Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2031
Revised: April 11, 2024
Accepted: May 27, 2024
Published online: July 27, 2024
Processing time: 151 Days and 9 Hours
The consistency of pancreatic apparent diffusion coefficient (ADC) values and intravoxel incoherent motion (IVIM) parameter values across different magnetic resonance imaging (MRI) devices significantly impacts the patient’s diagnosis and treatment.
To explore consistency in image quality, ADC values, and IVIM parameter values among different MRI devices in pancreatic examinations.
This retrospective study was approved by the local ethics committee, and informed consent was obtained from all participants. In total, 22 healthy volunteers (10 males and 12 females) aged 24-61 years (mean, 28.9 ± 2.3 years) underwent pancreatic diffusion-weighted imaging using 3.0T MRI equipment from three vendors. Two independent observers subjectively scored image quality and measured the pancreas’s overall ADC values and signal-to-noise ratios (SNRs). Subsequently, regions of interest (ROIs) were delineated for the IVIM parameters (true diffusion coefficient, pseudo-diffusion coefficient, and perfusion fraction) using post-processing software. These ROIs were on the head, body, and tail of the pancrease. The subjective image ratings were assessed using the kappa consistency test. Intraclass correlation coefficients (ICCs) and mixed linear models were used to evaluate each device’s quantitative parameter values. Finally, a pairwise analysis of IVIM parameter values across each device was performed using Bland-Altman plots.
The Kappa value for the subjective ratings of the different observers was 0.776 (P < 0.05). The ICC values for inter-observer and intra-observer agreements for the quantitative parameters were 0.803 [95% confidence interval (CI): 0.684-0.880] and 0.883 (95%CI: 0.760-0.945), respectively (P < 0.05). The ICCs for the SNR between different devices was comparable (P > 0.05), and the ICCs for the ADC values from different devices were 0.870, 0.707, and 0.808, respectively (P < 0.05). Notably, only a few statistically significant inter-device agreements were observed for different IVIM parameters, and among those, the ICC values were generally low. The mixed linear model results indicated differences (P < 0.05) in the f-value for the pancreas head, D-value for the pancreas body, and D-value for the pancreas tail obtained using different MRI machines. The Bland-Altman plots showed significant variability at some data points.
ADC values are consistent among different devices, but the IVIM parameters’ repeatability is moderate. Therefore, the variability in the IVIM parameter values may be associated with using different MRI machines. Thus, caution should be exercised when using IVIM parameter values to assess the pancreas.
Core Tip: The purpose of this study was to investigate whether the intravoxel incoherent motion (IVIM) parameter values of the normal pancreas are consistent when imaging with different magnetic resonance imaging (MRI) devices, and it was found that the variability of IVIM parameter values may be related to the use of different MRI machines.
- Citation: Liu X, Wang YF, Qi XH, Zhang ZL, Pan JY, Fan XL, Du Y, Zhai YM, Wang Q. Reproducibility study of intravoxel incoherent motion and apparent diffusion coefficient parameters in normal pancreas. World J Gastrointest Surg 2024; 16(7): 2031-2039
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2031.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2031
The microscopic movement of water in biological tissues typically includes the diffusion of water molecules and microcirculation perfusion. Diffusion-weighted imaging (DWI) is sensitive to proton displacement due to random Brownian motion and the overall displacement of protons within the capillaries[1]. In 1986, Le Bihan et al[2] introduced the concept of intravoxel incoherent motion (IVIM), which uses a bi-exponential decay model to capture tissue displacements attributed to microcirculatory perfusion and water molecule diffusion. IVIM involves acquiring DWI sequences with multiple b-values, which are processed using a bi-exponential model to obtain quantitative parameters, including the true diffusion coefficient (D), pseudo-diffusion coefficient associated with microperfusion, and perfusion fraction (f). These parameters are used to quantitatively analyze human tissues’ microstructural and pathophysiological status.
The increased magnetic resonance imaging (MRI) field strength and related technological innovations have recently led to a steady growth in IVIM analysis research. IVIM analysis is used to study human physiological and pathological processes, including the differentiation of benign and malignant tumors, pathological grading of malignant tumors, prognosis prediction, and many other aspects[3-6]. Moreover, research on IVIM sequence application in pancreatic diseases has also increased. IVIM multi-parameter analysis provides quantitative parameters for assessing pancreatic fat degeneration and fibrosis[7] and distinguishes between normal pancreatic tissue, pancreatic cancer, and pancreatic neuroendocrine tumors[8-10]. Furthermore, it has shown significant value for assessing the degree of pancreatic cancer differentiation[11] and predicting pancreatic cancer resectability[12].
A prerequisite for translating this growing research interest in IVIM multi-parameter analysis into clinical applications is its repeatability in different clinical settings. In clinical practice, the pancreas, a retroperitoneal organ, is often affected by respiratory motion and peristalsis during imaging. Moreover, different hospitals may have various MRI equipment, and even within the same hospital, different MRI devices may be introduced. The consistency of pancreatic IVIM parameter values across different MRI devices significantly impacts the patient’s diagnosis and treatment. Therefore, this study aimed to investigate whether the IVIM parameter values of the normal pancreas are consistent when imaged using different MRI devices.
This study included 22 physically healthy volunteers (10 males and 12 females) aged 24-61 years (mean: 28.9 ± 2.3 years). The inclusion criteria were as follows: (1) No history of drug or alcohol abuse; (2) no history of pancreatitis, diabetes, alcoholism, or abdominal surgery; and (3) no MRI contraindications (such as pacemaker implantation, metal implants, and claustrophobia). The exclusion criteria were as follows: (1) Failure to complete the DWI image examination; and (2) poor image quality (such as motion artifacts), which is insufficient for analysis. This prospective study was approved by the local ethics committee, and informed consent was obtained from all participants.
Before the examination, each volunteer fasted for 4-6 h. Subsequently, they were examined sequentially using three different 3.0T MRI devices denoted as A for the 3.0T Siemens Skyra, B for the 3.0T Philips Ingenia CX, and C for the domestic 3.0T United Imaging 780. In total, 66 examinations were performed (three examinations per volunteer). The volunteers were placed in a supine position, and the scanning range included the entire pancreas. The scan slice thickness was 5 mm for all MRI devices, and the scan sequences included T1-weighted Dixon, T2-weighted fat suppression, and plain IVIM sequences. IVIM sequence parameter values were calculated based on 12 b-values: 0, 10, 20, 30, 40, 60, 80, 100, 200, 500, 800, and 1200 s/mm². The number of excitations (NEX) for different b-values was as follows: NEX = 1 for b-values of 0-100 s/mm2, NEX = 2 for 200 s/mm2, NEX = 3 for 500 s/mm2, NEX = 5 for 800 s/mm2, and NEX = 6 for 1200 s/mm2. The settings of the different machines are listed in Table 1.
| Model | TR (ms) | TE (ms) | FOV (mm) | Matrix | Lamination thickness (mm) | Scanning time (min:s) |
| A | 6700 | 63 | 380 × 306 | 108 × 134 | 5 | 8:35 |
| B | 1897 | 75 | 380 × 297 | 128 × 98 | 5 | 3:36 |
| C | 4294 | 67 | 380 × 300 | 128 × 100 | 5 | 4:57 |
After completing the MRI examinations, two professionally trained radiologists experienced in diagnostic imaging subjectively and objectively assessed the images at a b-value of 1200 s/mm2. A 5-point rating scale was used for the subjective assessment of image quality, where 1 point represents the poorest and 5 points represent the best, with increasing degrees of quality from points 1 to 5.
Signal-to-noise ratio (SNR) is an objective assessment metric for image quality; it is calculated using the formula SNR = [S - average value (Sb)]/SD, where S represents the average signal value within the pancreatic region of interest (ROI), Sb represents the average background signal value, and SD represents the standard deviation of the signal within the pancreatic ROI. Images with a b-value of 1200 s/mm2 were selected to measure the pancreatic ROI signal average values, and circular ROIs with an area of approximately 50 mm² were drawn at three locations: The pancreatic head (PaH), pancreatic body (PaB), and pancreatic tail (PaT). The average values and standard deviations of the signal for each ROI were recorded, and the average values of the three ROIs were calculated. Circular regions were drawn in each of the four corners within the imaging field to measure the background signal values. Each region’s average signal values were recorded, and the Sb for the four regions was calculated. The SNR of the images was calculated using the formula above.
Parameter values were measured on different devices at their respective post-processing workstations. Images with b-values of 0 and 1200 s/mm2 were selected to generate the apparent diffusion coefficient (ADC) maps. The ADC values were measured separately for the PaH, PaB, and PaT, and the overall average ADC value for the pancreas was calculated. The delineation of the IVIM sequence ROI and the calculation of the parameter values were performed using the MITK-diffusion post-processing software of different devices to obtain the true D, false diffusion coefficient (D*), and f values. D represents the effect of pure molecular diffusion within a voxel, D* represents the diffusion effect associated with microcirculation perfusion, and f represents the volume fraction of the diffusion effects associated with microcirculation perfusion within a voxel, expressed as a percentage. Owing to the differences in the IVIM parameter values in different parts of the pancreas[13], the ROIs were delineated at the best-displayed levels for the PaH, PaB, and PaT. ROIs were drawn on axial images at b = 0 s/mm², with an area of 50 mm². The common bile duct and main pancreatic duct were avoided during the delineation. Notably, all quantitative parameter measurements were performed by two experienced radiologists, and one repeated the measurements for all quantitative parameters 1 mo later.
SAS 9.4 software, R language, and SPSS 26.0 were used for data collection and statistical analyses. Kappa analysis was used to compare the consistency of the subjective image quality scores between the observers. Kappa ≥ 0.75 indicated good consistency, 0.75 > Kappa ≥ 0.4 indicated general consistency, and kappa < 0.4 indicated poor consistency between the two observers. The intraclass correlation coefficient (ICC) was used to analyze the parameters between different observers and between two measurements from the same observer. Good consistency was indicated by ≥ 0.75, and 0.40-0.75 indicated moderate consistency. A value of < 0.40 indicated poor consistency.
The consistency of image SNR, ADC, and IVIM parameter values among different MRI devices was analyzed using ICC. The mean ± SD method was used to describe the IVIM parameter data between the different MRI devices. Differences between different devices were compared using a mixed linear model, and post-hoc pairwise comparisons were adjusted using the Bonferroni method, where the adjusted P-values were calculated by multiplying the original P-values by 3. Finally, Bland-Altman plots were used to evaluate the consistency of IVIM measurements across different devices. All statistical analyses and graphical representations were performed using GraphPad Prism 9.3 software, and statistical significance was set at P < 0.05.
The Kappa value for the subjective image quality assessment among the observers was 0.776 (P < 0.05). The ICC for all quantitative parameter values among different observers was 0.803 [95% confidence interval (CI): 0.684-0.880, P < 0.05]. The ICC for the quantitative parameter values measured by the same observer on two different occasions was 0.883 (95%CI: 0.760-0.945, P < 0.05).
Consistency analysis results of SNR and ADC values between different devices: The mean SNR values for MRI devices A, B, and C were 14.2 ± 3.1, 11.9 ± 2.4, and 15.5 ± 3.6, respectively. Device C had the highest SNR among the three devices. The ICC between different devices was not significant (P > 0.05; Table 2). The ICCs of the ADC values of devices A, B, and C were 0.870, 0.707, and 0.808, respectively (P < 0.05) (Table 2). The ICC between devices A and C was between 0.4 and 0.75, indicating that the repeatability of ADC value measurements between the two devices is moderate. However, for the comparisons between devices A and B and between devices B and C, the ICC was > 0.75, indicating good repeatability in ADC measurements between these device pairs.
| Machine A vs machine B | Machine A vs machine C | Machine B vs machine C | ||||
| ICC (95%CI) | P value | ICC (95%CI) | P value | ICC (95%CI) | P value | |
| D in PaH | 0.274 (-0.731, 0.697) | 0.232 | 0.342 (-0.411, 0.712) | 0.145 | 0.728 (0.358, 0.886) | 0.002 |
| D* in PaH | -0.186 (-1.545, 0.482) | 0.670 | 0.27 (-0.611, 0.685) | 0.220 | 0.578 (0.02, 0.822) | 0.022 |
| f in PaH | -0.209 (-1.818, 0.491) | 0.673 | 0.173 (-0.386, 0.582) | 0.272 | 0.19 (-0.541, 0.622) | 0.274 |
| D in PaB | 0.456 (-0.332, 0.776) | 0.089 | 0.683 (-0.026, 0.886) | 0.028 | 0.551 (-0.079, 0.814) | 0.037 |
| D* in PaB | -0.521 (-3.056, 0.393) | 0.815 | -0.023 (-1.636, 0.587) | 0.519 | -0.256 (-2.207, 0.491) | 0.691 |
| f in PaB | 0.385 (-0.407, 0.739) | 0.124 | -0.087 (-1.441, 0.534) | 0.580 | -0.332 (-2.715, 0.515) | 0.748 |
| D in PaT | 0.324 (-0.614, 0.718) | 0.186 | 0.286 (-0.557, 0.69) | 0.201 | 0.908 (0.781, 0.961) | < 0.001 |
| D* in PaT | 0.569 (-0.204, 0.836) | 0.060 | -0.289 (-2.251, 0.475) | 0.713 | -0.062 (-1.02, 0.504) | 0.569 |
| f in PaT | 0.531 (-0.144, 0.806) | 0.047 | 0.35 (-0.633, 0.735) | 0.174 | 0.486 (-0.275, 0.789) | 0.073 |
| SNR | 0.081 (-0.206, 0.421) | 0.312 | 0.178 (-0.238, 0.551) | 0.205 | 0.067 (-0.152, 0.364) | 0.301 |
| ADC value | 0.870 (0.691, 0.946) | < 0.001 | 0.707 (0.393, 0.871) | < 0.001 | 0.808 (0.590, 0.917) | < 0.001 |
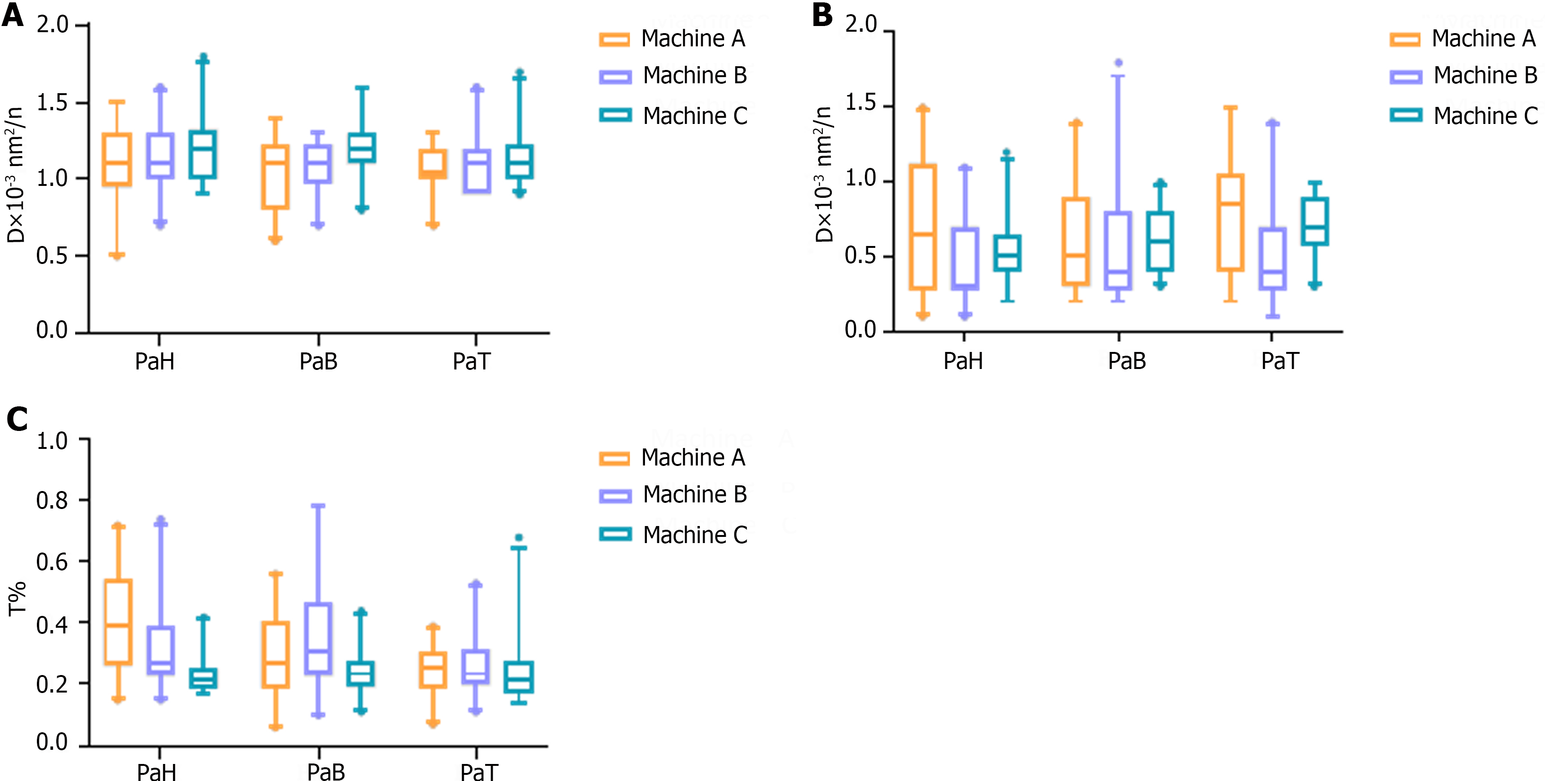
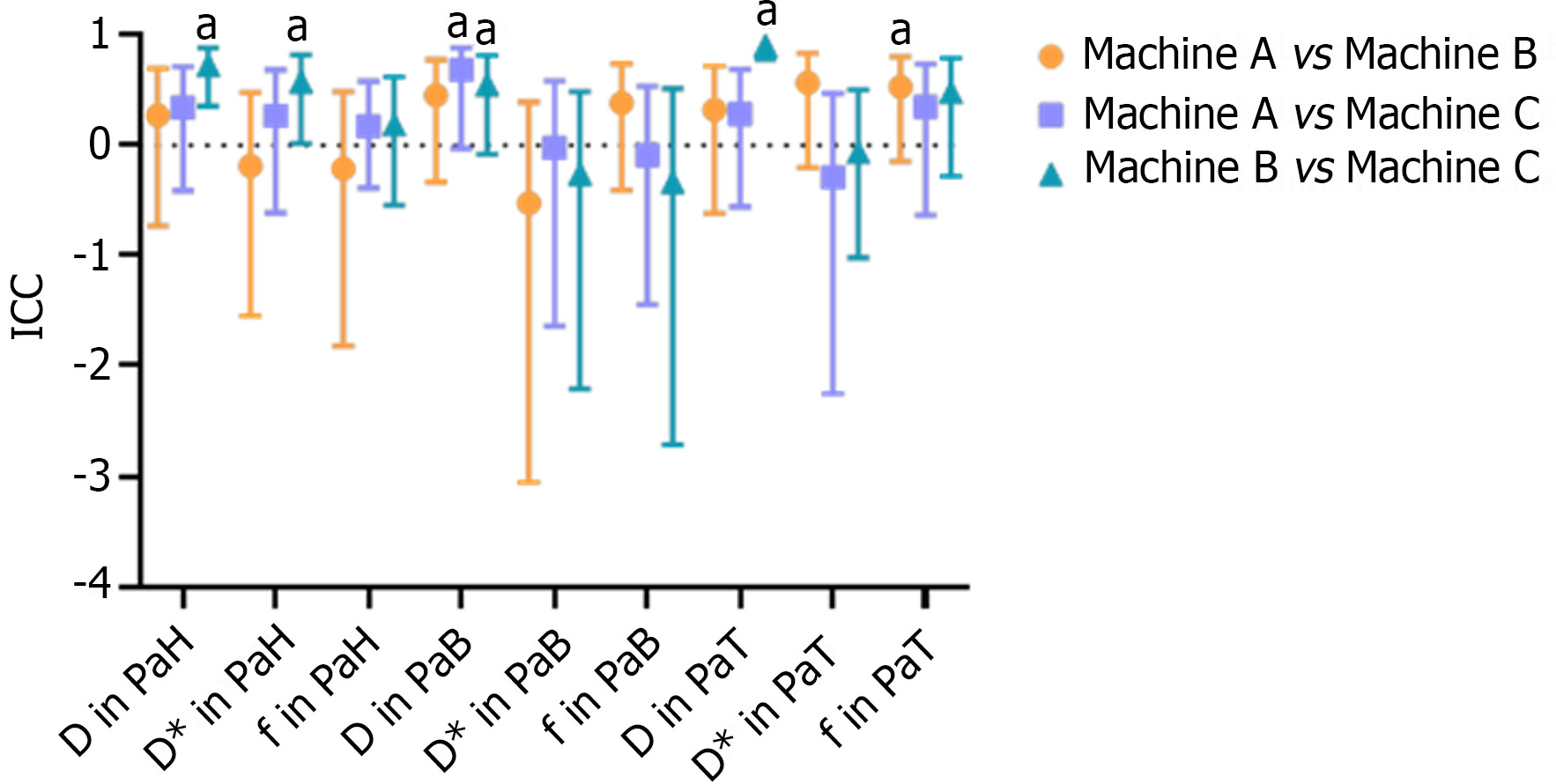
ICC results of IVIM parameters between different devices: The D, D*, and f values of the PaH, PaB, and PaT on different devices are statistically described using box plots (Figure 1). According to the ICC results, only a few differences between the devices were statistically significant. Devices A and B were statistically significant only for the f value of the PaT, with an ICC of 0.531 (P < 0.05). The difference in the D value of the PaB between devices A and C was statistically significant, and the ICC was 0.683 (P < 0.05). Devices B and C had more statistically significant differences. The two devices were statistically significant for the D and D* values of the PaH, D value of the PaB, and D value of the PaT. The ICC obtained was 0.728, 0.578, 0.551, and 0.908, respectively (P < 0.05). Similarly, from the ICC results, in the above statistically significant items, only the ICC of the D values of the PaT on devices B and C were > 0.75, and the ICC of the remaining items were between 0.4 and 0.75 (Table 2 and Figure 2).
Analysis of differences in IVIM parameter values among different pancreatic regions on different machines: The mixed linear model analysis results showed differences in the f value of the PaH, the D value of the PaB, and the D* value of the PaT among different devices (P < 0.05). The f value of the PaH on device C was lower than those on devices A and B (P < 0.05). The D value of the PaB on device C was higher than those on devices A and B (P < 0.05). The D* value of the PaT on device B was lower than that on device A (P < 0.05) (Table 3).
| Region and parameter | Machine A | Machine B | Machine C | Mixed liner model | Post-hoc test | 95% limits of agreement | ||
| F | P value | Pair comparison | P1 value | |||||
| PaH | ||||||||
| D | 1.08 ± 0.27 | 1.15 ± 0.22 | 1.22 ± 0.23 | 2.893 | 0.078 | A vs B | 0.986 | -0.56, 0.38 |
| A vs C | 0.140 | -0.47, 0.44 | ||||||
| B vs C | 0.311 | -0.32, 0.27 | ||||||
| D* | 0.07 ± 0.04 | 0.05 ± 0.03 | 0.05 ± 0.02 | 2.275 | 0.128 | A vs B | 0.139 | -0.13, 0.03 |
| A vs C | 0.327 | -0.11, 0.03 | ||||||
| B vs C | 0.531 | -0.05, 0.04 | ||||||
| f | 0.40 ± 0.18 | 0.33 ± 0.15 | 0.23 ± 0.07 | 15.725 | 0.001 | A vs B | 0.483 | -0.57, 0.16 |
| A vs C | 0.001 | -0.52, 0.00 | ||||||
| B vs C | 0.030 | -0.40, 0.06 | ||||||
| PaB | ||||||||
| D | 1.05 ± 0.24 | 1.09 ± 0.18 | 1.21 ± 0.20 | 10.060 | 0.001 | A vs B | 0.999 | -0.46, 0.27 |
| A vs C | 0.003 | -0.21, 0.33 | ||||||
| B vs C | 0.027 | -0.27, 0.31 | ||||||
| D* | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.06 ± 0.02 | 0.228 | 0.798 | A vs B | 0.999 | -0.12, 0.05 |
| A vs C | 0.999 | -0.08, 0.04 | ||||||
| B vs C | 0.999 | -0.09, 0.05 | ||||||
| f | 0.30 ± 0.15 | 0.37 ± 0.20 | 0.24 ± 0.07 | 3.174 | 0.062 | A vs B | 0.513 | -0.36, 0.27 |
| A vs C | 0.420 | -0.39, 0.11 | ||||||
| B vs C | 0.066 | -0.57, 0.10 | ||||||
| PaT | ||||||||
| D | 1.07 ± 0.16 | 1.12 ± 0.22 | 1.15 ± 0.18 | 2.438 | 0.112 | A vs B | 0.999 | -0.42, 0.28 |
| A vs C | 0.258 | -0.34, 0.29 | ||||||
| B vs C | 0.601 | -0.19, 0.14 | ||||||
| D* | 0.08 ± 0.04 | 0.05 ± 0.03 | 0.07 ± 0.02 | 9.451 | 0.001 | A vs B | 0.002 | -0.10, 0.00 |
| A vs C | 0.999 | -0.11, 0.04 | ||||||
| B vs C | 0.058 | -0.06, 0.06 | ||||||
| f | 0.25 ± 0.08 | 0.26 ± 0.10 | 0.25 ± 0.12 | 0.191 | 0.827 | A vs B | 0.999 | -0.20, 0.12 |
| A vs C | 0.999 | -0.25, 0.13 | ||||||
| B vs C | 0.999 | -0.27, 0.12 | ||||||
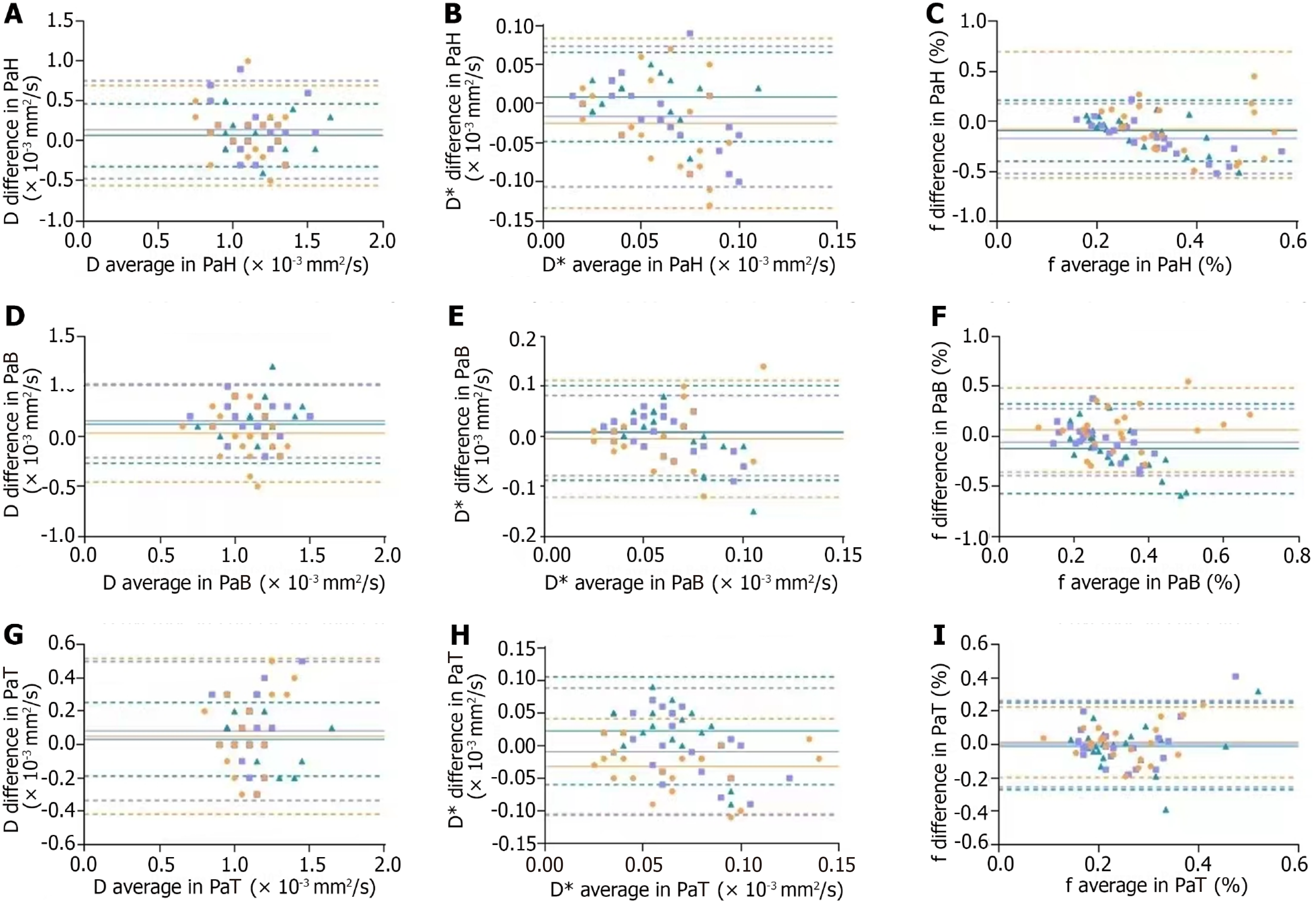
Bland-Altman plot analysis between different machines: In a Bland-Altman plot, if the majority of data points fall within the 95% limits of agreement (between the two dashed lines representing the mean ± 1.96 times the SD), and the maximum difference is clinically acceptable, it can be considered that the two methods exhibit good agreement and can be used interchangeably. As shown in Figure 3, there was one point beyond the 95% limits of agreement for devices A and B (orange), one point for devices A and C (purple), and two points for devices B and C (dark green).
With advancements in MRI equipment and technology, research on IVIM and its potential clinical applications has increased. This necessitates a systematic assessment of the variability of the measured diffusion parameters. IVIM is a novel MRI technique used for the non-invasive evaluation of molecular diffusion and perfusion within living tissues. It is considered an ideal method for the non-invasive assessment and differentiation of pancreatic cancer[14].
The image quality and parameter values of IVIM are influenced by various factors, such as the magnetic field strength, choice of b-values, selection of breathing techniques, image post-processing methods, and the use of MRI equipment manufactured by different vendors. Previous studies on the consistency of IVIM parameter values have primarily focused on exploring the impact of different breathing techniques, various b-values, and the selection of optimal b-values on ADC or IVIM parameters within the same device[15-17]. However, there have been relatively few studies on the consistency of IVIM parameter values between different devices. Therefore, our study used three MRI machines with a magnetic field strength of 3.0T, identical b-value selection, and respiratory-triggered scanning. The final step involved delineating ROIs of the same size (50 mm²) on images from all machines, minimizing interference from other factors to some extent.
In our study, the kappa coefficient for subjective image quality assessment at b = 1200 s/mm2 among different observers was 0.776, indicating a good consistency in subjective image quality ratings. The ICC for all the quantitative parameter values between different observers was 0.803, and the quantitative parameter value measured by the same observer on two different occasions was 0.883. Notably, both ICCs were > 0.75, indicating excellent consistency in the measurements of the quantitative parameter values among different observers and within the same observer across the two measurements.
We used images acquired at b = 0 and b = 1200 s/mm2 to analyze the ADC values between different devices. The ICC values for pairwise comparisons between different devices were 0.870, 0.707, and 0.808, respectively, indicating good consistency among the three devices. This finding is consistent with the results of previous studies[18,19]. However, the ICC statistical analysis of the SNR between different machines yielded P > 0.05, indicating no statistical significance. This could be attributed to differences in imaging parameters used by different devices during image acquisition.
In the consistency analysis of various IVIM parameter values, devices A and B showed statistical significance only for the f value of the PaT region, with an ICC of 0.531. Devices A and C exhibited statistical significance only for the D value of the PaB region, with an ICC value of 0.683. The remaining parameters between devices A and B and between devices A and C showed no statistically significant differences, indicating poor repeatability between devices A and B and between devices A and C. Devices B and C showed a statistical difference in the D and D* values in the PaH region, the D value in the PaB region, and the D value in the PaT region, with an ICC of 0.728, 0.578, 0.551, and 0.908, respectively. This indicates that the consistency between devices B and C was better than that between devices A and B and between devices A and C, and the repeatability of the D values in different pancreatic regions between devices B and C was relatively good. Considering the magnitude of the ICC among the statistically significant parameters mentioned above, only the ICC for the D value in the PaT region between devices B and C was > 0.75. The ICC for the other parameters was between 0.4 and 0.75, indicating some consistency between devices B and C; however, the degree of consistency was mostly moderate and did not reach a high level of agreement.
In the mixed linear model, when P < 0.05, a significant difference exists between the two data sets. According to the results of statistical analysis, there were differences in the IVIM parameters between different machines in the PaH (f value), PaB (D value), and PaT regions (D* value). Machine C had a lower f value in the PaH region than machines A and B. Machine C had a higher D value in the PaB region than machines A and B. Machine B had a lower D* value in the PaT region than the other machines. This may have been associated with using different post-processing software and algorithms in the IVIM numerical analysis on different machines.
Based on the Bland-Altman plot results for different machines, the approximate distribution of different parameters could be visually observed. These plots showed that only 1-2 data points fell outside the dashed lines (representing the 95%CI), indicating that only a few parameters had relatively large variability, whereas most parameters exhibited acceptable consistency. However, considering the statistical analysis results mentioned earlier, it should be noted that many IVIM parameters had ICC values that are not particularly high, and significant differences existed in some parameter values. In summary, the repeatability of the IVIM parameter values between different devices appeared to be moderate.
This study had some limitations. First, our study only included data from the pancreas of young and middle-aged individuals and did not include individuals of other age groups. However, the extended duration of the examination may have been challenging for children and older adults to endure, making them unable to complete all examination procedures. In addition, images of patients with lesions were not incorporated in our study. In clinical practice, patients with pancreatic cancer often have compromised health, and it can be challenging for them to undergo examinations simultaneously using three different devices. Moreover, there could have been a selection bias if patients with benign pancreatic lesions were selected.
Furthermore, we used MITK-diffusion post-processing software from different manufacturers for IVIM analysis. Therefore, while we could ensure consistency in ROI sizes between different devices, the ROI positions could only be approximately matched. This may have introduced some bias into the measurements. In addition, the lack of uniformity in the post-processing software could introduce variations in the algorithms used, potentially affecting the final results. In such cases, it is possible to standardize the differences between MRI devices through phantom calibration, which can then be used to correct the results. However, it is crucial to note that using phantoms for calibration inevitably increases the time and economic costs associated with the study.
Pancreatic scans were conducted in volunteers using different MRI devices, and we found that the variability in IVIM parameter values may be associated with using various MRI equipment. The consistency of the ADC values between different devices was good; however, the overall repeatability of the IVIM parameter values was moderate. Therefore, applying IVIM parameter values in diagnosing pancreatic cancer should be approached cautiously.
| 1. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2448] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 2. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2638] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 3. | Englund EK, Berry DB, Behun JJ, Ward SR, Frank LR, Shahidi B. IVIM Imaging of Paraspinal Muscles Following Moderate and High-Intensity Exercise in Healthy Individuals. Front Rehabil Sci. 2022;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Markiet K, Glinska A, Nowicki T, Szurowska E, Mikaszewski B. Feasibility of Intravoxel Incoherent Motion (IVIM) and Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) in Differentiation of Benign Parotid Gland Tumors. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Yang D, She H, Wang X, Yang Z, Wang Z. Diagnostic accuracy of quantitative diffusion parameters in the pathological grading of hepatocellular carcinoma: A meta-analysis. J Magn Reson Imaging. 2020;51:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol. 2019;29:5791-5803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ, Jang JY, Kannengiesser S, Han JK, Choi BI. Pancreatic Steatosis and Fibrosis: Quantitative Assessment with Preoperative Multiparametric MR Imaging. Radiology. 2016;279:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, Schad LR, Stieltjes B. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Warmuth-metz M. Imaging Differential Diagnosis of Pediatric CNS Tumors. Imaging Diagn Pediatric Brain Tumor Stud. 2017;. [DOI] [Full Text] |
| 10. | Kim B, Lee SS, Sung YS, Cheong H, Byun JH, Kim HJ, Kim JH. Intravoxel incoherent motion diffusion-weighted imaging of the pancreas: Characterization of benign and malignant pancreatic pathologies. J Magn Reson Imaging. 2017;45:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Ma W, Wei M, Han Z, Tang Y, Pan Q, Zhang G, Ren J, Huan Y, Li N. The added value of intravoxel incoherent motion diffusion weighted imaging parameters in differentiating high-grade pancreatic neuroendocrine neoplasms from pancreatic ductal adenocarcinoma. Oncol Lett. 2019;18:5448-5458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Bian H, Liu F, Chen S, Li G, Song Y, Sun M, Dong H. Intravoxel incoherent motion diffusion-weighted imaging evaluated the response to concurrent chemoradiotherapy in patients with cervical cancer. Medicine (Baltimore). 2019;98:e17943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Ma C, Liu L, Li YJ, Chen LG, Pan CS, Zhang Y, Wang H, Chen SY, Lu JP. Intravoxel incoherent motion MRI of the healthy pancreas: Monoexponential and biexponential apparent diffusion parameters of the normal head, body and tail. J Magn Reson Imaging. 2015;41:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology. 2014;270:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Ding X, Xu J, Zhou J, Long Q, Xu H. Effects of different breathing techniques on the IVIM-derived quantitative parameters of the normal pancreas. Eur J Radiol. 2021;143:109892. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Merisaari H, Toivonen J, Pesola M, Taimen P, Boström PJ, Pahikkala T, Aronen HJ, Jambor I. Diffusion-weighted imaging of prostate cancer: effect of b-value distribution on repeatability and cancer characterization. Magn Reson Imaging. 2015;33:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Wáng YXJ, Wang X, Wu P, Wang Y, Chen W, Chen H, Li J. Topics on quantitative liver magnetic resonance imaging. Quant Imaging Med Surg. 2019;9:1840-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Donati OF, Chong D, Nanz D, Boss A, Froehlich JM, Andres E, Seifert B, Thoeny HC. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014;270:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Barral M, Soyer P, Ben Hassen W, Gayat E, Aout M, Chiaradia M, Rahmouni A, Luciani A. Diffusion-weighted MR imaging of the normal pancreas: reproducibility and variations of apparent diffusion coefficient measurement at 1.5- and 3.0-Tesla. Diagn Interv Imaging. 2013;94:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |