Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2054
Revised: May 6, 2024
Accepted: May 27, 2024
Published online: July 27, 2024
Processing time: 147 Days and 3.8 Hours
Portal shunt and immune status related to the spleen are related to the occurrence of hepatic encephalopathy (HE). It is unknown whether spleen volume before transjugular intrahepatic portosystemic shunt (TIPS) is related to postoperative HE.
To investigate the relationship between spleen volume and the occurrence of HE.
This study included 135 patients with liver cirrhosis who underwent TIPS, and liver and spleen volumes were elevated upon computed tomography imaging. The Kaplan-Meier curve was used to compare the difference in the incidence rate of HE among patients with different spleen volumes. Univariate and multivariate Cox regression analyses were performed to identify the factors affecting overt HE (OHE). Restricted cubic spline was used to examine the shapes of the dose-response association between spleen volumes and OHE risk.
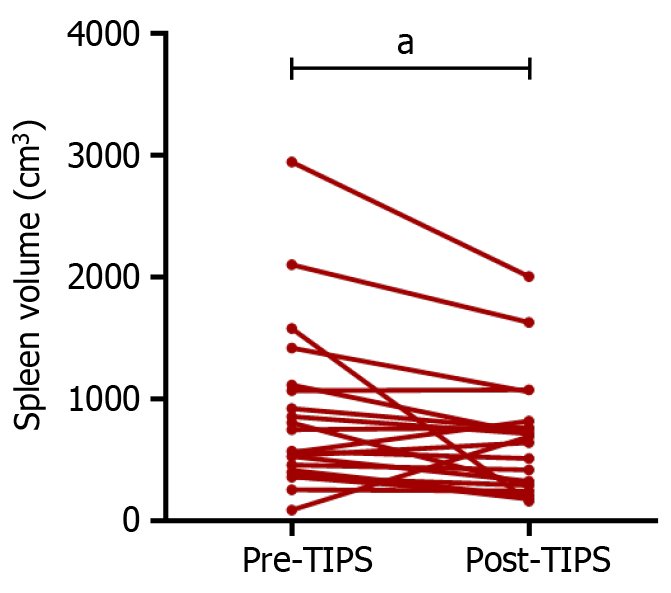
The results showed that 37 (27.2%) of 135 patients experienced OHE during a 1-year follow-up period. Compared with preoperative spleen volume (901.30 ± 471.90 cm3), there was a significant decrease in spleen volume after TIPS (697.60 ± 281.0 cm3) in OHE patients. As the severity of OHE increased, the spleen volume significantly decreased
Spleen volume is related to the occurrence of OHE after TIPS. Preoperative spleen volume is an independent risk factor for post-TIPS OHE.
Core Tip: This study included 135 patients with liver cirrhosis who underwent transjugular intrahepatic portosystemic shunt (TIPS), and spleen volumes were elevated by computed tomography-scan imaging. The results showed that there was a significant decrease in spleen volume after TIPS in overt hepatic encephalopathy (OHE) patients. The patients with a smaller spleen volume had a higher incidence of HE. Preoperative spleen volume was an independent risk factor for postoperative OHE in TIPS. In conclusion, spleen volume is closely related to the occurrence of OHE after TIPS. Preoperative spleen volume is an independent risk factor for post-TIPS OHE.
- Citation: Zhao CJ, Ren C, Yuan Z, Bai GH, Li JY, Gao L, Li JH, Duan ZQ, Feng DP, Zhang H. Spleen volume is associated with overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with portal hypertension. World J Gastrointest Surg 2024; 16(7): 2054-2064
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2054.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2054
Cirrhotic portal hypertension is a clinical syndrome characterized by esophageal and gastric variceal bleeding and ascites[1,2]. Transjugular intrahepatic portosystemic shunt (TIPS) is an effective treatment method for cirrhotic portal hypertension by reducing the gradient of portal venous pressure[3-7]. Hepatic encephalopathy (HE) is a common complication after TIPS[8,9]. The incidence rate of overt HE (OHE) after TIPS ranges from 15% to 48%[10]. OHE patients exhibit symptoms such as cognitive impairment, unclear language, drowsiness, and even coma[10,11]. It seriously hinders the quality of life of patients and is also the main reason for their readmission. Therefore, finding important factors affecting OHE after TIPS will help to screen high-risk groups, reduce the incidence rate of OHE after TIPS, and improve the survival prognosis of patients.
The occurrence of HE after TIPS is related to the shunt components and shunt flow rate[12,13]. The spleen, as an immune organ that interacts with the liver, participates in the progression of liver diseases[14,15]. The hemodynamic changes caused by TIPS affect the speed of splenic venous return into the liver, and components of the spleen entering the liver are also be affected. Previously, it was reported that peripheral interleukin (IL)-6 levels were associated with postoperative OHE after TIPS, and the total amount of venous return to the liver was a certain amount[16]. An increase in splenic venous return implies a decrease in various metabolites in superior mesenteric venous shunt, theoretically leading to a decrease in the incidence of HE. Therefore, abnormalities in the spleen may be one of the reasons affecting OHE after TIPS.
In recent years, with the development of imaging in the treatment of liver diseases, noninvasive assessment of spleen volume and stiffness based on ultrasound and computed tomography (CT) has been widely used in cirrhotic patients with portal hypertension[17-19]. However, there are few reports on whether noninvasive liver and spleen assessment based on imaging data can predict postoperative OHE. Therefore, the purpose of this study was to determine whether preoperative spleen size is related to the incidence of OHE after TIPS, and to stratify the risk of OHE in TIPS patients.
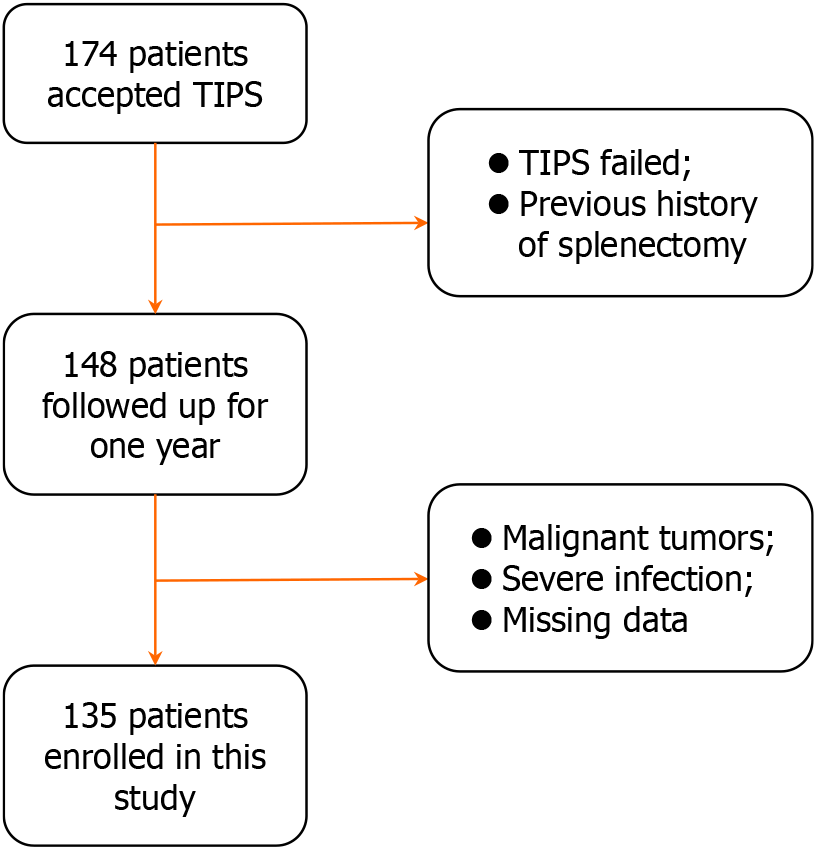
This study retrospectively analyzed 135 patients with liver cirrhosis who underwent TIPS treatment evaluated by interventional radiologists at the First Affiliated Hospital of Shanxi Medical University. We conducted a 1-year follow-up of these patients. The inclusion criteria were: (1) Meeting the indications for TIPS in liver cirrhosis; (2) TIPS treatment performed according to the guidelines between July 1, 2019 and May 31, 2022; and (3) Age > 18 years. The exclusion criteria (Figure 1) were: (1) Malignant tumors and hematological diseases; (2) Severe infection; (3) TIPS failure; (4) Previous history of splenectomy; and (5) Missing data.
The data involved in this study included: (1) Demographic data [age, gender, and body mass index (BMI)]; (2) Etiology [viral, alcohol, biliary, autoimmune hepatitis (AIH), and others]; (3) Laboratory examinations [white blood cell (WBC) count; platelet (PLT) count; international normalized ratio (INR); alanine aminotransferase (ALT); aspartate aminotransferase (AST); albumin (ALB); total bilirubin (TBIL); urea; and creatinine (CRE)]; (4) Liver cirrhosis scores [Child-Pugh[20] and model for end-stage liver disease (MELD)[21]]; (5) Imaging examinations [spleen volume, spleen length, liver volume, liver spleen volume ratio, portal vein blood flow velocity (PVV), portal vein diameter (PVD), spleen sound touch quantification (SSTQ), and spleen shear wave elastography (SSWE)]; (6) Hemodynamic examination [preoperative portal pressure gradient (PPG), postoperative PPG, PPG decrease value, and PPG decrease percentage]; and (7) Follow-up information: Presence or absence of HE, time of occurrence, and grade.
TIPS procedure was conducted as described previously[16]. All patients were implanted with an 8-mm-diameter poly-tetrafluoroethylene-covered stent (GORE VIATOOR).
The statistical analyses were performed using IBM SPSS (version 26.0) and GraphPad Prism (version 9.0). Continuous variables are expressed as the mean ± SD or median (interquartile range), and categorical variables as frequencies (proportions). The comparison between two groups was conducted using the two independent sample t-test, paired sample t-test, or Mann-Whitney U test. The χ2 test or Fisher’s exact test was used to compare categorical variables. The cumulative incidence of OHE after TIPS was compared using the Kaplan-Meier curve and the log-rank test. Cox regression model was used to evaluate the risk factors for OHE after TIPS. A restricted cubic spline model was utilized to evaluate the dose-response relationship. In all cases, two-sided P < 0.05 was considered statistically significant.
This study included 135 patients with liver cirrhosis who underwent TIPS, with a mean age of 57.8 years. Of the 135 participants, 96 (59.6%) were male. Hepatitis B virus cirrhosis (32.2%) and alcohol-associated cirrhosis (14.9%) were the two most common etiologies of liver cirrhosis. At the time of TIPS, nearly 60% (57.1%) of the enrolled patients had Child-Pugh B liver function.
During the 1-year follow-up period, 37 (27.2%) enrolled patients experienced OHE after TIPS. Based on the severity of OHE, most of the patients were categorized to have West-Haven grade 2 (19 patients, 13.2%), followed by 8 patients (5.9%) with grade 3, and 11 (8.1%) with grade 4. The baseline demographic characteristics and clinical data are detailed in Table 1.
| Parameter | All patients (n = 135) |
| Demographic characteristics | |
| Age, yr | 57.8 ± 24.4 |
| Gender, male | 96 (59.6) |
| BMI | 23.4 ± 9.4 |
| Etiology | |
| Viral (HAV/HBV/HCV) | 3/52/9 (1.8/32.2/5.5) |
| Alcohol | 24 (14.9) |
| Biliary | 15 (9.3) |
| AIH | 7 (4.3) |
| Others | 51 (31.7) |
| Scores | |
| Child-Pugh stage (A/B/C) | 39/92/30 (24.2/57.1/18.6) |
| Child-Pugh score | 7.9 ± 1.8 |
| MELD score | 13.6 ± 5.0 |
| Imaging parameters | |
| Spleen length, mm | 147.3 ± 29.1 |
| Spleen volume, cm3 | 853.0 ± 486.5 |
| Liver volume, cm3 | 1125.0 ± 406.1 |
| Liver/spleen volume | 2.0 ± 2.8 |
| OHE during follow-up | |
| OHE during follow-up | 37 (27.2) |
| OHE grade (2/3/4) | 18/8/11 (13.2/5.9/8.1) |
| Median months | 3 |
| Laboratory parameters | |
| WBC, 109/L | 3.8 ± 3.1 |
| PLT, 109/L | 78.0 ± 66.0 |
| INR | 2.0 ± 5.3 |
| ALT, U/L | 29.1 ± 41.1 |
| AST, U/L | 41.3 ± 71.7 |
| ALB, g/L | 34.3 ± 23.9 |
| TBIL, μmol/L | 35.2 ± 34.7 |
| Urea, mmol/L | 7.3 ± 10.7 |
| CRE, μmol/L | 81.4 ± 104.4 |
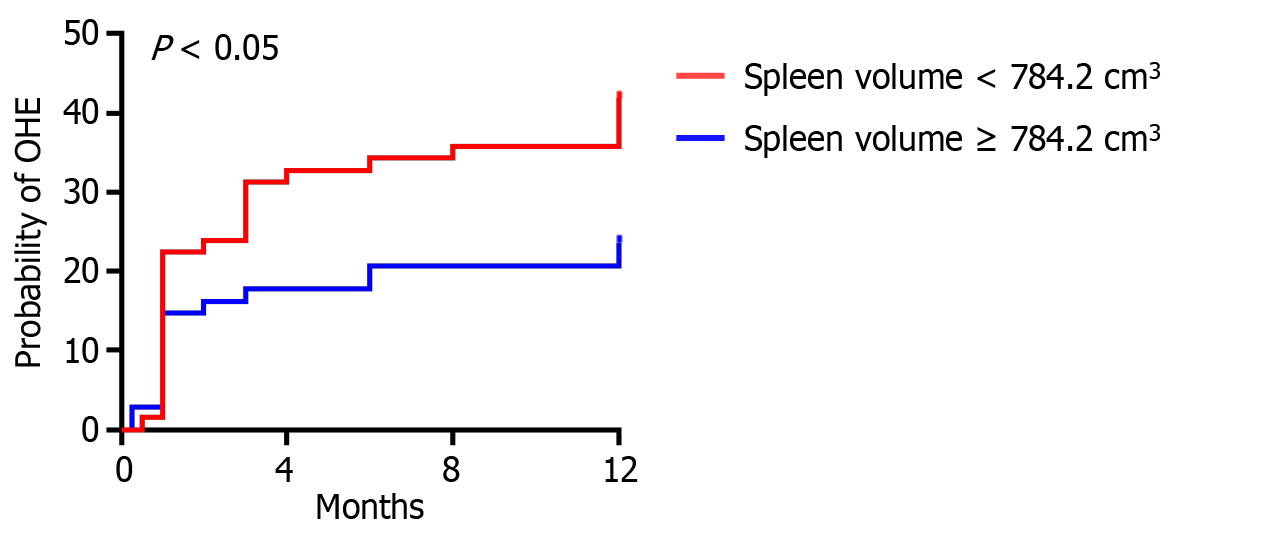
Based on the median splenic volume, we divided the patients into two groups: High splenic volume group (≥ 784.2 cm3) and low splenic volume group (< 784.2 cm3). As shown in Table 2, compared with the high spleen volume group, more patients in the low spleen volume group had OHE (40.3% vs 22.1%, P < 0.05). The results of Kaplan-Meier curve analysis suggested that the incidence rate of OHE was higher in patients with low spleen volume (P < 0.05; Figure 2). In addition, there were statistical differences in BMI, WBC count, and PLT count between the two groups, while there were no statistical differences in indicators such as age, gender, etiology of liver cirrhosis, Child-Pugh class, MELD score, INR, ALT, AST, ALB, TBIL, urea, or CRE between the two groups.
| Spleen volume ≥ 784.2 cm3 (n = 68) | Spleen volume < 784.2 cm3 (n = 67) | P value | |
| Demographic characteristics | |||
| Age, yr | 61.52 ± 0.42 | 57.07 ± 23.51 | 0.3427 |
| Gender, male | 43 (31.9) | 36 (26.7) | 0.2625 |
| BMI | 21.87 ± 4.32 | 23.51 ± 3.49 | < 0.05 |
| Etiology | 0.3532 | ||
| Viral | 26 (38.2) | 25 (37.3) | |
| Alcohol | 10 (14.7) | 10 (14.9) | |
| Biliary | 7 (10.3) | 8 (11.9) | |
| AIH | 3 (4.4) | 2 (3.0) | |
| Others | 22 (32.4) | 23 (34.3) | |
| Scores | |||
| Child-Pugh stage | 0.8530 | ||
| A | 17 (25.0) | 16 (23.9) | |
| B | 36 (53.9) | 38 (56.7) | |
| C | 15 (22.1) | 13 (19.4) | |
| Child-Pugh score | 8.07 ± 1.82 | 7.88 ± 1.82 | 0.5090 |
| MELD score | 13.39 ± 4.36 | 13.93 ± 5.57 | 0.5920 |
| OHE during follow-up | |||
| OHE grade | < 0.05 | ||
| 0/1 | 53 (77.9) | 40 (59.7) | |
| 2 | 7 (10.3) | 11 (16.4) | |
| 3 | 1 (1.5) | 9 (13.4) | |
| 4 | 7 (10.3) | 7 (10.4) | |
| Laboratory parameters | |||
| WBC, 109/L | 3.11 ± 2.63 | 4.18 ± 2.99 | < 0.05 |
| PLT, 109/L | 60.38 ± 57.97 | 91.77 ± 67.64 | < 0.01 |
| INR | 2.63 ± 8.05 | 1.56 ± 1.49 | 0.2941 |
| ALT, U/L | 29.55 ± 36.85 | 29.31 ± 51.22 | 0.9755 |
| AST, U/L | 34.09 ± 33.11 | 49.22 ± 105.00 | 0.2698 |
| ALB, g/L | 37.69 ± 36.24 | 31.80 ± 5.21 | 0.2000 |
| TBIL, μmol/L | 30.53 ± 17.13 | 41.71 ± 48.50 | 0.0845 |
| Urea, mmol/L | 5.61 ± 2.94 | 9.42 ± 15.95 | 0.0734 |
| CRE, μmol/L | 79.27 ± 82.35 | 78.97 ± 106.40 | 0.9861 |
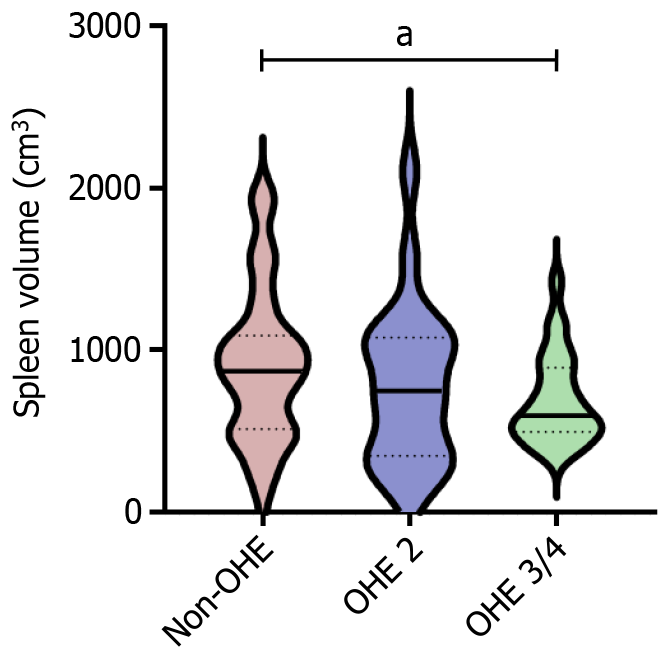
According to the West-Haven criteria, the severity of OHE was graded. We then compared the laboratory examination indicators and spleen measurement data between patients with different grades of OHE (Table 3 and Figure 3). The results showed that compared to non-OHE patients, those with high stage OHE (grades 3 + 4) had a smaller spleen volume (696.60 vs 901.30, P < 0.05). Compared with the preoperative value, spleen volume significantly decreased when OHE occurred after TIPS (Figure 4). The above results suggest that there is a close relationship between spleen volume and the incidence of OHE after TIPS, and preoperative low spleen volume is more likely to lead to post-TIPS OHE.
| Non-OHE (n = 99) | OHE/2 (n = 18) | OHE/3+4 (n = 19) | P value | |
| WBC, 109/L | 4.05 ± 3.44 | 2.51 ± 1.76 | 4.01 ± 2.44 | 0.1056 |
| PLT, 109/L | 78.61 ± 68.29 | 75.53 ± 75.37 | 74.96 ± 50.93 | 0.9579 |
| INR | 1.54 ± 1.39 | 1.38 ± 0.16 | 1.45 ± 0.23 | 0.8172 |
| ALT, U/L | 27.16 ± 29.84 | 17.29 ± 9.22 | 45.81 ± 78.55 | < 0.05 |
| AST, U/L | 35.91 ± 30.42 | 26.66 ± 19.07 | 74.35 ± 161.60 | < 0.05 |
| ALB, g/L | 35.21 ± 29.03 | 33.20 ± 4.87 | 32.03 ± 5.72 | 0.8144 |
| TBIL, μmol/L | 35.50 ± 39.82 | 28.60 ± 23.45 | 41.71 ± 31.00 | 0.4455 |
| Urea, mmol/L | 7.10 ± 9.17 | 9.78 ± 21.02 | 6.00 ± 3.30 | 0.4998 |
| CRE, μmol/L | 90.79 ± 127.20 | 66.29 ± 17.34 | 60.07 ± 16.12 | 0.3309 |
| Spleen length, mm | 150.90 ± 27.74 | 141.10 ± 35.44 | 143.00 ± 27.09 | 0.2619 |
| Spleen volume, cm3 | 901.30 ± 471.90 | 882.30 ± 704.60 | 697.60 ± 281.00 | < 0.05 |
| Liver volume, cm3 | 1120.00 ± 360.80 | 1070.00 ± 456.00 | 1153.00 ± 534.60 | 0.1891 |
| Liver/spleen volume | 2.14 ± 3.07 | 2.59 ± 2.41 | 1.66 ± 1.08 | 0.5066 |
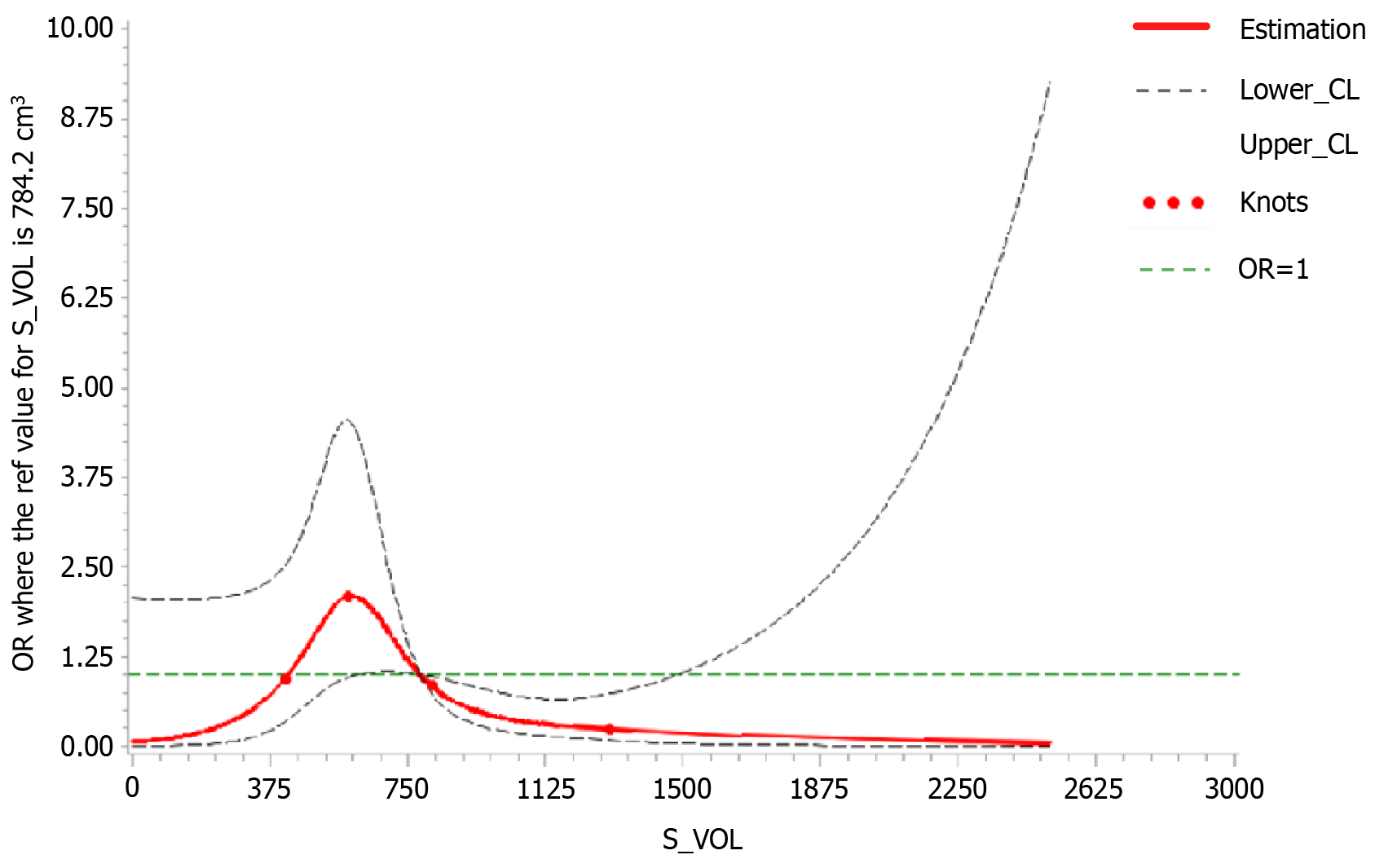
To further determine that spleen volume is an important factor affecting the occurrence of OHE, we used a Cox regression model to evaluate whether spleen volume is an independent risk factor affecting the occurrence of OHE in Table 4. Univariate Cox regression analysis demonstrated that higher age [hazard ratio (HR) = 1.762 (1.043-3.230), P < 0.05] and lower spleen volume [HR = 0.537 (0.283-0.986), P < 0.05] of patients predicted the incidence of OHE. Multivariate Cox regression analysis suggested that lower spleen volume could be an independent risk factor for OHE after excluding other factors [HR = 0.494 (0.234-0.929), P < 0.05]. Next, we used a restricted cubic spline model to further confirm the correlation between spleen volume and increased OHE risk by assessing the dose-response relationship (Figure 5). The results showed that with increasing spleen volume, the curve of post-TIPS OHE risk followed a first rising and then decreasing trend (P < 0.05).
| Univariable Cox | Multivariable Cox | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (≥ 60 yr) | 1.762 (1.043-3.230) | 0.049 | 1.473 (0.834-3.929) | 0.118 |
| Gender | 0.854 (0.449-1.575) | 0.618 | ||
| BMI (≥ 24.0) | 0.689 (0.331-1.329) | 0.288 | ||
| Etiology (HBV) | 1.799 (0.945-3.321) | 0.065 | ||
| Child-Pugh score | 1.259 (0.587-2.470) | 0.525 | ||
| MELD score | 1.279 (0.682-2.482) | 0.451 | ||
| Spleen volume | 0.537 (0.283-0.986) | 0.049 | 0.494 (0.234-0.929) | 0.042 |
| WBC, 109/L | 1.129 (0.616-2.095) | 0.696 | ||
| PLT, 109/L | 0.936 (0.512-1.707) | 0.828 | ||
To the best of our knowledge, we for the first time found a significant correlation between spleen volume and OHE risk after TIPS. Patients with a smaller preoperative spleen volume have a higher risk of OHE post-TIPS, which is different from the report of OHE in cirrhosis[22]. Patel et al[22] reported that compared with the normal liver group, the spleen volume of patients with liver cirrhosis significantly increased, but there was no correlation between spleen size and HE. We speculate that the pathogenesis of OHE after TIPS may not be the same as that of OHE in cirrhosis.
To investigate the pathogenesis of OHE after TIPS, we included multiple potential influencing factors such as age, gender, BMI, and spleen volume in the univariate and multivariate Cox regression analyses. The results showed that age and spleen volume had statistical significance in univariate Cox regression analysis. In multivariate Cox regression analysis, spleen volume was identified to be the main influencing factor of OHE after TIPS. Previous studies on the relationship between the spleen and postoperative HE after TIPS have not been extensive. Liu et al[23] found that the combination of Child-Pugh score and quantitative CT spleen volume can predict postoperative OHE in TIPS. Chen et al[24] used three-dimensional models based on liver and spleen indicators to predict the risk of OHE after TIPS, providing guidance for patients undergoing TIPS. Thus, spleen volume may serve as an independent risk factor for postoperative OHE after TIPS.
To explore the reasons why changes in spleen volume can affect postoperative OHE in TIPS, we investigated the differences in portal vein components before and after TIPS, as well as among individuals with different spleen volumes. First, we analyzed the changes in the components based on laboratory testing. Comparing the relevant indicators of the same patient before and after TIPS, it was found that spleen volume significantly decreased after TIPS, which is consistent with literature reports[23]. This indicates that TIPS leads to a decrease in portal vein pressure, which in turn leads to a decrease in spleen pressure and changes in liver hemodynamics. The spleen, as an immune organ, may undergo changes in its inflammatory microenvironment due to changes in spleen volume and hardness[25]. In addition, some inflammation-related factors, such as IL-6[16], are closely related to HE after TIPS. Therefore, the impact of TIPS on the inflammatory microenvironment of the spleen may be one of the reasons for the occurrence of HE after TIPS. However, in our study, WBC count, PLT count, and other indicators related to immune inflammation did not show significant changes in OHE after TIPS surgery (Supplementary Table 1). This may indicate that changes in portal vein components due to changes in spleen volume are not the main cause of OHE after TIPS.
In addition to portal vein components, we also analyzed the changes in portal vein flow velocity[26,27] and spleen hardness[28,29]. We compared the PVV, PVD, SSTQ, and SSWE before and after surgery in patients with OHE after TIPS. The results showed that the PPV of the small-volume spleen group was significantly reduced, while PVD, SSTQ, and SSWE did not show significant changes (Supplementary Figure 1). Therefore, changes in portal vein flow velocity due to changes in spleen volume may be the main cause of OHE after TIPS. Based on this, unlike the traditional definitions of type B and type C OHE, we believe that postoperative OHE in TIPS tends to be a combination of type B and type C.
In addition, this study investigated the differences in spleen size and laboratory indicators among patients with OHE of different etiologies and severities of liver cirrhosis. The study found that there was no significant change in spleen volume among different etiologies (Supplementary Table 2) and severities of liver cirrhosis (Supplementary Tables 3 and 4). In addition to spleen volume, we also compared laboratory indicators. The results showed significant statistical differences in WBC count and PLT count among different etiologies, with a significant increase in AIH type. Compared with Child-Pugh A and B groups, Child-Pugh C group had the highest WBC count and TBIL (Supplementary Table 3). In the MELD grading, the cutoff value was 12, and the comparison results showed that there was no statistically significant difference in the indicators between patients with different MELD grades (Supplementary Table 4). The above results indicate that changes in spleen volume are not affected by the etiology and severity of liver cirrhosis, and there are differences in some laboratory indicators. There is no direct correlation between spleen volume and some indicators. It should be noted that some of the results are different from previous studies[30,31], perhaps mainly due to the inclusion of patients with portal hypertension who met the indications for TIPS.
The advantage of our research was that each case had complete preoperative clinical, laboratory, and imaging data. We also acknowledged the limitations of our research. First, this was a single center retrospective study without validation from a multicenter prospective cohort study. Second, some cases lacked immediate CT and laboratory examinations during the onset of HE after TIPS. Nevertheless, we believe that our research findings have clinical implications for post-TIPS OHE in patients with liver cirrhosis.
In conclusion, in a well-characterised patient population, we showed that spleen volume correlates significantly with post-TIPS OHE. Spleen volume can serve as an independent non-invasive predictor of OHE after TIPS.
| 1. | Dib N, Oberti F, Calès P. Current management of the complications of portal hypertension: variceal bleeding and ascites. CMAJ. 2006;174:1433-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Guixé-Muntet S, Quesada-Vázquez S, Gracia-Sancho J. Pathophysiology and therapeutic options for cirrhotic portal hypertension. Lancet Gastroenterol Hepatol. 2024;9(7):646-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB; Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022;20:1636-1662.e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 4. | Larrue H, D'Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, Sauerbruch T, Hernandez-Gea V, Han G, Reiberger T, Thabut D, Vinel JP, Péron JM, García-Pagán JC, Bureau C. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 5. | Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, Casanovas G, Bosch J, Lv Y, Thabut D, Fan D, Hernández-Gea V, García-Pagán JC; Preemptive TIPS Individual Data Metanalysis, International Variceal Bleeding Study and Baveno Cooperation Study groups. Effects of Early Placement of Transjugular Portosystemic Shunts in Patients With High-Risk Acute Variceal Bleeding: a Meta-analysis of Individual Patient Data. Gastroenterology. 2021;160:193-205.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 6. | Jin YN, Zhang W. Transjugular intrahepatic portosystemic shunt: A promising therapy for recompensation in cirrhotic patients. World J Gastroenterol. 2024;30:2285-2286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (3)] |
| 7. | Melandro F, Parisse S, Ginanni Corradini S, Cardinale V, Ferri F, Merli M, Alvaro D, Pugliese F, Rossi M, Mennini G, Lai Q. Transjugular Intrahepatic Portosystemic Shunt as a Bridge to Abdominal Surgery in Cirrhosis. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Lv Y, Chen H, Luo B, Bai W, Li K, Wang Z, Xia D, Guo W, Wang Q, Li X, Yuan J, Cai H, Xia J, Yin Z, Fan D, Han G. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A randomized controlled trial. Hepatology. 2022;76:676-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Schindler P, Heinzow H, Trebicka J, Wildgruber M. Shunt-Induced Hepatic Encephalopathy in TIPS: Current Approaches and Clinical Challenges. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2:100122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Ridola L, Faccioli J, Nardelli S, Gioia S, Riggio O. Hepatic Encephalopathy: Diagnosis and Management. J Transl Int Med. 2020;8:210-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Nolte W, Wiltfang J, Schindler C, Münke H, Unterberg K, Zumhasch U, Figulla HR, Werner G, Hartmann H, Ramadori G. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology. 1998;28:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Li W, Duan Y, Liu Z, Lu X, She J, Qing J, Zhang C. Clinical value of hemodynamic changes in diagnosis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Scand J Gastroenterol. 2022;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 14. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Dajti E, Ravaioli F, Zykus R, Rautou PE, Elkrief L, Grgurevic I, Stefanescu H, Hirooka M, Fraquelli M, Rosselli M, Chang PEJ, Piscaglia F, Reiberger T, Llop E, Mueller S, Marasco G, Berzigotti A, Colli A, Festi D, Colecchia A; Spleen Stiffness—IPD-MA Study Group. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 16. | Li J, Liu Y, Li M, Rong X, Yuan Z, Ren C, Liu S, Li L, Zhao C, Gao L, Feng D. Association of preoperative IL-6 levels with overt HE in patients with cirrhosis after TIPS. Hepatol Commun. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Romero-Cristóbal M, Clemente-Sánchez A, Ramón E, Téllez L, Canales E, Ortega-Lobete O, Velilla-Aparicio E, Catalina MV, Ibáñez-Samaniego L, Alonso S, Colón A, Matilla AM, Salcedo M, Albillos A, Bañares R, Rincón D. CT-derived liver and spleen volume accurately diagnose clinically significant portal hypertension in patients with hepatocellular carcinoma. JHEP Rep. 2023;5:100645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Yu Q, Xu C, Li Q, Ding Z, Lv Y, Liu C, Huang Y, Zhou J, Huang S, Xia C, Meng X, Lu C, Li Y, Tang T, Wang Y, Song Y, Qi X, Ye J, Ju S. Spleen volume-based non-invasive tool for predicting hepatic decompensation in people with compensated cirrhosis (CHESS1701). JHEP Rep. 2022;4:100575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 19. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1507] [Article Influence: 502.3] [Reference Citation Analysis (2)] |
| 20. | Johnson PJ, Pinato DJ, Kalyuzhnyy A, Toyoda H. Breaking the Child-Pugh Dogma in Hepatocellular Carcinoma. J Clin Oncol. 2022;40:2078-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Pagliaro L. MELD: the end of Child-Pugh classification? J Hepatol. 2002;36:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Patel M, Tann M, Liangpunsakul S. CT-scan Based Liver and Spleen Volume Measurement as a Prognostic Indicator for Patients with Cirrhosis. Am J Med Sci. 2021;362:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 23. | Liu J, Ma J, Yang C, Ye T, Meng J, Shi Q, Xiong B. Impact of TIPS on Splenic Volume and Thrombocytopenia. AJR Am J Roentgenol. 2021;216:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Chen X, Wang T, Ji Z, Luo J, Lv W, Wang H, Zhao Y, Duan C, Yu X, Li Q, Zhang J, Chen J, Zhang X, Huang M, Zhou S, Lu L, Huang M, Fu S. 3D automatic liver and spleen assessment in predicting overt hepatic encephalopathy before TIPS: a multi-center study. Hepatol Int. 2023;17:1545-1556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 25. | Chien CH, Lin YL, Chien RN, Hu CC, Yen CL, Lee TS, Hsieh PJ, Lin CL. Transient Elastography for Spleen Stiffness Measurement in Patients With Cirrhosis: Role in Degree of Thrombocytopenia. J Ultrasound Med. 2016;35:1849-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Hu XG, Dai JJ, Lu J, Li G, Wang JM, Deng Y, Feng R, Lu KP. Efficacy of transjugular intrahepatic portosystemic shunts in treating cirrhotic esophageal-gastric variceal bleeding. World J Gastrointest Surg. 2024;16:471-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Han H, Yang J, Jin WK, Li X, Zhang F, Zhuge YZ, Wu M, Yang B. Diagnostic value of conventional ultrasound and shear wave elastography in detecting transjugular intrahepatic portosystemic shunt dysfunction. Acta Radiol. 2021;62:1575-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Jansen C, Bogs C, Verlinden W, Thiele M, Möller P, Görtzen J, Lehmann J, Vanwolleghem T, Vonghia L, Praktiknjo M, Chang J, Krag A, Strassburg CP, Francque S, Trebicka J. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: A prospective multicentre study. Liver Int. 2017;37:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Franchi-Abella S, Corno L, Gonzales E, Antoni G, Fabre M, Ducot B, Pariente D, Gennisson JL, Tanter M, Corréas JM. Feasibility and Diagnostic Accuracy of Supersonic Shear-Wave Elastography for the Assessment of Liver Stiffness and Liver Fibrosis in Children: A Pilot Study of 96 Patients. Radiology. 2016;278:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Kashani A, Salehi B, Anghesom D, Kawayeh AM, Rouse GA, Runyon BA. Spleen size in cirrhosis of different etiologies. J Ultrasound Med. 2015;34:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Cacciottolo TM, Kumar A, Godfrey EM, Davies SE, Allison M. Spleen Size Does Not Correlate With Histological Stage of Liver Disease in People With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2023;21:535-537.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |