, and the occurrence of gastroesophageal reflux disease can be predicted when the age is over 60 years in the general population[20,21]. This study showed that in patients with uremia undergoing hemodialysis, age is an independent risk factor for gastrointestinal symptoms; the older the patient, the higher the risk of gastrointestinal symptoms (odds ratio [OR]: 2.671; 95% confidence interval [CI]: 1.875-3.937). In contrast, the lower the BMI, the higher the possibility of gastrointestinal symptoms (OR: 0.168; 95%CI: 0.037-0.716); high BMI is an independent protective factor for gastrointestinal symptoms. This may be because the older the patient, the less he eats, which leads to insufficient protein intake and increased occurrence of gastrointestinal symptoms.
In patients with uremia undergoing hemodialysis, the more drugs they take, the more serious gastrointestinal sym
In addition, this study showed that corticosteroids, diabetes mellitus, hemodialysis time, PTH, and CRP were independent risk factors for patients with uremia undergoing hemodialysis, which was consistent with the results of previous retrospective studies[23-25]. These observations may be due to the many adverse reactions of glucocorticoids, in which the digestive system stimulates the gastric mucosa and promotes the secretion of gastric acid and pepsin, thus reducing the resistance of the gastric mucosa. Glucocorticoids aggravate or even induce the occurrence of bleeding and perforation caused by peptic ulcer. Moreover, in patients with diabetes, hyperglycemia leads to the weakening of gastric motility and gastric secretion disorders, resulting in corresponding gastrointestinal symptoms. The longer the hemo
The independent influencing factors identified in this paper are of great significance for healthcare professionals in the symptom management and screening program of uremic hemodialysis patients. For example, patients with higher BMI may need more active diet management and exercise programs to optimize weight control. Elderly patients may need to monitor age-related complications more frequently and slowly adjust dialysis parameters to avoid intolerance. The number of patients taking drugs may increase side effects, and healthcare professionals should provide guidance on the importance of insisting on taking drugs and the risk of missing doses. Dialysis time and urea clearance index are the key to maintain electrolyte balance and urination. Healthcare professionals should closely monitor these parameters to ensure effective dialysis and avoid any episodes of hyperkalemia or hypotension. It may be necessary to adjust the dialysis time or dialyzer type according to the patient's individual response. Hb level is a key index of anemia management in hemo
This study has some limitations. First, this is a retrospective cohort study that may be susceptible to selection bias. Second, this is a relatively small sample size, single-center clinical study. In addition, this study only collected the incidence of gastrointestinal symptoms in patients with uremia undergoing hemodialysis over the past 2 wk, and the long-term impact remains unclear. Long-term, multicenter clinical studies are needed to confirm these findings in the future.
This study showed that age, number of tablets, dialysis time, glucocorticoid, PTH, combined diabetes mellitus and CRP were independent risk factors for gastrointestinal symptoms in patients with uremia undergoing hemodialysis, while BMI, Hb, and urea clearance index were independent protective factors. Therefore, the implementation of personalized preventive measures in the early stage is critical to reducing the occurrence of gastrointestinal symptoms in patients with uremia undergoing hemodialysis.
| 1. | Bello AK, Ronksley PE, Tangri N, Kurzawa J, Osman MA, Singer A, Grill A, Nitsch D, Queenan JA, Wick J, Lindeman C, Soos B, Tuot DS, Shojai S, Brimble S, Mangin D, Drummond N. Prevalence and Demographics of CKD in Canadian Primary Care Practices: A Cross-sectional Study. Kidney Int Rep. 2019;4:561-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Yi C, Wang X, Ye H, Lin J, Yang X. Patient-reported gastrointestinal symptoms in patients with peritoneal dialysis: the prevalence, influence factors and association with quality of life. BMC Nephrol. 2022;23:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Sharma S, Raman P, Deo AS. Comparative preoperative sonological assessment of gastric contents in patients with chronic kidney disease versus those with normal renal function - A prospective observational study. Indian J Anaesth. 2023;67:503-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Roza K, George JC, Bermudez M, Mehta Z. Uremic Calciphylaxis #325. J Palliat Med. 2017;20:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Grooteman M, Nubé M. Reappraisal of Hemodiafiltration for Managing Uremic Complications. Clin J Am Soc Nephrol. 2021;16:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Drüeke TB, Ikizler TA. Is hemodiafiltration superior to hemodialysis in patients with kidney failure? Kidney Int. 2023;104:874-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Wang C, Chen C, Wang J, Guo X, Deng YC, Liu L, Zhao C. Delayed gastric emptying in nondiabetic patients with end-stage kidney disease. Ren Fail. 2022;44:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Mortazavi M, Adibi P, Hassanzadeh Keshteli A, Feizi A, JameShorani M, Soodavi M, Jafari M. Comparison of Gastrointestinal Symptoms between Patients Undergoing Hemodialysis and Healthy Population. Middle East J Dig Dis. 2022;14:310-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Khalid MA, Iqbal J, Memon HL, Hanif FM, Butt MOT, Luck NH, Majid Z. Dyspepsia Amongst End Stage Renal Disease Undergoing Hemodialysis: Views from a Large Tertiary Care Center. J Transl Int Med. 2018;6:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Gupta R, Pokhriyal AS, Jindal P, Sarpal R, Goyal M. Evaluation of Gastric Emptying by Ultrasonography after Recommended Fasting Period and Administration of Prokinetic in End-stage Renal Disease Patients. Anesth Essays Res. 2020;14:42-48. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Vanholder R, Argilés A, Jankowski J; European Uraemic Toxin Work Group (EUTox). A history of uraemic toxicity and of the European Uraemic Toxin Work Group (EUTox). Clin Kidney J. 2021;14:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Cameron JS. The prehistory of haemodialysis as a treatment for uraemia. G Ital Nefrol. 2016;33 Suppl 66:33.S66.2. [PubMed] |
| 13. | Souza GS, Sardá FA, Giuntini EB, Gumbrevicius I, Morais MB, Menezes EW. TRANSLATION AND VALIDATION OF THE BRAZILIAN PORTUGUESE VERSION OF THE GASTROINTESTINAL SYMPTOM RATING SCALE (GSRS) QUESTIONNAIRE. Arq Gastroenterol. 2016;53:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Xu T, Wang D, Cong G. Efficacy of hemodialysis combined with hemofiltration in the treatment of uremia complicated with intractable hypertension. Am J Transl Res. 2023;15:3521-3529. [PubMed] |
| 15. | Shaki Z, Ghaffari F, Alijaniha F, Kamalinejad M, Kazemnejad A, Daneshfard B, Naseri M, Heidari MR. Effect of Dill (Anethum graveolens) Oil on Pruritus and Quality of Life of Hemodialysis Patients: A Randomized Double-Blind Three-Arm Controlled Trial. Evid Based Complement Alternat Med. 2024;2024:3077603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Yu J, Liu S, Fang XC, Zhang J, Gao J, Xiao YL, Zhu LM, Chen FR, Li ZS, Hu PJ, Ke MY, Hou XH. Gastrointestinal symptoms and associated factors in Chinese patients with functional dyspepsia. World J Gastroenterol. 2013;19:5357-5364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Sun Y, Zhang Z, Zheng CQ, Sang LX. Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review. World J Gastroenterol. 2021;27:2963-2978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Changa AR, Rucker JC, Drummond PS. Opsoclonus in Uremia With Resolution After Hemodialysis. J Neuroophthalmol. 2022;42:e448-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Misra M. Home Hemodialysis. Adv Chronic Kidney Dis. 2021;28:123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Blankestijn PJ, Vernooij RWM, Hockham C, Strippoli GFM, Canaud B, Hegbrant J, Barth C, Covic A, Cromm K, Cucui A, Davenport A, Rose M, Török M, Woodward M, Bots ML; CONVINCE Scientific Committee Investigators. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N Engl J Med. 2023;389:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 122] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 21. | Guedes M, Vernooij RWM, Davenport A, Kuhlmann MK, Aregger F, Pecoits-Filho R. Clinical performance, intermediate and long-term outcomes of high-volume hemodiafiltration in patients with kidney failure. Semin Dial. 2022;35:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Caskey FJ, Procter S, MacNeill SJ, Wade J, Taylor J, Rooshenas L, Liu Y, Annaw A, Alloway K, Davenport A, Power A, Farrington K, Mitra S, Wheeler DC, Law K, Lewis-White H, Ben-Shlomo Y, Hollingworth W, Donovan J, Lane JA. The high-volume haemodiafiltration vs high-flux haemodialysis registry trial (H4RT): a multi-centre, unblinded, randomised, parallel-group, superiority study to compare the effectiveness and cost-effectiveness of high-volume haemodiafiltration and high-flux haemodialysis in people with kidney failure on maintenance dialysis using linkage to routine healthcare databases for outcomes. Trials. 2022;23:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Ellis P. An overview of haemodialysis. Br J Nurs. 2023;32:356-360. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Brunet P. [Treatment of chronic kidney failure by haemodialysis]. Soins. 2018;63:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Hegbrant J, Bernat A, Del Castillo D, Pizarro JL, Caparros S, Gaspar M, Jarava C, Strippoli GFM, Daugirdas JT. Residual Renal Phosphate Clearance in Patients Receiving Hemodialysis or Hemodiafiltration. J Ren Nutr. 2023;33:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |