Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2012
Revised: May 6, 2024
Accepted: May 28, 2024
Published online: July 27, 2024
Processing time: 169 Days and 17.8 Hours
With the continuous progress of surgical technology and improvements in medi
To compare the surgical safety, clinical efficacy, and safety of double-channel anastomosis and tubular gastroesophageal anastomosis in proximal gastrectomy.
The clinical and follow-up data of 99 patients with proximal gastric cancer who underwent proximal gastrectomy and were admitted to our hospital between January 2018 and September 2023 were included in this retrospective cohort study. According to the different anastomosis methods used, the patients were divided into a double-channel anastomosis group (50 patients) and a tubular ga
In the double-channel anastomosis cohort, there were 35 males (70%) and 15 females (30%), 33 (66.0%) were under 65 years of age, and 37 (74.0%) had a body mass index ranging from 18 to 25 kg/m2. In the group undergoing tubular gastroesophageal anastomosis, there were eight females (16.3%), 21 (42.9%) individuals were under the age of 65 years, and 34 (69.4%) had a body mass index ranging from 18 to 25 kg/m2. The baseline data did not sig
The safety of double-channel anastomosis in proximal gastric cancer surgery is equivalent to that of tubular gastric surgery. Compared with tubular gastric surgery, double-channel anastomosis is a preferred surgical technique for proximal gastric cancer. It offers advantages such as less esophageal reflux and improved quality of life.
Core Tip: This study compared the clinical efficacy and safety of double-channel anastomosis and tubular gastroesophageal anastomosis in proximal gastrectomy. The clinical data of the patients, including surgical indications, surgical methods, postoperative complications, and follow-up results, were retrospectively analyzed to evaluate the differences in surgical treatment effect and postoperative safety between the two anastomotic methods. This study focused on indicators such as the postoperative complication rate, incidence of anastomotic fistula, postoperative discharge time, nutritional status, and quality of life during long-term follow-up to comprehensively compare the advantages and disadvantages of the two surgical methods and provide a reference for clinicians to choose the best treatment plan.
- Citation: Liu BY, Wu S, Xu Y. Clinical efficacy and safety of double-channel anastomosis and tubular gastroesophageal anastomosis in gastrectomy. World J Gastrointest Surg 2024; 16(7): 2012-2022
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2012.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2012
The main surgical procedures for proximal gastric cancer include total gastrectomy and proximal gastrectomy[1-3]. Proximal gastrectomy, which preserves at least half of the stomach, is generally used only for early-stage upper gastric cancer. According to the 6th edition of the Japanese Guidelines for the Treatment of Gastric Cancer, proximal gastrectomy is a weak recommendation for esophagogastric junction cancer, but total gastrectomy is recommended for patients with a large tumor diameter and gastric invasion[4]. The high incidence of reflux esophagitis after proximal gastrectomy has severely limited the application of proximal gastrectomy[5]. With the development of the concept of functional preservation surgery in recent years, an increasing number of proximal gastrectomy and digestive tract reconstruction procedures, including double-channel anastomosis, tubular gastroesophageal anastomosis, jejunal interposition, and double muscle flap reconstruction, have been applied in clinical practice[6]. However, the antireflux effect and adva
Among the reconstruction methods mentioned above, tubular gastroesophageal anastomosis and double-channel anastomosis are two relatively mature antireflux surgery methods[8-10]. Compared with traditional residual gastroesophageal anastomosis, tubular gastroesophageal anastomosis excises part of the gastric antrum, reduces gastric acid secretion, increases the gastric acid reflux path, and significantly improves long-term postoperative quality of life[11]. However, the current literature reports that the postoperative antireflux effect is not ideal, and the incidence of reflux symptoms is as high as 14%-35%. Double-channel anastomosis avoids direct entry of gastric acid into the esophagus, and the incidence of reflux symptoms reported in the literature ranges from 1.1% to 10.0%[12]. However, in some cases, when double-channel anastomosis occurs, food will escape from the jejunal path and not enter the residual gastric path, which leads to the possibility that double-channel reconstruction may not be beneficial to patients[13-15].
At present, most studies on the quality of life of patients after proximal gastrectomy have focused on comparisons between traditional esophagogastric anastomosis, double-channel anastomosis, and double muscle flap anastomosis. However, there are few reports comparing tubular gastroesophageal anastomosis and double-channel anastomosis[16-18]. Therefore, the present study retrospectively analyzed the effects of double-channel anastomosis and tubular gastroesophageal anastomosis on the quality of life of patients with stages I and II proximal gastric cancer after proximal gastrectomy to provide a further reference for the selection of digestive tract reconstruction methods for proximal gastric cancer.
A retrospective cohort study design was used in this study. The inclusion criteria were as follows: (1) Primary gastric adenocarcinoma was confirmed by preoperative endoscopic pathological biopsy, and the clinical stage of gastric cancer was stage I or stage II according to the 8th edition of the American Cancer Federation; (2) Preoperative computed tomography and other imaging examinations confirmed that the tumor was located in the upper 1/3 of the stomach; and (3) The surgical method was proximal gastrectomy, and the digestive tract reconstruction method was double-channel anastomosis or tubular gastroesophageal anastomosis. The exclusion criteria were as follows: (1) Received preoperative neoadjuvant therapy; (2) Had tumors at other sites; (3) Did not achieve R0 excision; and (4) Had incomplete postoperative follow-up data.
According to the above criteria, the clinicopathological data of 99 patients with upper gastric adenocarcinoma admitted to the General Surgery Center of Fujian Provincial Hospital between January 2018 and September 2023 were retr
| Group | Cases | Age | Gender | Body mass index | ||||||
| ≤ 65 years old | > 65 years old | Male | Female | < 18 kg/m² | 18-25 kg/m² | > 25 kg/m² | ||||
| Dual-channel anastomosis group | 50 | 33 (66.0) | 17 (34.0) | 35 (70.0 | 15 (30.0) | 1 (2.0) | 37 (74.0) | 12 (24.0) | ||
| Tubular gastroesophageal anastomosis group | 49 | 21 (42.9) | 28 (57.1) | 41 (83.7) | 8 (16.3) | 0 | 34 (69.4) | 15 (30.6) | ||
| χ2 | 5.346 | 2.594 | 1.392 | |||||||
| P value | 0.021 | 0.107 | 0.577 | |||||||
| Group | Cases | American Society of Anesthesiologists Classification | Hypertension | Coronary heart disease | Diabetes | |||||
| I | Ⅱ | Yes | No | Yes | No | Yes | No | |||
| Dual-channel anastomosis group | 50 | 37 (74.0) | 13 (26.0) | 18 (36.0) | 32 (64.0) | 1 (2.0) | 49 (98.0) | 6 (12.0) | 44 (88.0) | |
| Tubular gastroesophageal anastomosis group | 49 | 36 (73.5) | 13 (26.5) | 16 (32.7) | 33 (67.3) | 0 | 49 (100) | 6 (12, 2) | 43 (87.8) | |
| χ2 | 0.004 | 0.123 | 0.99 | 0.001 | ||||||
| P value | 0.952 | 0.726 | 0.32 | 0.97 | ||||||
| Group | Cases | Tumor T-stage | Tumor N-stage | Tumor TNM-stage | Tumor diameter | |||||
| Tl | T2 | T3 | NO | N1 | I | Ⅱ | < 4 cm | 24 cm | ||
| Dual-channel anastomosis group | 50 | 36 (72.0) | 11 (22.0) | 3 (6.0) | 46 (92.0) | 4 (8.0) | 42 (84.0) | 8 (16.0) | 49 (98.0) | 1 (2.0) |
| Tubular gastroesophageal anastomosis group | 49 | 34 (69.4) | 6 (12.2) | 9 (18.4) | 49 (100) | 0 | 40 (81.6) | 9 (18.4) | 44 (89.9) | 5 (10.2) |
| χ2 | 4.518 | 2.282 | 0.098 | 1.662 | ||||||
| P value | 0.104 | 0.131 | 0.755 | 0.197 | ||||||
All patients and their families provided informed consent before the operation. The Ethics Committee of Fujian Provincial Hospital granted approval for this study.
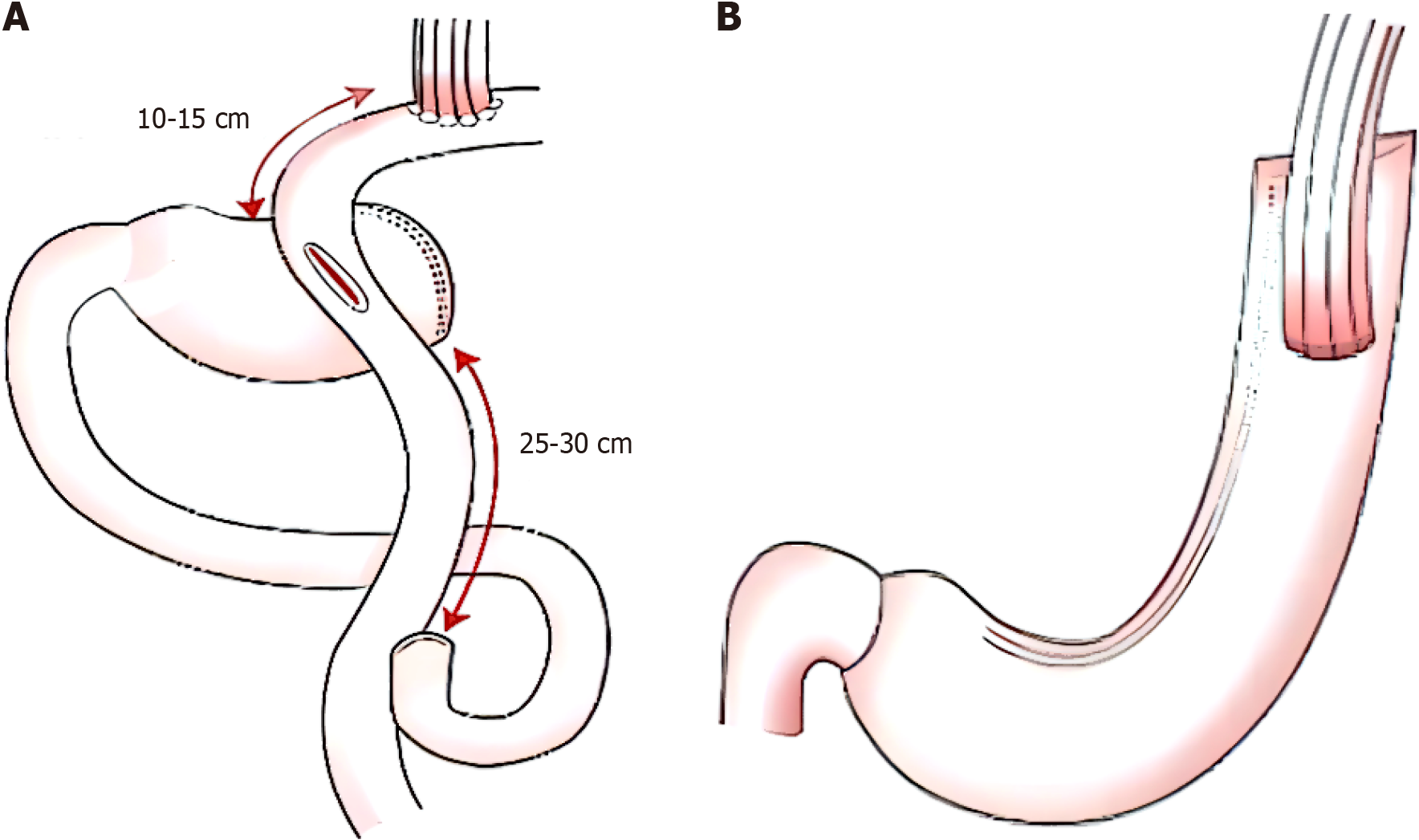
All patients were treated with D1, D1+, and D2 Lymph node dissection according to the requirements for proximal gastrectomy. The perigastric vessels were cut, the omentum was excised, and the proximal esophagus was cut as needed. The methods used for distal digestive tract reconstruction in the two groups were as follows: (1) In the double-channel anastomosis group, the distal end was separated from the tumor margin by > 5 cm. Esophagojejunal anastomosis was performed at a distance of 10-15 cm below the Treitz's ligament, and jejjunojejunal anastomosis was performed at a distance of 25-30 cm from the proximal jejunal anastomosis (Figure 1A); and (2) In the tubular gastroplasty group, tubular gastroplasty was performed on the distal stump of the stomach, and direct anastomosis was performed between the posterior wall of the esophagus and the anterior wall of the gastric tube using a 45-mm linear stapler (Figure 1B).
Outcome measures: Main outcome measure was quality of life 1 year after surgery. Secondary outcome measures in
Evaluation criteria: (1) Quality of life: The post-gastrectomy syndrome assessment scale (PSAS-45), designed by the Chinese version of the Japan Working Group on Post-Gastrectomy Syndrome, was used to measure the intensity of various symptoms after gastrectomy and explain to what extent they affect the patient's life. The scale mainly consists of the symptom domain, life state domain, and life quality domain. According to the different domains, the relevant problems were graded according to different degrees, in which the higher the score of body mass change, food intake per meal, meal quality subscale, total physical health measurement, and total mental health measurement, the better the situation, and the higher the score of other indicators, the worse the situation; (2) Nutritional status: Nutritional indicators including hemoglobin, serum albumin (ALB), and serum total protein levels were evaluated. Hemoglobin < 120 g/L in men and < 110 g/L in women is defined as anemia. A serum total protein concentration < 60 g/L or a serum ALB concentration < 25 g/L is considered hypoproteinaemia; and (3) Postoperative long-term complications: Complications in
Follow-up method: Postoperative follow-up was conducted for 1 year, and postoperative review was performed at 1, 3, 6, and 12 months. The review included physical examination, laboratory examination (including routine blood tests, bio
All the data were processed using SPSS 26.0. Normally distributed measurement data are expressed as the mean ± SD, and two independent samples t-tests were used for comparisons between groups. Nonnormally distributed data are expressed as M (Q1, Q3), and the Mann-Whitney U test was used for comparisons between groups. Count data are represented by cases (%). χ2 tests were used for comparisons between nonranked count data groups, and Mann-Whitney U tests were used for comparisons between ranked count data groups. Repeated measures analysis of variance was used to compare nutrition-related indicators before surgery and 1, 3, 6, and 12 months after surgery. P < 0.05 indicated that the difference was statistically significant.
The operation was successful in both groups, and R0 resection was performed in both groups. There were no statistically significant differences between the two surgical methods in terms of intraoperative blood loss, number of lymph nodes dissected, operation time, length of hospital stay, or incidence of recent postoperative complications (P > 0.05), as shown in Table 2. All the patients with anastomotic bleeding were hemostatic under endoscopy, and the remaining complications were resolved by conservative symptomatic treatment; no patients who underwent a second operation were re
| Group | Cases | Surgical methods | Intraoperative bleeding volume (mL) | Number of lymph nodes resected | Operative time (h) | Hospital stay (d) | Short-term complications after anastomotic surgery (%) | ||||||
| Laparoscopic | Open | Total | Anastomotic bleeding | Anastomotic leakage | Gastroparesis | Pneumonia | Infect | ||||||
| Dual-channel anastomosis group | 50 | 16 (32.0) | 34 (68.0) | 200 (100, 300) | 17 (15, 21) | 4 (3, 4) | 16 (15, 18) | 5 (10.0) | 1 | 2 | 1 | 0 | 1 |
| Tubular gastroesophageal anastomosis group | 49 | 10 (20.4) | 39 (79.6) | 200 (100, 200) | 20 (16, 25) | 3.6 (3.1, 4.0) | 14 (13, 18) | 4 (8.2) | 1 | 1 | 1 | 1 | 0 |
| Statistics | χ2 = 1.717 | Z = 0.245 | Z = 1.866 | Z = 0.102 | Z = 1.463 | χ2 = 0.101 | |||||||
| P value | 0.19 | 0.807 | 0.062 | 0.918 | 0.144 | 0.751 | |||||||
At 1 year after surgery, compared with the tubular gastroesophageal anastomosis group, the double-channel anastomosis group had better scores in terms of esophageal reflux, eating discomfort, constipation, and total symptom score in the somatic symptom domain. Patients with tubular gastroesophageal anastomosis were more likely to have anal exhaust but were less likely to have loose stools. The differences were statistically significant (P < 0.05) (Table 3).
| Group | Dual-channel anastomosis group | Tubular gastroesophageal anastomosis group | Z value | P value | |
| Cases | 50 | 49 | |||
| Somatic symptoms | Esophageal reflux scale | 2.8 (2.3, 4.0) | 4.8 (3.8, 5.0) | 3.489 | < 0.001 |
| Abdominal pain scale | 2.0 (1.3, 3.0) | 1.7 (1.3, 2.3) | 0.358 | 0.72 | |
| Eating distress subscale | 2.7 (1.7, 3.0) | 3.3 (2.7, 4.0) | 3.393 | 0.001 | |
| Digestive dysfunction scale | 3.0 (2.5, 3.8) | 2.8 (2.0, 3.8) | 0.79 | 0.43 | |
| Diarrhea subscale | 1.3 (13, 1.7) | 1.3 (13, 1.3) | 0.143 | 0.886 | |
| Constipation subscale | 1.3 (1.3, 1.3) | 1.3 (1.3, 1.3) | 2.004 | 0.045 | |
| Dumping scale | 1.3 (1.3, 13) | 3 (1.3, 1.3) | 0.802 | 0.422 | |
| Total symptom scale | 2.3 (1.7, 2.7) | 2.5 (2.2, 2.9) | 2.243 | 0.025 | |
| Other outcome indicators | Increased anal exhaust | 3.5 (2.0, 5.0) | 3.0 (2.0, 4.0) | 2.345 | 0.019 |
| Loose stool | 2.0 (1.0, 2.0) | 2.0 (2.0, 2.0) | 2.397 | 0.017 | |
| Living conditions | Changes in body mass | 13.7 (6.3, 18.6) | 12.9 (8.7, 16.5) | 0.042 | 0.967 |
| Food intake per meal | 6.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 1.924 | 0.054 | |
| Necessity of additional meals and meal quality | 5.0 (3.0, 5.0) | 5.0 (5.0, 5.0) | 1.488 | 0.137 | |
| Meal quality quantity scale | 4.3 (3.7, 4.3) | 4.0 (3.3, 4.3) | 2.666 | 0.008 | |
| Quality of life | Working ability | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.0) | 1.362 | 0.173 |
| Dissatisfied with symptoms | 2.0 (1.0, 3.0) | 2.0 (2.0, 3.0) | 2.127 | 0.033 | |
| Discontent with dining | 2.0 (1.0, 2.0) | 2.0 (2.0, 3.0) | 3.976 | < 0.001 | |
| Dissatisfied with work | 1.0 (1.0, 2.0) | 2.0 (1.0, 20) | 2.279 | 0.023 | |
| Scale of dissatisfaction | 1.7 (13, 2.0) | 2.0 (2.0, 2.3) | 3.95 | < 0.001 | |
| Total measurement of physical health | 83.8 (77.5, 88.8) | 82.5 (77.5, 88.8) | 1.702 | 0.089 | |
| Overall measurement of mental health | 93.8 (87.5, 100.0) | 91.7 (83.3, 100.0) | 0.412 | 0.68 | |
At 1 year after surgery, reflux esophagitis was less severe in the double-channel anastomosis group than in the tubular gastroesophageal anastomosis group, and the difference was statistically significant (P < 0.05). There was no significant difference in the incidence of postoperative anastomotic stenosis, intestinal obstruction, or gastric emptying disorder between the two groups (P > 0.05), as shown in Table 4. In the double-channel anastomosis group, one patient with a small intestinal obstruction was treated via surgery. In the double-channel anastomosis group and the tubular gastroesophageal anastomosis group, 3 and 7 patients with anastomotic stenosis, respectively, had endoscopic anastomotic dilation. The other patients received treatment based on their symptoms. Both groups of patients with long-term complications had a good prognosis after treatment.
| Group | Cases | Reflux esophagitis | Anastomotic stenosis | Intestinal obstruction | Delayed gastric emptying | ||||
| Total | A | B | C | D | |||||
| Dual channel matching group | 50 | 2 (4.0) | 2 (4.0) | 0 | 0 | 0 | 6 (12.0) | 1 (2.0) | 0 |
| Tubular gastroesophageal anastomosis group | 49 | 13 (26.5) | 6 (12.2) | 5 (10.2) | 1 (2.0) | 1 (2.0) | 12 (24.5) | 0 | 2 (4.1) |
| Statistical value | χ2 = 13.507 | Z = 3.177 | χ2 = 2.595 | χ2 < 0.001 | χ2 = 0.531 | ||||
| P value | 0.009 | 0.001 | 0.107 | > 0.999 | 0.466 | ||||
There were no significant differences in the levels of hemoglobin, serum ALB, or total serum protein between the two groups at any time point after surgery (P > 0.05). From 1 to 12 months after the operation, the hemoglobin and serum total protein levels of patients in the tubular gastroesophageal anastomosis group tended to increase. In the double-channel anastomosis group, the above indices showed an increasing trend at 1 to 6 months after the operation, and the serum ALB and total protein concentrations showed a slight decreasing trend at 6 to 12 months after the operation.
Reconstruction of the digestive tract after radical resection of proximal gastric cancer has been a hot topic for clinicians[19]. In the past, Roux-en-Y anastomosis was the surgeon's first choice for total gastrectomy, but patients were prone to malnutrition after this procedure. Although traditional proximal gastrectomy with residual gastroesophageal an
This study is the first to report the use of tubular gastroesophagostomy for the treatment of early proximal gastric ca
The aim of this study was to systematically evaluate the quality of life of patients after proximal gastrectomy with double-channel anastomosis and tubular gastroesophageal anastomosis[30]. Compared with previous studies, PSAS-45, designed by the Japan Post-Gastrectomy Syndrome Working Group, was comprehensively used in this study to evaluate the postoperative quality of life of patients in the proximal gastrectomy double-channel anastomosis and tubular ga
Postoperative nutritional status is also an important factor in the selection of proximal gastrectomy for gastrointestinal reconstruction[37]. The gastroduodenal channel was kept open with the tubular gastroesophageal anastomosis, and the physiological and anatomical structures of the stomach were mostly kept intact. Moreover, the pepsinogen and intrinsic factor secreted by the residual stomach can also promote the digestion and absorption of food. The preserved residual stomach can help move and mix food and bile, and some of the food can travel straight into the jejunum[38]. This can help with the slow emptying or sticking of the stomach that occurs after a vagotomy. Theoretically, both methods can improve the postoperative nutritional status of patients. In this study, hemoglobin, serum ALB, and total serum protein in both groups recovered to higher levels 1 year after surgery. In addition, postoperative gastrointestinal angiography revealed that 33.3% (8/24) of patients in the double-channel anastomosis group underwent single-channel angiography. According to previous studies[39-41], in some patients the food directly entered the jejunum after surgery without entering the residual stomach, which may lead to insufficient absorption of food nutrients. One year after surgery, the patients in the double-channel anastomosis group were more likely to have increased anal exhaust than were those in the tubular gastroesophageal anastomosis group. This may be because food moves more quickly into the jejunum, according to the quality of life assessment. These factors may be responsible for the slight decrease in the serum ALB and total protein concentrations at 6 to 12 months after surgery in patients who underwent double-channel anastomosis. The results of this study showed that there was no significant difference in postoperative nutritional status between patients who underwent proximal gastrectomy with tubular gastroesophageal anastomosis and those who underwent proximal gastrectomy with double-channel anastomosis.
Safe and feasible surgery and radical resection of tumors are prerequisites for treatment. All patients in this study underwent R0 excision, and postoperative pathological margins were negative. There was no significant difference in the number of lymph nodes dissected between the two groups. Compared with total gastrectomy, the number of lymph nodes examined was lower. Considering that distal lymph node metastasis of proximal gastric cancer is less common, it is still suitable for proximal gastrectomy of stages I and II gastric cancer patients. To improve the accuracy of lymph node staging, the lymph nodes should be completely cleaned, and the number of examined lymph nodes should be increased according to surgical requirements. The results of this study showed that the surgical conditions and perioperative complication rates of the two groups were similar, and the incidence of anastomotic leakage in the double-channel an
In summary, for stages I and II proximal gastric cancer, double-channel anastomosis is superior to tubular gastroesophageal anastomosis in terms of long-term quality of life and can significantly improve postoperative esophageal reflux symptoms and eating discomfort, with surgical safety not inferior to tubular gastroesophageal anastomosis. The results of this study can provide some guidance for surgeons in choosing surgical methods. However, considering that this was a single-center retrospective study with a limited number of included patients and insufficient follow-up time, further multicenter, large-sample prospective studies are needed for further verification of our findings.
| 1. | Yang J, Zheng S, Li JJ, Li YL, Su R, Zheng X, Liu P, Zhao EH. Clinical application of laparoscopic continuous interposition jejunostomy with double-tract anastomosis and esophagogastric anastomosis: a retrospective study. Eur Rev Med Pharmacol Sci. 2023;27:9324-9332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Park DJ, Han SU, Hyung WJ, Hwang SH, Hur H, Yang HK, Lee HJ, Kim HI, Kong SH, Kim YW, Lee HH, Kim BS, Park YK, Lee YJ, Ahn SH, Lee I, Suh YS, Park JH, Ahn S, Park YS, Kim HH. Effect of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction vs Total Gastrectomy on Hemoglobin Level and Vitamin B12 Supplementation in Upper-Third Early Gastric Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2256004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Lu S, Ma F, Zhang Z, Peng L, Yang W, Chai J, Liu C, Ge F, Ji S, Luo S, Chen X, Hua Y. Various Kinds of Functional Digestive Tract Reconstruction Methods After Proximal Gastrectomy. Front Oncol. 2021;11:685717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Li L, Cai X, Liu Z, Mou Y, Wang Y. Digestive tract reconstruction after laparoscopic proximal gastrectomy for Gastric cancer: A systematic review. J Cancer. 2023;14:3139-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Hipp J, Hillebrecht HC, Kalkum E, Klotz R, Kuvendjiska J, Martini V, Fichtner-Feigl S, Diener MK. Systematic review and meta-analysis comparing proximal gastrectomy with double-tract-reconstruction and total gastrectomy in gastric and gastroesophageal junction cancer patients: Still no sufficient evidence for clinical decision-making. Surgery. 2023;173:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 6. | Sun KK, Wu YY. Current status of laparoscopic proximal gastrectomy in proximal gastric cancer: Technical details and oncologic outcomes. Asian J Surg. 2021;44:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Li X, Gong S, Lu T, Tian H, Miao C, Liu L, Jiang Z, Hao J, Jing K, Yang K, Guo T. Proximal Gastrectomy Versus Total Gastrectomy for Siewert II/III Adenocarcinoma of the Gastroesophageal Junction: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2022;26:1321-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 9. | Talavera-Urquijo E, Davies AR, Wijnhoven BPL. Prevention and treatment of a positive proximal margin after gastrectomy for cardia cancer. Updates Surg. 2023;75:335-341. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB 2024; 10: 1256. [DOI] [Full Text] |
| 11. | Li L, Liu ZH, Cai XF, Jiang QT, Mou YP, Wang YY. Cardia function-preserving surgery and anti-reflux anastomotic method after proximal gastrectomy for gastric cancer: Current status and future perspectives. Front Oncol. 2022;12:1000719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Shaibu Z, Chen Z, Mzee SAS, Theophilus A, Danbala IA. Effects of reconstruction techniques after proximal gastrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 14. | Yamamoto M, Omori T, Shinno N, Hara H, Fujii Y, Mukai Y, Sugase T, Takeoka T, Asukai K, Kanemura T, Hasegawa S, Akita H, Haraguchi N, Nishimura J, Wada H, Matsuda C, Yasui M, Miyata H, Ohue M. Laparoscopic Proximal Gastrectomy with Novel Valvuloplastic Esophagogastrostomy vs. Laparoscopic Total Gastrectomy for Stage I Gastric Cancer: a Propensity Score Matching Analysis. J Gastrointest Surg. 2022;26:2041-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Matsuo K, Shibasaki S, Suzuki K, Serizawa A, Akimoto S, Nakauchi M, Tanaka T, Inaba K, Uyama I, Suda K. Efficacy of minimally invasive proximal gastrectomy followed by valvuloplastic esophagogastrostomy using the double flap technique in preventing reflux oesophagitis. Surg Endosc. 2023;37:3478-3491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 16. | Huang QZ, Wang PC, Chen YX, Lin S, Ye K. Comparison of proximal gastrectomy with double-flap technique and double-tract reconstruction for proximal early gastric cancer: a meta-analysis. Updates Surg. 2023;75:2117-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Yuan Z, Cui H, Xu Q, Gao J, Liang W, Cao B, Lin X, Song L, Huang J, Zhao R, Li H, Yu Z, Du J, Wang S, Chen L, Cui J, Zhao Y, Wei B. Total versus proximal gastrectomy for proximal gastric cancer after neoadjuvant chemotherapy: a multicenter retrospective propensity score-matched cohort study. Int J Surg. 2024;110:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 19. | Tian P, Liu Y, Bian S, Li M, Zhang M, Liu J, Jin L, Zhang P, Zhang Z. Laparoscopic Proximal Gastrectomy Versus Laparoscopic Total Gastrectomy for Proximal Gastric Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:607922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 21. | Lu S, Ma F, Yang W, Peng L, Hua Y. Is single tract jejunal interposition better than double tract reconstruction after proximal gastrectomy? Updates Surg. 2023;75:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Schrope B, Coons B, Rosario V, Toledano S. Proximal Gastrectomy Is a Viable Alternative to Total Gastrectomy in Early Stage Proximal Gastric Cancer. JSLS. 2021;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Irfan A, Yang T, Bowring M, Blair AB, Duncan M. Proximal vs. Total Gastrectomy: Is There a Difference in Quality of Life for Patients? Am Surg. 2023;89:401-406. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Greenan G, Rogers BD, Gyawali CP. Proximal Gastric Pressurization After Sleeve Gastrectomy Associates With Gastroesophageal Reflux. Am J Gastroenterol. 2023;118:2148-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Hipp J, Kuvendjiska J, Martini V, Hillebrecht HC, Fichtner-Feigl S, Diener MK. Proximal gastrectomy and double-tract reconstruction vs total gastrectomy in gastric and gastro-esophageal junction cancer patients - a systematic review and meta-analysis protocol (PROSPERO registration number: CRD42021291500). Syst Rev. 2023;12:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Ishida M, Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Hato S, Kimura T, Muraoka A, Tanakaya K, Matsuda T, Takashima H, Nonaka Y, Ishii H, Shirakawa Y, Kamikawa Y, Fujiwara T. Incidence of Metachronous Remnant Gastric Cancer after Proximal Gastrectomy with the Double-flap Technique (rD-FLAP-rGC Study): A Multicenter, Retrospective Study. Ann Surg Oncol. 2023;30:2307-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Peng R, Yue C, Wei W, Zhou B, Wen X, Gu RM, Ming XZ, Li G, Chen HQ, Xu ZK. Proximal gastrectomy may be a reasonable choice for patients with selected proximal advanced gastric cancer: A propensity score-matched analysis. Asian J Surg. 2022;45:1823-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Wei J, Yang P, Huang Q, Chen Z, Zhang T, He F, Hu H, Zhong J, Li W, Wei F, Wang Q, Cao J. Proximal versus total gastrectomy for proximal gastric cancer: a Surveillance, Epidemiology, and End Results Program database analysis. Future Oncol. 2021;17:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Xu Y, Gao J, Wang Y, Tan Y, Xi C, Ye N, Wu D, Xu X. Validation of a novel reconstruction method of laparoscopic gastrectomy for proximal early gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Checcacci P, Feleppa C, Berti S. Laparoscopic proximal gastrectomy for leiomyosarcoma of the stomach (with video). J Visc Surg. 2022;159:523-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Peng W, Yan S, Huang Y, Cheng M, Liu T, Ren R, Chen Q, Zhang J, Gong W, Xing C, Wu Y. Laparoscopic proximal gastrectomy with right-sided overlap and single-flap valvuloplasty (ROSF): a case-series study. BMC Surg. 2023;23:90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 34. | Fu J, Li Y, Liu X, Jiao X, Wang Y, Qu H, Niu Z. Clinical outcomes of proximal gastrectomy with gastric tubular reconstruction and total gastrectomy for proximal gastric cancer: A matched cohort study. Front Surg. 2022;9:1052643. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Li Z, Ma Y, Liu G, Fang M, Xue Y. Proximal gastrectomy with gastric tube reconstruction or jejunal interposition reconstruction in upper-third gastric cancer: which offers better short-term surgical outcomes? BMC Surg. 2021;21:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 36. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Kano Y, Ohashi M, Ida S, Kumagai K, Sano T, Hiki N, Nunobe S. Laparoscopic proximal gastrectomy with double-flap technique versus laparoscopic subtotal gastrectomy for proximal early gastric cancer. BJS Open. 2020;4:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 38. | Lee S, Son WJ, Roh YH, Song JH, Park SH, Cho M, Kim YM, Hyung WJ, Kim HI. Indication of Proximal Gastrectomy for Advanced Proximal Gastric Cancer Based on Lymph Node Metastasis at the Distal Part of the Stomach. Ann Surg Open. 2021;2:e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Xiang R, Song W, Ren J, Lu W, Zhang H, Fu T. Proximal gastrectomy with double-tract reconstruction versus total gastrectomy for proximal early gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e27818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Ying K, Bai W, Yan G, Xu Z, Du S, Dang C. The comparison of long-term oncological outcomes and complications after proximal gastrectomy with double tract reconstruction versus total gastrectomy for proximal gastric cancer. World J Surg Oncol. 2023;21:101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Ko HJ, Kim KH, Lee SH, Choi CW, Kim SJ, In Choi C, Kim DH, Kim DH, Hwang SH. Can Proximal Gastrectomy with Double-Tract Reconstruction Replace Total Gastrectomy? A Propensity Score Matching Analysis. J Gastrointest Surg. 2020;24:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |