Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2065
Revised: May 6, 2024
Accepted: May 7, 2024
Published online: July 27, 2024
Processing time: 145 Days and 1.2 Hours
Acute appendicitis (AA) is the most common cause of acute abdomen in children. Anesthesia significantly influences the surgical treatment of AA in children, making the scientific and effective selection of anesthetics crucial.
To assess the clinical effect of atropine (ATR) in combination with remifentanil (REMI) in children undergoing surgery for AA.
In total, 108 cases of pediatric AA treated between May 2020 and May 2023 were selected, 58 of which received ATR + REMI [research group (RG)] and 50 who received REMI [control group (CG)]. Comparative analyses were conducted on the time to loss of eyelash reflex, pain resolution time, recovery time from anes
Compared with the CG, the RG showed significantly shorter time to loss of eyelash reflex, pain resolution, recovery from anesthesia, and safe departure from the operating room. Furthermore, the incidence rates of overall AEs (head shaking, limb activity, etc.) were lower, and influences on intraoperative hemody
ATR + REMI is superior to REMI alone in children undergoing AA surgery, with a lower incidence of AEs, fewer influences on hemodynamics and stress responses, and better post-anesthesia recovery.
Core Tip: Pediatric acute appendicitis (AA) is usually treated surgically (appendectomy), and anesthesia has a significant impact intraoperatively. Scientific and effective selection of anesthetics is crucial in preventing postoperative adverse events (AEs) and improving surgical outcomes for pediatric AA. This study compared and analyzed the clinical effects of atropine (ATR) combined with remifentanil (REMI) and REMI monotherapy in children undergoing surgery for AA. ATR combined with REMI exhibited a better anesthetic effect than REMI alone, with a lower incidence of AEs, relatively fewer influences on hemodynamics, milder stress responses, and faster recovery from anesthesia.
- Citation: Li YJ, Chen YY, Lin XL, Zhang WZ. Evaluation of the clinical effects of atropine in combination with remifentanil in children undergoing surgery for acute appendicitis. World J Gastrointest Surg 2024; 16(7): 2065-2072
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2065.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2065
Acute appendicitis (AA) is the most common cause of acute abdomen, especially in children[1,2]. Women have a statistically lower lifetime risk of AA than men but a higher risk of appendectomy[3]. AA may present atypical symptoms, especially in preschoolers, and the typical symptoms include anorexia, fever, periumbilical pain that moves to the right iliac fossa, and tenderness of the right iliac fossa[4]. Pediatric AA is generally treated surgically (appendectomy), and the use of anesthetic greatly influences the operation[5,6]. Thus, the scientific and effective selection of anesthetics aids significantly in preventing adverse events (AEs) in children after AA surgery and improving surgical outcomes[7].
Remifentanil (REMI), a fast-acting (1–2 min) opioid with an elimination half-life of 3–10 minutes, exerts analgesic and sedative effects and can significantly enhance clinical anesthesia when used in combination with propofol[8,9]. The use of REMI in general anesthesia for pediatric patients undergoing dental surgery and transthoracic device closure of ventricular septal defects has demonstrated clinical efficacy and safety and positive contributions to higher recovery quality[10,11]. Atropine (ATR), an anticholinergic agent, can work synergistically with REMI in providing optimal intubation conditions for neonates and reducing potential negative hemodynamic effects of anesthesia induction[12,13]. This study analyzed the clinical effect of ATR + REMI in children undergoing AA surgery to address the research gap on the subject and help optimize the selection of anesthesia schemes in pediatric AA surgery.
One hundred and eight pediatric patients with AA treated at Shanxi Provincial Children’s Hospital between May 2020 and May 2023 were enrolled. The research group (RG; n = 58) received ATR + REMI, whereas the control group (CG; n = 50) received REMI alone. No significant differences were noted in general data between the RG and CG (P > 0.05).
Inclusion criteria: All children that met the diagnostic criteria for pediatric appendicitis and the indications of laparoscopic surgery, with intact medical records, with normal communication and cognitive abilities, and ability to cooperate with the study were enrolled.
Exclusion criteria: Children with complications of congenital heart disease, malignant hyperthermia, autoimmune deficiency, or blood coagulation dysfunction were excluded, as well as those with allergic constitution or allergies to the study medication.
The RG received total intravenous anesthesia with ATR and REMI: 0.02 mg/kg of ATR was administered intravenously 30 min before the operation. Then, routine anesthesia induction was performed, with intravenous administration of midazolam (0.1 mg/kg), sufentanil (2 μg/kg), propofol (2 mg/kg), and cisatracurium (0.1 mg/kg), administered sequentially. An endotracheal tube was inserted with a video laryngoscope 3 min later, and mechanical ventilation was initiated using an anesthesia machine after confirmation by auscultation of normal respiratory sounds in both lungs. Respiration was controlled with a tidal volume of 8–10 mL/kg, frequency of 14–20 times/min, airway pressure of 15–20 cmH2O, and end-tidal carbon dioxide of 35–40 mmHg. For anesthesia maintenance, propofol was administered at 3–5 mg/(kg·h) and REMI at 0.2–0.3 μg/(kg·min) using intravenous infusion pumps.
The CG was given REMI: The children were given an intravenous injection of normal saline at the same dose as ATR 30 min before surgery. Other procedures, such as anesthesia induction, tracheal intubation, mechanical ventilation, and anesthesia maintenance, were the same as those performed in the RG.
Both groups of patients fasted for 12 h, and water was withdrawn 8 h before surgery. In both groups, mask oxygen was given continuously during the operation with an oxygen flow rate of 1 L/min. The anesthesia recovery time was recorded after natural recovery.
The time to loss of eyelash reflex, pain resolution, and recovery from anesthesia were observed and recorded.
Incidence of AEs: The incidence of postoperative adverse reactions such as respiratory depression, hypoxemia, bradycardia, nausea and vomiting, and hypotension were monitored and recorded.
Intraoperative responses: The occurrence of head shaking and limb activity during the operation was observed and recorded, and the orientation recovery time and safe departure time from the operating room were measured.
Hemodynamic parameters: The mean blood oxygen saturation (SPO2), mean arterial pressure (MAP), heart rate (HR), and respiratory rate (RR) before, during, and after the operation were measured by color Doppler ultrasound.
Postoperative sedation score: Patients were assessed using the Ramsay score for sedation: 1 point, restless and agitated; 2 points, tranquil and cooperative; 3 points, sleepiness and response to commands; 4 points, sleeping but easily aroused; 5 points: sluggish response to verbal stimulus; 6 points: deep sleep and no response to verbal stimulus. We employed the Face, Legs, Activity, Cry, Consolability (FLACC) Behavioral Scale to evaluate the degree of pain: 0, painless; 1–3, mild pain that can be tolerated; 4–6, pain that affects sleep but is still tolerable; 7–10: severe and unbearable pain.
All calculations were performed using SPSS 21.0 software under a significance threshold of P < 0.05. Age, body mass, and other measurement data were expressed as mean ± SD and compared between groups using the independent sample t-test. Sex, American Society of Anesthesiologists (ASA) physical status classification, incidence of AEs, and other count data were expressed as n (%) and analyzed using the χ2 test.
RG and CG did not differ significantly in age, sex, body weight, disease course, ASA classification, and other general data (P > 0.05; Table 1).
| Factors | Research group (n = 58) | Control group (n = 50) | χ2/t value | P value |
| Age (yr) | 8.71 ± 2.15 | 8.58 ± 2.09 | 0.317 | 0.752 |
| Sex (male/female) | 33/25 | 30/20 | 0.106 | 0.744 |
| Body mass (kg) | 23.20 ± 4.90 | 23.05 ± 4.77 | 0.161 | 0.873 |
| Disease course (d) | 2.97 ± 1.01 | 2.72 ± 0.90 | 1.348 | 0.180 |
| ASA classification (I/II) | 43/15 | 40/10 | 0.519 | 0.471 |
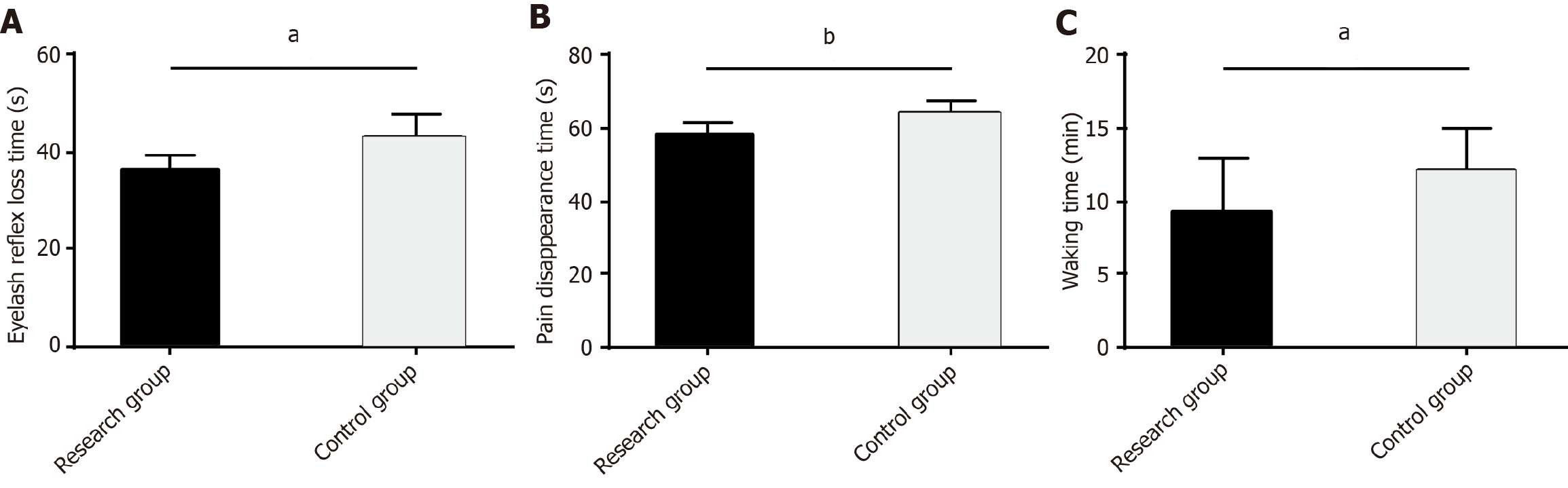
The time to loss of eyelash reflex, pain resolution, and recovery from anesthesia were recorded to evaluate the anesthetic efficacy of the two anesthesia protocols. The data showed significantly lower values of the three indexes in the RG than in the CG (P < 0.05; Figure 1).
The overall incidence of AEs, such as respiratory depression, hypoxemia, bradycardia, nausea and vomiting, and hypotension, were significantly lower in the RG than in the CG (P < 0.05; Table 2).
| Factors | Research group (n = 58) | Control group (n = 50) | χ2 | P value |
| Respiratory depression | 0 (0.00) | 1 (2.00) | ||
| Hypoxemia | 0 (0.00) | 2 (4.00) | ||
| Bradycardia | 2 (3.45) | 2 (4.00) | ||
| Nausea and vomiting | 1 (1.72) | 4 (8.00) | ||
| Hypotension | 1 (1.72) | 4 (8.00) | ||
| Total | 4 (6.90) | 13 (26.00) | 7.388 | 0.007 |
Head shaking, limb activity, orientation recovery, and safe departure time from the operating room were monitored in the patients to evaluate the influence of the two anesthetic interventions on the responses of the pediatric patients during AA surgery. The incidence rates of head shaking and limb activity and shorter orientation recovery and safe departure time from the operating room were significantly lower in the RG than in the CG (P < 0.05; Table 3).
| Factors | Research group (n = 58) | Control group (n = 50) | χ2/t value | P value |
| Head shaking, n (%) | 2 (3.45) | 7 (14.00) | 3.914 | 0.048 |
| Limb activity, n (%) | 1 (1.72) | 6 (12.00) | 4.678 | 0.031 |
| Orientation recovery time (min) | 10.21 ± 2.11 | 14.88 ± 3.37 | 8.753 | < 0.001 |
| Safe departure time from the operating room (min) | 32.40 ± 4.02 | 41.36 ± 5.38 | 9.883 | < 0.001 |
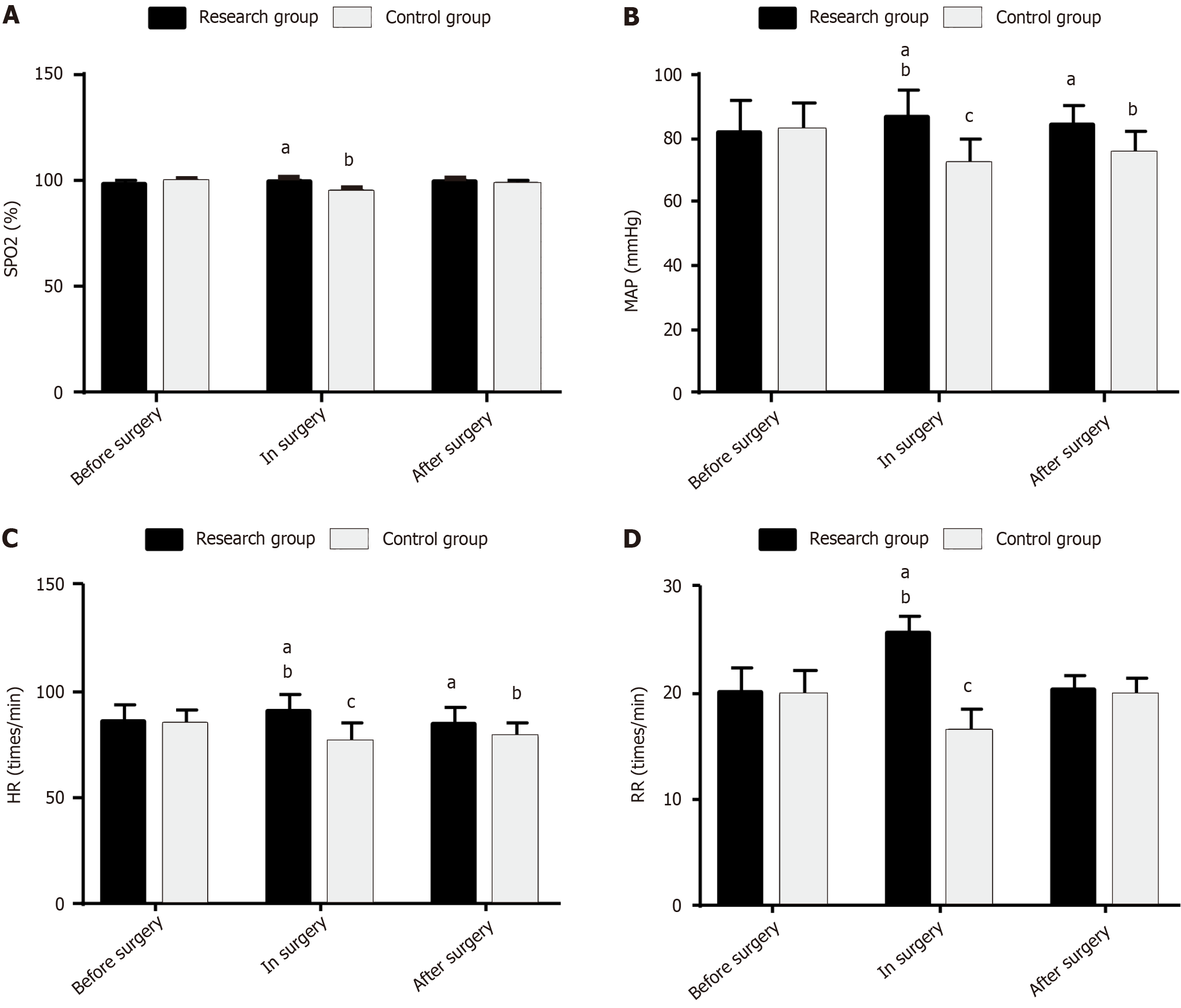
Hemodynamic parameters, such as SPO2, MAP, HR, and RR, of both groups were measured to evaluate the influence of the two anesthetic interventions on the intraoperative hemodynamics in children undergoing AA surgery. SPO2 changed little before, during, or after surgery in the RG (P > 0.05), whereas it decreased intraoperatively in the CG (P < 0.05). Moreover, the postoperative SPO2 was higher in the RG than in the CG (P < 0.05). In the RG, the intraoperative MAP, HR, and RR increased significantly (P < 0.05), whereas the postoperative levels did not differ significantly from the baseline (P > 0.05). In the CG, the intraoperative MAP, HR, and RR decreased significantly (P < 0.05), and the postoperative levels remained significantly lower than the baseline (P < 0.05). Furthermore, the RG had higher intra- and postoperative MAP and HR and higher intraoperative RR than the CG (P < 0.05; Figure 2).
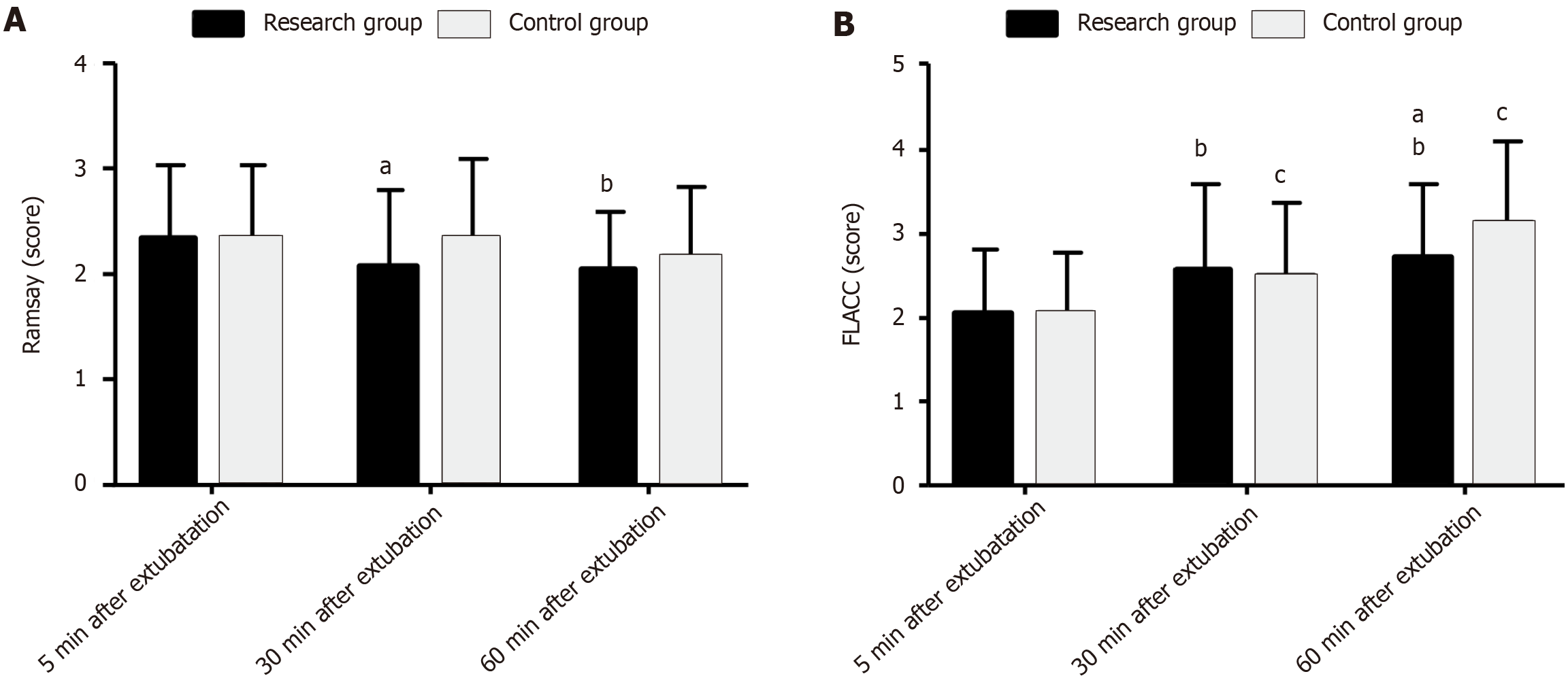
The Ramsay and FLACC scores were measured at 5, 30, and 60 min after extubation to evaluate the effects of the two anesthetic regimens on postoperative sedation and pain levels in children undergoing AA surgery. The two groups exhibited similar scores for the two scales at 5 min after extubation (P > 0.05). After extubation, the Ramsay scores of the two groups gradually decreased over time, whereas the FLACC scores gradually increased. The Ramsay score was lower in the RG than in the CG at 30 min after extubation (P < 0.05), but the post-extubation FLACC scores were similar (P > 0.05). At 60 min after extubation, the two groups showed similar Ramsay scores (P > 0.05), but the RG showed a lower FLACC score than the CG (P < 0.05; Figure 3).
AA is a common abdominal surgical emergency in pediatrics and may be associated with the interaction of many factors, such as heredity, environment, eating habits, bacterial infection, and secondary inflammatory reaction[14,15]. Appropriate anesthesia selection must be ensured to prevent complications associated with surgery for pediatric AA[16].
Many studies have analyzed anesthesia selection for pediatric AA surgery and have provided many useful references and insights for the optimization of anesthesia management. For example, Kaszyński et al[17] pointed out that intravenous lidocaine can effectively relieve pain while reducing opioid use in children undergoing laparoscopic appendectomy. Ergün et al[18] reported that intraperitoneal injection of bupivacaine in children undergoing laparoscopic appendectomy can effectively reduce postoperative shoulder pain and postoperative analgesia demand. Elnabtity et al[19] found that the addition of dexmedetomidine and intraperitoneal bupivacaine in children undergoing laparoscopic appendectomy can further promote the postoperative analgesic effect, prolong the first rescue analgesia time, reduce the rescue analgesia dose, shorten the hospitalization time, and improve parental satisfaction. The present study comparatively analyzed the clinical advantages of ATR + REMI vs REMI alone in pediatric AA surgery. The results showed that the RG had evidently shorter time to loss of eyelash reflex, pain resolution, and recovery from anesthesia than the CG, suggesting that ATR + REMI can establish a good anesthetic effect in pediatric AA surgery. The major AEs observed after AA surgery in children were respiratory depression, hypoxemia, bradycardia, nausea and vomiting, and hypotension, similar to the results reported by Kaszyński et al[20]. RG had a lower overall incidence of AEs than CG, indicating the increased benefit conferred by ATR + REMI, thereby preventing postoperative AEs in pediatric patients undergoing surgery for AA. Furthermore, ATR helps prevent bradycardia events during routine anesthesia in critically ill children and is also effective in mitigating bradycardia and hypotension in critically ill patients with fingolimod overdose[21,22]. This may be related to the ability of ATR to prophylactically ameliorate cardiac output reduction and alleviate vasodilation responses[23,24].
The incidence of head shaking and limb activity was markedly lower in the RG than in the CG, and the orientation recovery time and safe departure time from the operating room were significantly shorter. A meta-analysis by Shi et al[25] showed that ATR for pediatric sedation reduced saliva secretion in children and was comparable to ketamine in reducing agitation symptoms. The present results confirm that the use of ATR + REMI for anesthetic intervention in pediatric AA surgery can significantly alleviate intraoperative responses. In the RG, the hemodynamic evaluation showed that the SPO2 showed no significant changes before, during, or after surgery, whereas the intraoperative MAP, HR, and RR increased significantly but recovered to preoperative levels postoperatively. In the CG, the intraoperative SPO2, MAP, HR, and RR all decreased significantly, with only SPO2 and RR returning to preoperative levels postoperatively. Thus, ATR + REMI has a lesser effect on hemodynamics than REMI. In addition, the Ramsay score of the RG was significantly lower than that of the CG at 30 min after extubation, whereas the FLACC score was significantly lower at 60 min after extubation, indicating that ATR + REMI provides better sedation and pain relief than REMI alone. This benefit may be attributed to the ability of ATR to inhibit parasympathetic nerves, relieve smooth muscle spasms, inhibit glandular secretions, and reduce respiratory secretions[26,27].
This study has some limitations. First, the single-center study design and relatively small sample size may have reduced the universality of the study results. Second, the factors affecting the safety of pediatric patients with AA undergoing surgery were not analyzed. A safety evaluation is required to avoid related AEs and optimize surgical management in a more targeted manner. Finally, the underlying anesthetic mechanisms of ATR and REMI were not evaluated. This assessment is needed to further deepen our understanding of the efficacy of these two anesthetics. A more in-depth analysis should be conducted in the future based on the above points to confirm and even improve on the current findings.
Taken together, ATR + REMI is superior to REMI in pediatric AA surgery. It contributes to shorter time to loss of eyelash reflex, pain resolution and recovery from anesthesia, lower risk of postoperative AEs, milder intraoperative responses, and less impact on hemodynamics, with better sedative and analgesic effects, which deserves clinical popularization.
| 1. | Perez KS, Allen SR. Complicated appendicitis and considerations for interval appendectomy. JAAPA. 2018;31:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Omling E, Salö M, Saluja S, Bergbrant S, Olsson L, Persson A, Björk J, Hagander L. Nationwide study of appendicitis in children. Br J Surg. 2019;106:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Téoule P, Laffolie J, Rolle U, Reissfelder C. Acute Appendicitis in Childhood and Adulthood. Dtsch Arztebl Int. 2020;117:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Stringer MD. Acute appendicitis. J Paediatr Child Health. 2017;53:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | de Almeida Leite RM, Seo DJ, Gomez-Eslava B, Hossain S, Lesegretain A, de Souza AV, Bay CP, Zilberstein B, Marchi E, Machado RB, Barchi LC, Ricciardi R. Nonoperative vs Operative Management of Uncomplicated Acute Appendicitis: A Systematic Review and Meta-analysis. JAMA Surg. 2022;157:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (102)] |
| 6. | Zuo Y, Chang Y, Thirupathi A, Zhou C, Shi Z. Prenatal sevoflurane exposure: Effects of iron metabolic dysfunction on offspring cognition and potential mechanism. Int J Dev Neurosci. 2021;81:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Nepomuceno H, Pearson EG. Nonoperative management of appendicitis in children. Transl Gastroenterol Hepatol. 2021;6:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Grillot N, Lebuffe G, Huet O, Lasocki S, Pichon X, Oudot M, Bruneau N, David JS, Bouzat P, Jobert A, Tching-Sin M, Feuillet F, Cinotti R, Asehnoune K, Roquilly A; Atlanrea Study GroupSociété Française d’Anesthésie Réanimation (SFAR) Research Network. Effect of Remifentanil vs Neuromuscular Blockers During Rapid Sequence Intubation on Successful Intubation Without Major Complications Among Patients at Risk of Aspiration: A Randomized Clinical Trial. JAMA. 2023;329:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Sridharan K, Sivaramakrishnan G. Comparison of Fentanyl, Remifentanil, Sufentanil and Alfentanil in Combination with Propofol for General Anesthesia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr Clin Pharmacol. 2019;14:116-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Yu LS, Xie WP, Liu JF, Wang J, Cao H, Wang ZC, Chen Q. A comparison of the outcomes of dexmedetomidine and remifentanil with sufentanil-based general anesthesia in pediatric patients for the transthoracic device closure of ventricular septal defects. J Cardiothorac Surg. 2021;16:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Nourbakhsh N, Kaviani N, Salari-Moghaddam R, Marzoughi S. Effects of remifentanil on the recovery quality among pediatric candidates for dental procedures under general anesthesia. Dent Res J (Isfahan). 2022;19:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Chollat C, Maroni A, Aubelle MS, Guillier C, Patkai J, Zana-Taïeb E, Keslick A, Torchin H, Jarreau PH. Efficacy and Safety Aspects of Remifentanil Sedation for Intubation in Neonates: A Retrospective Study. Front Pediatr. 2019;7:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Poterman M, Scheeren TWL, van der Velde MI, Buisman PL, Allaert S, Struys MMRF, Kalmar AF. Prophylactic atropine administration attenuates the negative haemodynamic effects of induction of anaesthesia with propofol and high-dose remifentanil: A randomised controlled trial. Eur J Anaesthesiol. 2017;34:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Glass CC, Rangel SJ. Overview and diagnosis of acute appendicitis in children. Semin Pediatr Surg. 2016;25:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. 2015;386:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 684] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 16. | Shi M, Miao S, Gu T, Wang D, Zhang H, Liu J. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Devel Ther. 2019;13:897-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Kaszyński M, Lewandowska D, Sawicki P, Wojcieszak P, Pągowska-Klimek I. Efficacy of intravenous lidocaine infusions for pain relief in children undergoing laparoscopic appendectomy: a randomized controlled trial. BMC Anesthesiol. 2021;21:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ergün E, Gurbanov A, Gollu G, Ates U, Bingöl-Koloğlu M, Çakmak A, Can ÖS. Effects of intraperitoneal bupivacaine injection in laparoscopic appendectomy in children on post-operative pain: A controlled randomized double-blinded study. Ulus Travma Acil Cerrahi Derg. 2022;28:974-978. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Elnabtity AM, Ibrahim M. Intraperitoneal dexmedetomidine as an adjuvant to bupivacaine for postoperative pain management in children undergoing laparoscopic appendectomy: A prospective randomized trial. Saudi J Anaesth. 2018;12:399-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Kaszyński M, Stankiewicz B, Pałko KJ, Darowski M, Pągowska-Klimek I. Impact of lidocaine on hemodynamic and respiratory parameters during laparoscopic appendectomy in children. Sci Rep. 2022;12:14038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Jones P, Dauger S, Peters MJ. Bradycardia during critical care intubation: mechanisms, significance and atropine. Arch Dis Child. 2012;97:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Stephenson M, Wong A, Rotella JA, Crump N, Kerr F, Greene SL. Deliberate fingolimod overdose presenting with delayed hypotension and bradycardia responsive to atropine. J Med Toxicol. 2014;10:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Shim JG, Cho EA, Ryu KH, Lee SH, Kim JI, Kim D, Oh EJ, Ahn JH. Effects of prophylactic atropine on the time to tracheal intubation with the pre-administration of remifentanil. Acta Anaesthesiol Scand. 2021;65:335-342. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Choi WJ, Lee K, Kim YK, Song KJ, Jeong SM, Hwang GS. Vagolytic atropine attenuates cerebral vasodilation response during acute orthostatic hypotension. Korean J Anesthesiol. 2015;68:594-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Shi J, Li A, Wei Z, Liu Y, Xing C, Shi H, Ding H, Pan D, Ning G, Feng S. Ketamine versus ketamine pluses atropine for pediatric sedation: A meta-analysis. Am J Emerg Med. 2018;36:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Botha C, Farmer AD, Nilsson M, Brock C, Gavrila AD, Drewes AM, Knowles CH, Aziz Q. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut. 2015;64:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Liu X, Yu HK, Gu LX, Chen JK, Wen ZB. Atropine Premedication Facilitates Ultrasound-Guided Reduction by Saline Enema in Children With Intussusception. Front Pharmacol. 2019;10:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |