Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2135
Revised: May 11, 2024
Accepted: June 4, 2024
Published online: July 27, 2024
Processing time: 109 Days and 21.3 Hours
Colorectal cancer (CRC) is a prevalent cancer type in clinical settings; its early signs can be difficult to detect, which often results in late-stage diagnoses in many patients. The early detection and diagnosis of CRC are crucial for improving treatment success and patient survival rates. Recently, imaging techniques have been hypothesized to be essential in managing CRC, with magnetic resonance imaging (MRI) and spiral computed tomography (SCT) playing a significant role in enhancing diagnostic and treatment approaches.
To explore the effectiveness of MRI and SCT in the preoperative staging of CRC and the prognosis of laparoscopic treatment.
Ninety-five individuals admitted to Zhongshan Hospital Xiamen University underwent MRI and SCT and were diagnosed with CRC. The precision of MRI and SCT for the presurgical classification of CRC was assessed, and pathological staging was used as a reference. Receiver operating characteristic curves were used to evaluate the diagnostic efficacy of blood volume, blood flow, time to peak, permeability surface, blood reflux constant, volume transfer constant, and extracellular extravascular space volume fraction on the prognosis of patients with CRC.
Pathological biopsies confirmed the following CRC stages: 23, 23, 32, and 17 at T1, T2, T3, and T4, respectively. There were 39 cases at the N0 stage, 22 at N1, 34 at N2, 44 at M0 stage, and 51 at M1. Using pathological findings as the benchmark, the combined use of MRI and SCT for preoperative TNM staging in patients with CRC demonstrated superior sensitivity, specificity, and accuracy compared with either modality alone, with a statistically significant difference in accuracy (P < 0.05). Receiver operating characteristic curve analysis revealed the predictive values for laparoscopic treatment prognosis, as indicated by the areas under the curve for blood volume, blood flow, time to peak, and permeability surface, blood reflux constant, volume transfer constant, and extracellular extravascular space volume fraction were 0.750, 0.683, 0.772, 0.761, 0.709, 0.719, and 0.910, respectively. The corresponding sensitivity and specificity values were also obtained (P < 0.05).
MRI with SCT is effective in the clinical diagnosis of patients with CRC and is worthy of clinical promotion.
Core Tip: Colorectal cancer (CRC) is frequently encountered as a malignant neoplasm in clinical settings. The initial signs are typically subtle, leading to the majority of cases being identified during the intermediate to advanced phases. Early detection and accurate diagnosis of CRC are important for improving treatment efficacy and patient prognosis. We examined a cohort of 95 individuals diagnosed with CRC and assessed the practical effectiveness of magnetic resonance imaging with spiral computed tomography in evaluating the preoperative staging and prognosis of laparoscopic treatment for CRC. The combination was found to be effective and of clinical significance.
- Citation: Bai LN, Zhang LX. Effectiveness of magnetic resonance imaging and spiral computed tomography in the staging and treatment prognosis of colorectal cancer. World J Gastrointest Surg 2024; 16(7): 2135-2144
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2135.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2135
Colorectal cancer (CRC) is one of the most common malignant neoplasms in clinical practice. Approximately 90% of colorectal lesions develop from normal epithelium to hyperplastic epithelium, and adenoma occurs when the disease develops until it becomes cancerous. During its initial phase, CRC frequently occurs unnoticed because most individuals do not display clear signs. However, the symptoms that emerge can differ significantly depending on whether the cancer is located in the colon or rectum[1,2].
Prompt identification and diagnosis are crucial for designing effective clinical interventions to improve a patient’s condition and prognosis. Surgical intervention is a proven approach for managing CRC, and accurate preoperative staging is an effective basis for guiding the formulation of surgical plans[3]. Recently, imaging technology has rapidly developed and significantly influenced the differential diagnosis of CRC.
Magnetic resonance imaging (MRI) is distinguished by its high-resolution capabilities and elevated rate of lesion identification. MRI can accurately show the relationship between the tumor and colorectal wall as well as the speed and process of contrast agent clearance from the lesion, intuitively reflecting the microcirculation of the lesion. It has a significant use in the presurgical staging and prognostic assessment of CRC[4].
Spiral computed tomography (SCT) can clearly show the secondary signs of soft tissue mass and distal metastasis during CRC examination, effectively predicting the metastasis or local invasion of cancer cells. It measures the density and time change of the contrast agent in the target detection tissue to reflect the tissue perfusion state[5,6]. However, patients undergoing SCT are exposed to a large dose of X-ray ionizing radiation during the examination and the resolution of soft tissue images is not as good as that when using MRI. Therefore, a combination of the two methods can compensate for these deficiencies and improve the accuracy of diagnosis.
Therefore, this study aimed to explore the effectiveness of combining MRI and SCT in evaluating preoperative primary tumor (T stage), lymph node metastasis (N stage), and distant metastasis (M stage) (TNM) staging and the prognosis of laparoscopic CRC treatment.
We retrospectively reviewed the medical records of 95 patients who underwent surgery for CRC at Zhongshan Hospital Xiamen University between March 2020 and August 2023. This group comprised 61 males and 34 females aged 43-80 years, with a mean age of 66.65 years and a standard deviation of 9.13 years.
The inclusion criteria included the following: (1) Each patient received a CRC diagnosis through biopsy; (2) All participants underwent both MRI and SCT; and (3) Patients had complete follow-up data. The exclusion criteria were as follows: (1) Individuals lacking comprehensive medical records; (2) Patients who received preoperative chemoradiotherapy; (3) Individuals with critical heart and brain conditions or other systemic illnesses; and (4) Patients with severe immune system diseases or systemic or local infections.
The study gathered information on pathology reports, MRI, SCT, and patient follow-ups from the hospital’s digital medical record database. A two-person dual-core working mode was adopted to avoid errors in the process of data extraction.
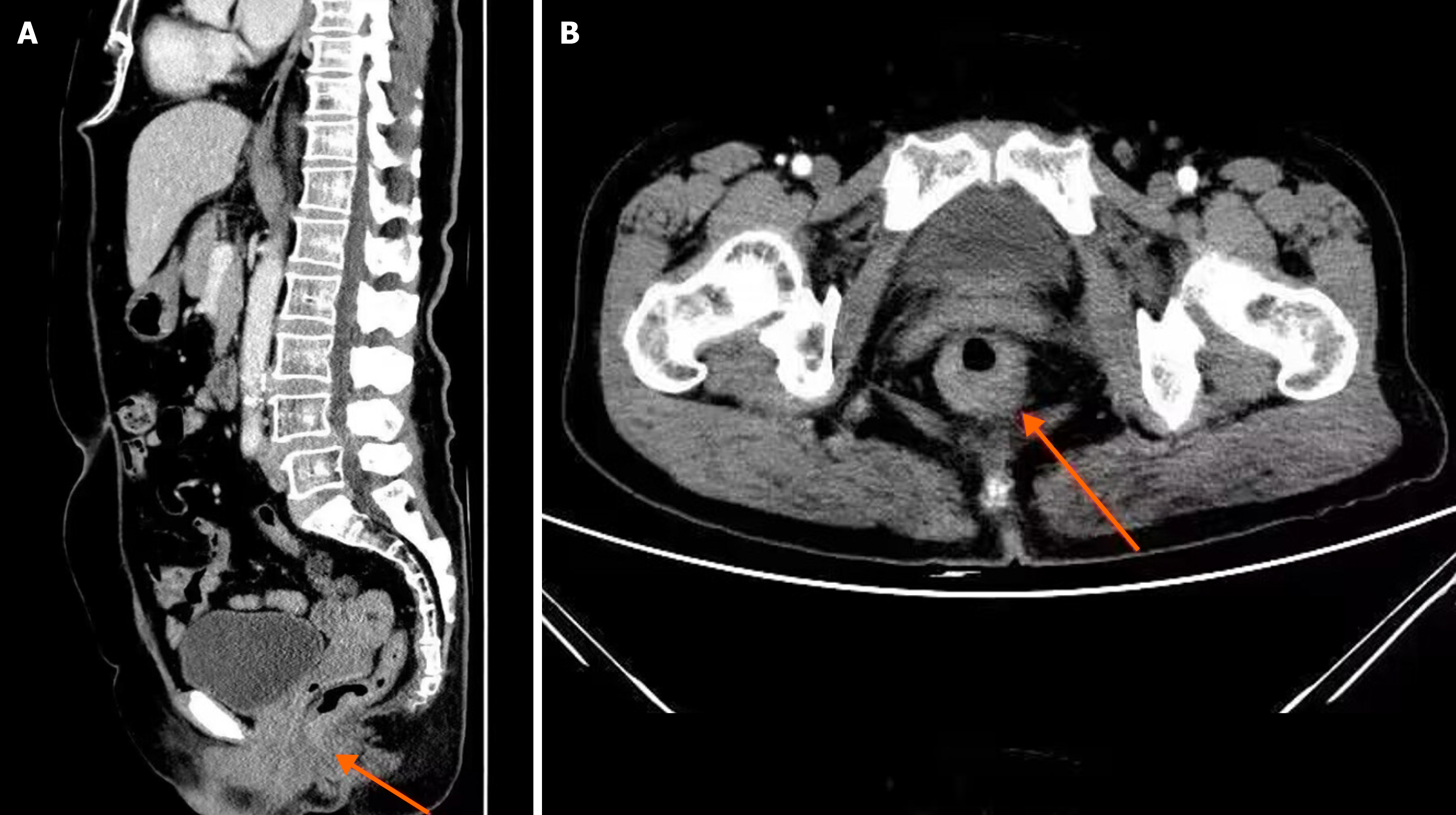
Two radiologists, each with more than a decade of professional experience, were chosen from our hospital to interpret the images using a double-blind procedure. Colorectal tumors surrounding the tissue structures and lymph nodes were carefully observed and analyzed. Where there were differences in opinion, the two radiologists discussed to obtain a unified result. A sample image from the digital medical record database is shown in Figure 1. Based on the diagnosing imaging results, the primary tumor (T stage), lymph node metastasis (N stage), and distant metastasis (M stage) of the CRC were determined, and the SCT perfusion and MRI quantitative parameters were measured using the software in the workstation. The SCT perfusion parameters included the blood volume (BV), blood flow (BF), time to peak (TTP), and permeability surface (PS). The quantitative MRI parameters included the blood reflux constant (Kep), volume transfer constant (Ktrans), and extracellular extravascular space volume fraction (Ve).
(1) Assessment was conducted to determine the performance of SCT and MRI in diagnosing the TNM stages by calculating their respective diagnostic sensitivity, specificity, and accuracy. Sensitivity = true positive / (true positive + false negative) × 100 %. Specificity = true negative / (true negative + false positive) × 100 %. Accuracy = (true positive + true negative) / total number of cases × 100 %; and (2) To determine the significance of perfusion and quantitative parameters for the prognosis of patients with CRC.
SPSS 23.0 was chosen as the statistical tool for the analysis. Frequency data were presented as case counts (n) and percentages (%). The χ2 test was used for categorical comparisons. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value of both perfusion and quantitative metrics in patients with CRC. Statistical significance was set at P < 0.05.
Pathological biopsies confirmed 23, 23, 32, and 17 cases of T1, T2, T3, and T4 CRC, respectively. Regarding nodal involvement, 39, 22, and 34 patients had N0, N1, and N2 CRC stages, respectively. Regarding metastases, 44 and 51 cases were classified as M0 and M1, respectively.
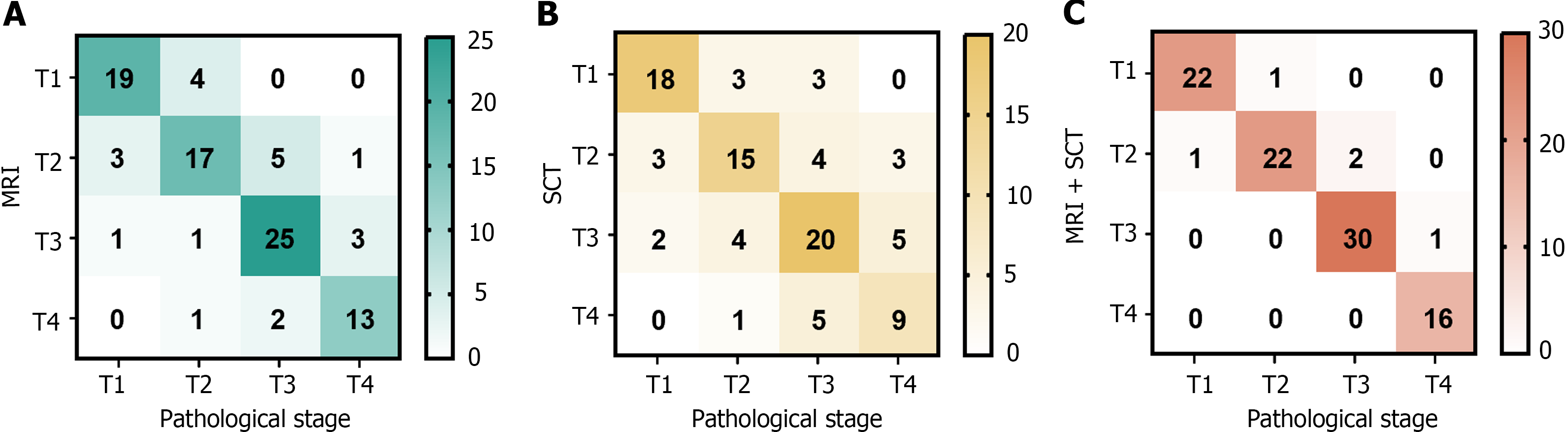
A total of 95 patients were examined using MRI, including 23, 26, 30, and 16 cases of T1, T2, T3, and T4, respectively. Via SCT, a total of 95 patients were diagnosed with T1 (24 cases), T2 (25 cases), T3 (31 cases), or T4 (15 cases). Via MRI and SCT together, 23, 25, 31, and 16 patients were diagnosed with T1, T2, T3, and T4, respectively (Figure 2).
Using pathological findings as the benchmark, the combination of MRI and SCT demonstrated superior sensitivity, specificity, and accuracy for preoperative T staging in CRC compared to only SCT or MRI. This enhancement in accuracy was statistically significant (P < 0.05) (Table 1).
| Index | Pathological stage | |||
| T1 | T2 | T3 | T4 | |
| MRI | ||||
| Sensitivity | 82.61 (19/23) | 73.91 (17/23) | 78.13 (25/32) | 76.47 (13/17) |
| Specificity | 94.44 (68/72) | 87.50 (63/72) | 92.06 (58/63) | 96.15 (75/78) |
| Accuracy | 91.58 (87/95)a | 84.21 (80/95) a | 87.37 (83/95)a | 92.63 (88/95)a |
| SCT | ||||
| Sensitivity | 78.26 (18/23) | 65.22(15/23) a | 62.50 (20/32)a | 52.94 (9/17)a |
| Specificity | 91.67 (66/72)a | 86.11 (62/72)a | 82.54 (52/63)a | 92.31 (72/78)a |
| Accuracy | 88.42 (84/95)a | 81.05 (77/95)a | 75.79 (72/95)a | 85.26 (81/95)a |
| MRI + SCT | ||||
| Sensitivity | 95.65 (22/23) | 95.65 (22/23) | 93.75 (30/32) | 94.12 (16/17) |
| Specificity | 98.61(71/72) | 95.83 (69/72) | 98.41 (62/63) | 100.00 (78/78) |
| Accuracy | 97.89 (93/95) | 95.79 (91/95) | 96.84 (92/95) | 98.95 (94/95) |
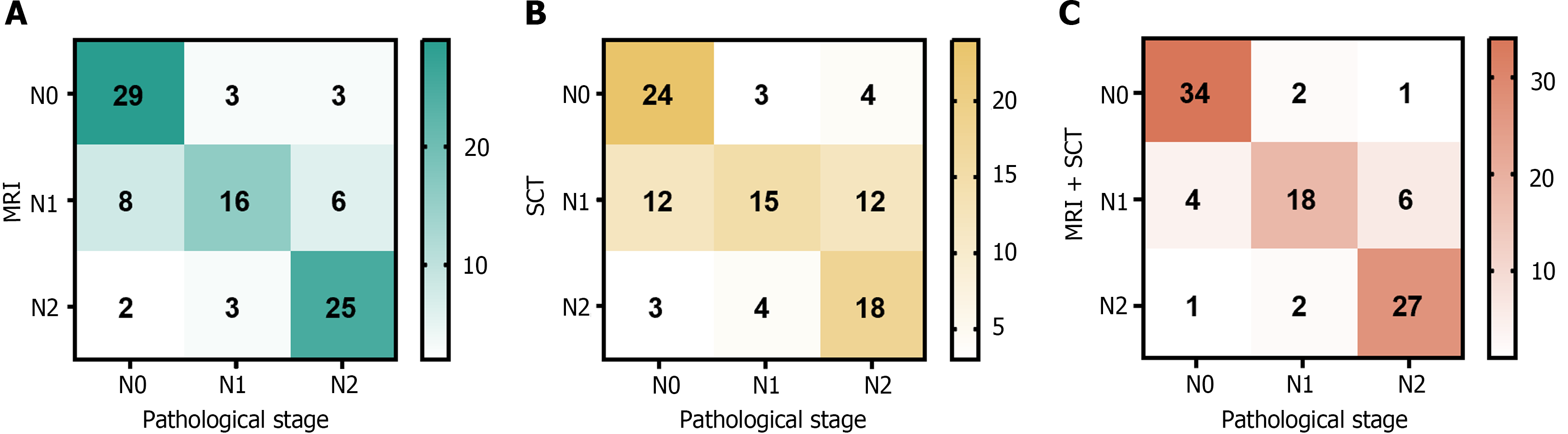
A total of 95 patients were examined using MRI, and 35, 30, and 30 cases of N0, N1, and N2, respectively, were diagnosed. According to the SCT, 31, 39, and 25 cases of the N0, N1, and N2 stages, respectively, were diagnosed in the 95 patients. Via combined MRI and SCT, 37, 28, and 30 cases were diagnosed as N0, N1, and N2 stages, respectively (Figure 3).
Using pathological outcomes as the reference standard, the combined application of MRI and SCT for preoperative N staging in patients with CRC yielded increased sensitivity, specificity, and accuracy over the use of SCT or MRI alone for diagnosis. A significant difference was observed between the accuracy of combined MRI and SCT and that of a single diagnosis of SCT (P < 0.05). The details of this analysis are shown in Table 2.
| Index | Pathological stage | ||
| N0 | N1 | N2 | |
| MRI | |||
| Sensitivity | 74.36 (29/39) | 72.73 (16/22) | 73.53 (25/34) |
| Specificity | 89.29 (50/56) | 80.82 (59/73) | 91.80 (56/61) |
| Accuracy | 83.16 (79/95) | 77.95 (75/95) | 85.26 (81/95) |
| SCT | |||
| Sensitivity | 61.54 (24/39)a | 68.18 (15/22) | 52.94 (18/34)a |
| Specificity | 87.50 (49/56) | 67.12 (49/73)a | 88.52 (54/61) |
| Accuracy | 76.84 (73/95) | 67.37 (64/95)a | 75.79 (72/95)a |
| MRI + SCT | |||
| Sensitivity | 87.18 (34/39) | 81.81 (18/22) | 79.41 (27/34) |
| Specificity | 94.64 (53/56) | 86.30 (63/73) | 95.08 (58/61) |
| Accuracy | 91.58 (87/95) | 85.26 (81/95) | 89.69 (85/95) |
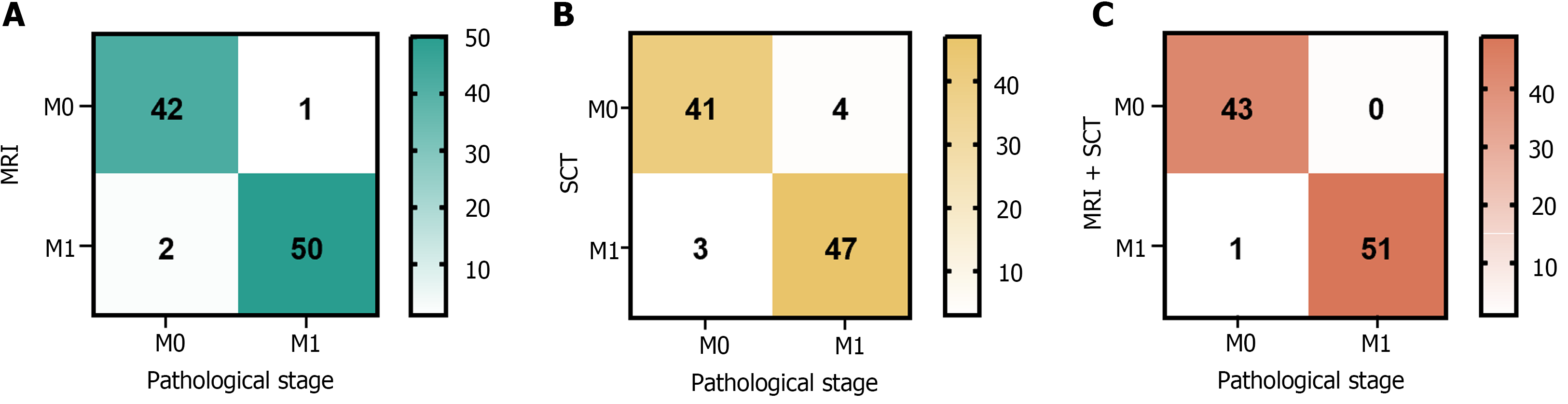
Ninety-five patients underwent MRI assessments, and 43 and 52 cases of M0 and M1 stages, respectively, were revealed. SCT of the same cohort identified 45 M0 and 50 M1 cases. Using both MRI and SCT together confirmed 43 cases as M0 and 52 as M1 (Figure 4).
Using pathological findings as the benchmark, the diagnostic performance for preoperative M staging in CRC, as measured by sensitivity, specificity, and accuracy, was enhanced when MRI was used with SCT compared with using either SCT or MRI alone. Among these changes, the accuracy of SCT diagnosis alone and MRI combined with SCT diagnosis was significantly different (P < 0.05). The details of this analysis are shown in Table 3.
| Index | Pathological stage | |
| M0 | M1 | |
| MRI | ||
| Sensitivity | 95.45 (42/44) | 98.04 (50/51) |
| Specificity | 98.04 (50/51) | 95.45 (42/44) |
| Accuracy | 96.84 (92/95) | 96.84 (92/95) |
| SCT | ||
| Sensitivity | 93.18 (41/44) | 92.16 (47/51)a |
| Specificity | 92.16 (47/51) | 93.18 (41/44) |
| Accuracy | 92.63 (88/95) | 92.63 (88/95)a |
| MRI + SCT | ||
| Sensitivity | 97.73 (43/44) | 100.00 (51/51) |
| Specificity | 96.08 (49/51) | 97.73 (43/44) |
| Accuracy | 96.84 (92/95) | 98.95 (94/95) |
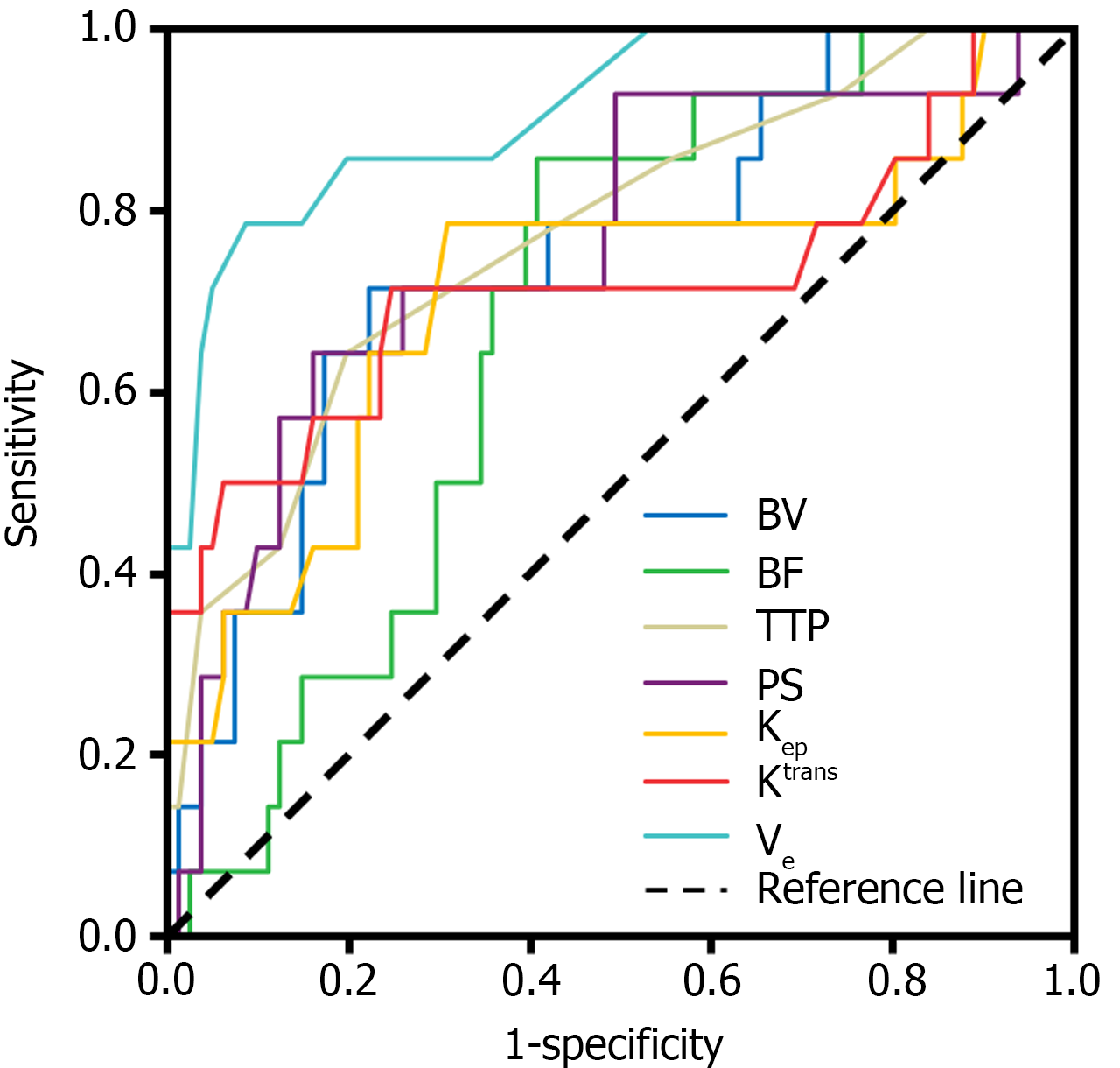
SCT perfusion and the quantitative parameters of MRI were used as test variables and the ROC curve was drawn with a prognosis of 6 mo after the operation as the state variable. The ROC curve analysis demonstrated that the prognostic predictive power, as indicated by the area under the curve values, of BV, BF, TTP, PS, Kep, Ktrans, and Ve for the outcomes of post-laparoscopic patients with CRC was 0.750, 0.683, 0.772, 0.761, 0.709, 0.719, and 0.910, respectively. The sensitivity was 0.714, 0.857, 0.643, 0.643, 0.786, 0.714, and 0.786, respectively, and the specificity was 0.778, 0.593, 0.802, 0.840, 0.691, 0.753, and 0.914, respectively (Table 4 and Figure 5).
| Index | AUC | 95%CI | Sensitivity | Specificity | P value |
| BV | 0.750 | 0.612-0.889 | 0.714 | 0.778 | 0.003 |
| BF | 0.683 | 0.559-0.806 | 0.857 | 0.593 | 0.030 |
| TTP | 0.772 | 0.633-0.910 | 0.643 | 0.802 | 0.001 |
| PS | 0.761 | 0.615-0.906 | 0.643 | 0.840 | 0.002 |
| Kep | 0.709 | 0.538-0.880 | 0.786 | 0.691 | 0.013 |
| Ktrans | 0.719 | 0.536-0.902 | 0.714 | 0.753 | 0.009 |
| Ve | 0.910 | 0.825-0.995 | 0.786 | 0.914 | < 0.001 |
Oncological research has indicated that the clinical and pathological characteristics of CRC are complex and often lack distinct signs during the initial phase of the disease. Moreover, the lesion site of CRC is difficult to locate. Consequently, most patients are diagnosed at the advanced stage, while some patients have metastasis and diffusion of cancer cells that negatively impacts their system and function, causing a variety of symptoms. CRC is associated with a high degree of malignancy, poor postoperative recovery, and a low 5-year survival rate[7,8]. Therefore, accurate preoperative staging is particularly important when formulating treatment plans. Imaging technology has rapidly developed in recent years. The extensive use of MRI and SCT in clinical settings has significantly enhanced the identification of CRC lesions, offering robust support for extending patient survival and enhancing their quality of life[9,10].
Currently, the clinical treatment of CRC is personalized. Medical staff remove cancerous tissues while ensuring that the quality of life and basic life function of patients are met as much as possible. Therefore, accurately evaluating and analyzing the patient’s condition before surgery and clarifying the treatment plan is necessary. Currently, SCT and MRI are often used for the preoperative diagnosis of patients with CRC[11]. MRI uses electromagnetic signals when scanning the human body, establishing the information parameters of tissues and organs based on the obtained electromagnetic signals, observing changes in the internal characteristics of the body in real-time, and offering a dependable foundation for medical diagnosis and treatment creation strategies[12]. Compared with SCT, the MRI principle is more complex and can provide diagnostic information from more angles and levels. MRI creates high-resolution images of colorectal soft tissue, can implement cross-sectional, coronal, and sagittal imaging simultaneously, and can clearly show the structures of the rectal, muscularis, and sub-mucosae, the muscularis propria, and serosa. MRI can clearly show the nature of the tumor and the adjacent tissue structure and can effectively detect the degree of tumor invasion, i.e. whether the surrounding tissue is infiltrated. It possesses considerable benefits in distinguishing between benign and malignant growth and in accurately determining the tumor stage. However, MRI is expensive, which makes it unconducive for the examination of distant metastatic lesions[13].
SCT has a wide scanning range and fast scanning speed. It can scan lesions and tissues around the intestine with high definition and resolution. In addition, SCT can reveal the location, size, and shape of the lesion, which is necessary for identifying the metastasis or local invasion of cancer cells[14]. However, SCT examinations have poor clarity and cannot accurately distinguish between the hierarchical structure of the intestinal wall and the internal structure of the lymph nodes. It is impossible to determine whether the tumor involves the submucosa and muscle layer. Additionally, risks, such as ionizing radiation damage, limit the clinical application of SCT[15].
The findings of this study indicated that the combined use of MRI and SCT for preoperative TNM staging in patients with CRC demonstrated superior sensitivity, specificity, and accuracy compared to either SCT or MRI alone. Moreover, statistical analysis revealed a significant difference in the level of accuracy (P < 0.05). This is because the T stage determines the depth of the intestinal wall tumor invasion and the N stage determines lymph node metastasis, location, density, size, and other information. Therefore, the use of MRI can be more intuitive to observe colorectal tumor infiltration and to observe whether inflammation or fibrosis is present in the surrounding normal tissue[16]. There needs to be a high degree of patient cooperation during SCT and, if necessary, a better three-dimensional reconstruction image should be obtained using computer technology for clinical diagnosis and treatment. However, patients undergoing SCT are exposed to a large dose of X-ray ionizing radiation during the examination, and the resolution of soft tissue images is not as good as that when using MRI[17]. Therefore, a combination of the two can compensate for these deficiencies and improve the accuracy of diagnosis.
Laparoscopic radical resection of CRC is a minimally invasive surgery that began to be used in the late 1980s. With the advancement of laparoscopic techniques and the accumulation of surgical experience, good results have been obtained. Studies have shown that the therapeutic effect of laparoscopic radical resection is comparable to that of open surgery. Laparoscopic radical resection of CRC is characterized by minimal pain, small incisions, and rapid recovery; therefore, it is widely used[18-20]. Although the treatment of CRC through laparoscopy can enhance patient survival rates, metastasis and recurrence after surgery, which affect patient prognosis, are still risks.
The findings of this study revealed that the predictive accuracies for the prognosis of patients with CRC undergoing laparoscopic treatment, as measured by the area under the curve values for BV, BF, TTP, PS, Kep, Ktrans, and Ve, were 0.750, 0.683, 0.772, 0.761, 0.709, 0.719, and 0.910, respectively. The corresponding sensitivity values were 0.714, 0.857, 0.643, 0.643, 0.786, 0.714, and 0.786, respectively, and the specificity values were 0.778, 0.593, 0.802, 0.840, 0.691, 0.753, and 0.914, respectively.
International research suggests that Ktrans is a reliable prognostic marker for cancer patient outcomes. Martens et al[21] discovered that Ktrans on dynamic contrast-enhanced MRI is a predictor of local recurrence in patients with cell carcinoma. A high Ktrans value indicated a good prognosis. Kep, calculated using Ktrans, denotes the rate at which the contrast agent recirculates from the extracellular extravascular space back into the bloodstream. A high value indicates that the microvessels in the cancer tissue area have high permeability and that the speed of the contrast agent backflow to the blood vessels is accelerated.
In this study, the specificity of Ve in predicting the prognosis of patients was the highest, reaching 0.914. The value of Ve is the ratio of Ktrans to Kep and represents the volume ratio of extravascular extracellular space[11]. Therefore, Ve is related to the activity of the cell environment: The smaller the Ve value, the higher the malignancy of cancer. In patients who have a poor prognosis, there are usually immature new capillaries in the lesion area. This exacerbates the degree of barrier destruction and so the proportion of extracellular space outside the blood vessels and, consequently, Ve increase. This is believed to be the reason for the link between poor prognosis and high Ve values[22,23]. In a study on dynamic contrast enhanced-MRI quantitative parameters for predicting the efficacy of concurrent chemoradiotherapy for locally advanced cancer, Liu et al[24] discovered tumor regression when Ve was low, which could be used as a potential indicator to predict the treatment response of locally advanced cancer.
The BF directly reflects the state of tissue perfusion. TTP refers to the time from the beginning of injection to the peak of tissue enhancement and is also an indicator of blood flow velocity and tissue perfusion. PS reflects tumor maturity and vascular network tightness. An abundant blood supply is necessary for the rapid proliferation of malignant tumor cells. A malignant tumor angiogenesis network is developed, the blood flow rate is high, and the lesion is in a high perfusion state. These patients typically have serious conditions. The space available for tumor survival in the organ is limited; therefore, the pressure in the tissue increases, compressing the capillaries and slowing down the blood flow velocity, thus prolonging the peak time[25]. Therefore, perfusion and quantitative parameters are useful in the prognosis of laparoscopic treatment in patients with CRC.
This paper reported on a single-center, retrospective analysis with a limited sample size, so the findings may be subject to bias. Future endeavors will include a large-scale, multicenter investigation to establish a solid and dependable framework for the evaluation technique under study.
In summary, the diagnostic efficacy of the preoperative TNM staging of CRC using MRI with SCT was better than that of any single imaging method, especially in terms of improved accuracy. In addition, by analyzing perfusion and quantitative parameters, MRI with SCT can effectively predict the outcomes of patients with CRC after laparoscopic treatment, which provides an important basis for clinical treatment decisions.
| 1. | Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 300] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 2. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 376] [Article Influence: 125.3] [Reference Citation Analysis (6)] |
| 3. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 4. | Li Y, Xin J, Sun Y, Han T, Zhang H, An F. Magnetic resonance imaging-guided and targeted theranostics of colorectal cancer. Cancer Biol Med. 2020;17:307-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Anyene I, Caan B, Williams GR, Popuri K, Lenchik L, Giri S, Chow V, Beg MF, Cespedes Feliciano EM. Body composition from single vs multi-slice abdominal computed tomography: Concordance and associations with colorectal cancer survival. J Cachexia Sarcopenia Muscle. 2022;13:2974-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 6. | Lee DH, Kim B, Lee JM, Lee ES, Choi MH, Kim H. Multidetector CT of Extrahepatic Bile Duct Cancer: Diagnostic Performance of Tumor Resectability and Interreader Agreement. Radiology. 2022;304:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao X, Lu Y. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci. 2021;17:3837-3849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 8. | Wang H. MicroRNAs and Apoptosis in Colorectal Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Shin J, Seo N, Baek SE, Son NH, Lim JS, Kim NK, Koom WS, Kim S. MRI Radiomics Model Predicts Pathologic Complete Response of Rectal Cancer Following Chemoradiotherapy. Radiology. 2022;303:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 10. | Klassen P, Baracos V, Gramlich L, Nelson G, Mazurak V, Martin L. Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Inchingolo R, Maino C, Cannella R, Vernuccio F, Cortese F, Dezio M, Pisani AR, Giandola T, Gatti M, Giannini V, Ippolito D, Faletti R. Radiomics in colorectal cancer patients. World J Gastroenterol. 2023;29:2888-2904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 12. | Zugni F, Padhani AR, Koh DM, Summers PE, Bellomi M, Petralia G. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening in asymptomatic subjects of the general population: review and recommendations. Cancer Imaging. 2020;20:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Zins M, Santiago IAGP. MRI for T Restaging of Locally Advanced Rectal Cancer Following Neoadjuvant Chemotherapy and Radiation Therapy. Radiology. 2022;305:373-374. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Dercle L, Zhao B, Gönen M, Moskowitz CS, Connors DE, Yang H, Lu L, Reidy-Lagunes D, Fojo T, Karovic S, Maitland ML, Oxnard GR, Schwartz LH. An imaging signature to predict outcome in metastatic colorectal cancer using routine computed tomography scans. Eur J Cancer. 2022;161:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Vernuccio F, Mercante I, Tong XX, Crimì F, Cillo U, Quaia E. Biliary complications after liver transplantation: A computed tomography and magnetic resonance imaging pictorial review. World J Gastroenterol. 2023;29:3257-3268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 16. | Fair ES, Duvnjak P. Utilization of Pelvic MRI for Nodal Staging in Rectal Cancer Staging. Acad Radiol. 2020;27:1718-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Fan S, Li X, Cui X, Zheng L, Ren X, Ma W, Ye Z. Computed Tomography-Based Radiomic Features Could Potentially Predict Microsatellite Instability Status in Stage II Colorectal Cancer: A Preliminary Study. Acad Radiol. 2019;26:1633-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Becattini C, Pace U, Pirozzi F, Donini A, Avruscio G, Rondelli F, Boncompagni M, Chiari D, De Prizio M, Visonà A, De Luca R, Guerra F, Muratore A, Portale G, Milone M, Castagnoli G, Righini M, Martellucci J, Persiani R, Frasson S, Dentali F, Delrio P, Campanini M, Gussoni G, Vedovati MC, Agnelli G. Rivaroxaban vs placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Blood. 2022;140:900-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Gehrman J, Angenete E, Björholt I, Lesén E, Haglind E. Cost-effectiveness analysis of laparoscopic and open surgery in routine Swedish care for colorectal cancer. Surg Endosc. 2020;34:4403-4412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Ueda Y, Shiraishi N, Kawasaki T, Akagi T, Ninomiya S, Shiroshita H, Etoh T, Inomata M. Short- and long-term outcomes of laparoscopic surgery for colorectal cancer in the elderly aged over 80 years old versus non-elderly: a retrospective cohort study. BMC Geriatr. 2020;20:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Martens RM, Koopman T, Lavini C, Ali M, Peeters CFW, Noij DP, Zwezerijnen G, Marcus JT, Vergeer MR, Leemans CR, de Bree R, de Graaf P, Boellaard R, Castelijns JA. Multiparametric functional MRI and (18)F-FDG-PET for survival prediction in patients with head and neck squamous cell carcinoma treated with (chemo)radiation. Eur Radiol. 2021;31:616-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Zhu Y, Jiang Z, Wang B, Li Y, Jiang J, Zhong Y, Wang S, Jiang L. Quantitative Dynamic-Enhanced MRI and Intravoxel Incoherent Motion Diffusion-Weighted Imaging for Prediction of the Pathological Response to Neoadjuvant Chemotherapy and the Prognosis in Locally Advanced Gastric Cancer. Front Oncol. 2022;12:841460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Qu C, Zeng P, Wang H, Guo L, Zhang L, Yuan C, Yuan H, Xiu D. Preoperative Multiparametric Quantitative Magnetic Resonance Imaging Correlates with Prognosis and Recurrence Patterns in Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Liu B, Sun Z, Ma WL, Ren J, Zhang GW, Wei MQ, Hou WH, Hou BX, Wei LC, Huan Y, Zheng MW. DCE-MRI Quantitative Parameters as Predictors of Treatment Response in Patients With Locally Advanced Cervical Squamous Cell Carcinoma Underwent CCRT. Front Oncol. 2020;10:585738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Chen SH, Miles K, Taylor SA, Ganeshan B, Rodriquez M, Fraioli F, Wan S, Afaq A, Shortman R, Walls D, Hoy L, Endozo R, Bhargava A, Hanson M, Huang J, Raouf S, Francis D, Siddiqi S, Arulampalam T, Sizer B, Machesney M, Reay-Jones N, Dindyal S, Ng T, Groves AM. FDG-PET/CT in colorectal cancer: potential for vascular-metabolic imaging to provide markers of prognosis. Eur J Nucl Med Mol Imaging. 2021;49:371-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |