Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2003
Revised: May 6, 2024
Accepted: May 30, 2024
Published online: July 27, 2024
Processing time: 139 Days and 8.1 Hours
Necrotising enterocolitis (NEC) is a critical gastrointestinal emergency affecting premature and low-birth-weight neonates. Serum amyloid A (SAA), procalcitonin (PCT), and high-mobility group box 1 (HMGB1) have emerged as potential biomarkers for NEC due to their roles in inflammatory response, tissue damage, and immune regulation.
To evaluate the diagnostic value of SAA, PCT, and HMGB1 in the context of NEC in newborns.
The study retrospectively analysed the clinical data of 48 newborns diagnosed with NEC and 50 healthy newborns admitted to the hospital. Clinical, radio
The study demonstrated significantly elevated levels of serum SAA, PCT, and HMGB1 Levels in newborns diagnosed with NEC compared with healthy controls. The correlation analysis indicated strong positive correlations among serum SAA, PCT, and HMGB1 Levels and the presence of NEC. ROC analysis revealed promising sensitivity and specificity for serum SAA, PCT, and HMGB1 Levels as potential diagnostic markers. The combined model of the three biomarkers demonstrating an extremely high area under the curve (0.908).
The diagnostic value of serum SAA, PCT, and HMGB1 Levels in NEC was highlighted. These biomarkers potentially improve the early detection, risk stratification, and clinical management of critical conditions. The findings suggest that these biomarkers may aid in timely intervention and the enhancement of outcomes for neonates affected by NEC.
Core Tip: This study presents a retrospective analysis of the diagnostic significance of serum amyloid A (SAA), procalcitonin (PCT), and high-mobility group box 1 (HMGB1) as potential biomarkers for necrotising enterocolitis (NEC) in newborns. The findings demonstrate significantly elevated levels of these biomarkers in newborns diagnosed with NEC compared to healthy controls. Furthermore, the study reveals strong positive correlations between serum SAA, PCT, and HMGB1 levels, and the presence of NEC, indicating the potential utility of these markers for early detection and accurate diagnosis of NEC. Combining these biomarkers provides a high area under the curve, suggesting their synergistic diagnostic potential.
- Citation: Guo LM, Jiang ZH, Liu HZ, Zhang L. Diagnostic significance of serum levels of serum amyloid A, procalcitonin, and high-mobility group box 1 in identifying necrotising enterocolitis in newborns. World J Gastrointest Surg 2024; 16(7): 2003-2011
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2003.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2003
Necrotising enterocolitis (NEC) is a devastating intestinal disease that primarily affects premature and low-birth-weight neonates, with an incidence ranging from 1% to 7% in neonatal intensive care units[1-3]. With a mortality rate of up to 30%, NEC is one of the most critical gastrointestinal emergencies in the neonatal population[4]. This acute inflammatory intestinal condition is characterised by the development of necrosis and inflammation in the intestinal mucosa, which may progress rapidly to intestinal perforation, sepsis, and multiorgan failure if not promptly diagnosed and treated[5,6].
The clinical presentation of NEC varies widely, ranging from nonspecific signs and symptoms, such as feeding intolerance, abdominal distension, and bloody stools, to severe systemic manifestations, such as hypotension, metabolic acidosis, and respiratory failure[7]. Currently, the diagnosis of NEC is based on a combination of clinical, radiological, and laboratory findings, including abdominal radiographs, pneumatosis intestinalis, portal venous gas, and elevated inflammatory markers such as C-reactive protein (CRP) and white blood cell (WBC) count[8].
However, the lack of specific and sensitive biomarkers for the early detection and accurate diagnosis of NEC remains a major challenge in clinical practice, leading to delayed treatment and increased risk of disease progression and associated complications. Thus, novel biomarkers that can aid in the early diagnosis and risk stratification of NEC in newborns are urgently needed.
Serum amyloid A (SAA), procalcitonin (PCT), and high-mobility group box 1 (HMGB1) have emerged as potential biomarkers for NEC because of their roles in inflammatory response, tissue damage, and immune regulation. SAA is an acute-phase protein that is rapidly produced and secreted by hepatocytes and monocytes in response to proinflammatory cytokines, and its levels are significantly elevated in various inflammatory conditions, including sepsis and tissue injury[9,10].
PCT, a precursor of calcitonin, is another promising biomarker that has been extensively studied for its diagnostic and prognostic value in neonatal sepsis and other infectious conditions[11]. PCT levels rise rapidly in response to bacterial infections and systemic inflammation, serving as a valuable marker for differentiating infectious from non-infectious causes of systemic inflammation[12].
HMGB1, a highly conserved nuclear protein that can be released by activated immune cells and damaged tissues, has recently attracted considerable interest as a key mediator of inflammation and tissue injury in various pathological conditions, including sepsis, ischaemia–reperfusion injury, and autoimmune disorders[13,14]. It is a potential biomarker for NEC and other inflammatory gastrointestinal diseases because of its extracellular release and proinflammatory properties[15]. In this study, we aimed to review and analyse existing evidence regarding the diagnostic value of serum SAA, PCT, and HMGB1 Levels in the context of NEC in newborns.
Overall, understanding the diagnostic value of serum SAA, PCT, and HMGB1 in NEC offers valuable insights for improving the early detection, risk stratification, and management of this life-threatening condition, ultimately enhancing outcomes for affected neonates.
The study analysed the clinical data of newborns diagnosed with NEC and admitted to our hospital from June 2021 to June 2023. These newborns were included in an NEC group (n = 48). Additionally, a control group of healthy newborns admitted during the same period were included in the present study (n = 50). This study was approved by the Ethics Committee of Qingdao Women and Children’s Hospital, and written informed consent was obtained from the parents or guardians of all children.
The inclusion criteria comprised newborns diagnosed with NEC; age of 0–28 d upon admission; and single births.
The exclusion criteria included the presence of the congenital malformations of the digestive tract, such as congenital oesophageal atresia and pyloric obstruction, and concomitant congenital immunodeficiency diseases or hereditary metabolic diseases; recent treatment with albumin or platelet transfusion; other types of infectious diseases, such as neonatal pneumonia or peritonitis; inadequate clinical data; and abnormal coagulation function.
The detection methods involved collecting 5 mL of fasting venous blood from the study group of paediatric patients before treatment, and 6 mL of fasting venous blood from the control group of newborns 3 d after birth. The samples were placed in anticoagulant tubes and centrifuged at a rate of 3500 r/min with a centrifuge (KA-2200, Kubota, Japan). The obtained supernatant was stored at low temperature for subsequent use. Serum SAA levels were measured using an automatic biochemical analyser (7060, Hitachi, Japan) with ab100635 kit from Abcam, USA. Serum HMGB-1 and PCT levels were determined using enzyme-linked immunosorbent assay (ELISA) kits (ab18256 and ab181208, Abcam, United States).
Three millilitres of fasting venous blood was drawn from the patients. After serum separation, leukocyte count, haemoglobin level, platelet count, lymphocyte count, and serum albumin level were examined using a fully automatic biochemical analyser (BS-280, Mindray, China). CRP levels were measured using ELISA, and neutrophil count was determined using a fully automatic blood cell analyser (XE2100, SYSMEX, Japan).
Baseline data, including gender distribution, age distribution, body mass distribution, obstetric asphyxia during childbirth, and delivery method, were collected for both groups. WBC count, neutrophil count, lymphocyte count, platelet count, serum albumin level, CRP level, and haemoglobin level were determined through laboratory examinations. Additionally, the serum levels of SAA, PCT, and HMGB1 were measured. All data were collected by trained healthcare professionals following standard protocols and guidelines.
The data were analysed using SPSS 25.0 statistical software (SPSS Inc., Chicago, IL, United States). For categorical data, n (%) is used for representation. A chi-square test was applied when the sample size was ≥ 40, and the theoretical frequency T was ≥ 5. Test statistic is represented by χ2. When the sample size was ≥ 40 but the theoretical frequency T was ≥ 1 but < 5, the chi-square test was adjusted using the correction formula. At a sample size of < 40 or theoretical frequency T of < 1, statistical analysis was conducted using the Fisher’s exact probability method. For normally distributed continuous data, the mean ± SD is employed. Non-normally distributed data were analysed using the Wilcoxon rank-sum test. Using NEC in newborns as the diagnostic variable and serum SAA, PCT, and HMGB1 as state variables, we constructed receiver operating characteristic (ROC) curves to calculate sensitivity and specificity, determine the area under the curve (AUC), Youden index, and optimal predictive threshold, and analyse the predictive value of serum SAA, PCT, and HMGB1 for NEC in newborns. A combined predictive model of serum SAA, PCT, and HMGB1 was developed, and the K-nearest neighbour classification algorithm was utilised to compute its AUC value. A P-value < 0.05 was considered statistically significant.
The comparison of baseline data between healthy newborns and the NEC group revealed no significant differences in gender distribution (χ2 = 0.000, P = 0.997), with 50.00% males and 50.00% females in the healthy group vs 47.92% males and 52.08% females in the NEC group (Table 1). Similarly, the age distribution showed no significant differences (χ2 = 0.038, P = 0.846), and 46.00% of healthy newborns and 50.00% of NEC cases were over 6 d old. The body mass distribution revealed no significant differences (χ2 = 2.562, P = 0.109), and 40.00% of healthy newborns and 22.92% of NEC cases weighed ≥ 2500 g. Furthermore, obstetric asphyxia during childbirth did not significantly differ (χ2 = 2.663, P = 0.103) between the two groups but affected 44.00% of healthy newborns and 62.50% of NEC cases. Finally, no significant difference was observed in delivery method between the two groups (χ2 = 0.000, P = 0.997), and 50.00% of healthy newborns and 47.92% of NEC cases were delivered through natural childbirth. Overall, these results indicated that the baseline characteristics of the healthy and NEC groups were comparable.
| Parameter | Healthy newborns (n = 50) | NEC group (n = 48) | t/χ2 | P value |
| Gender | ||||
| Male | 25 (50.00) | 23 (47.92) | 0.000 | 0.997 |
| Female | 25 (50.00) | 25 (52.08) | ||
| Age (d) | ||||
| ≥ 6 | 23 (46.00) | 24 (50.00) | 0.038 | 0.846 |
| < 6 | 27 (54.00) | 24 (50.00) | ||
| Body mass (g) | ||||
| ≥ 2500 | 20 (40.00) | 11 (22.92) | 2.562 | 0.109 |
| < 2500 | 30 (60.00) | 37 (77.08) | ||
| Obstetric asphyxia during childbirth | ||||
| Yes | 22 (44.00) | 30 (62.50) | 2.663 | 0.103 |
| No | 28 (56.00) | 18 (37.50) | ||
| Delivery method | ||||
| Natural childbirth | 25 (50.00) | 23 (47.92) | 0.000 | 0.997 |
| Caesarean section | 25 (50.00) | 25 (52.08) |
The comparison of laboratory examination between healthy newborns and the NEC group revealed significant differences in several parameters (Table 2). The NEC group exhibited a significantly higher WBC count (18.96 ± 4.25 × 109/L) compared with healthy newborns (16.57 ± 3.68 × 109/L; t = 2.970, P = 0.004). Moreover, neutrophil percentage (%) was markedly elevated in the NEC group (68.25% ± 6.48%) compared with healthy newborns (62.51% ± 5.32%; t = 4.778, P < 0.001). Similarly, lymphocyte percentage (%) was significantly lower in the NEC group (34.63% ± 5.53%) compared with healthy newborns (41.38% ± 6.27%; t = 5.659, P < 0.001). However, no statistically significant differences were observed in platelet count, serum albumin levels, or haemoglobin between the two groups. Notably, CRP levels were significantly higher in the NEC group (18.75 ± 5.62 mg/L) compared with healthy newborns (16.14 ± 5.08 mg/L; t = 2.410, P = 0.018). These results indicate the potential impact of NEC on newborns.
| Parameter | Healthy newborns (n = 50) | NEC group (n = 48) | t value | P value |
| WBC count (× 109/L) | 16.57 ± 3.68 | 18.96 ± 4.25 | 2.970 | 0.004 |
| Neutrophil percentage (%) | 62.51 ± 5.32 | 68.25 ± 6.48 | 4.778 | < 0.001 |
| Lymphocyte percentage (%) | 41.38 ± 6.27 | 34.63 ± 5.53 | 5.659 | < 0.001 |
| Platelet count (× 109/L) | 210.75 ± 45.19 | 200.35 ± 38.57 | 1.227 | 0.223 |
| Serum albumin (g/L) | 30.64 ± 3.83 | 29.42 ± 3.69 | 1.608 | 0.111 |
| CRP level (mg/L) | 16.14 ± 5.08 | 18.75 ± 5.62 | 2.410 | 0.018 |
| Haemoglobin | 10.87 ± 1.54 | 10.36 ± 1.84 | 1.483 | 0.141 |
The comparison of serum biomarkers between healthy newborns and the NEC group revealed substantial differences (Table 3). The NEC group exhibited significantly elevated levels of serum SAA (15.67 ± 3.28 mg/L) compared with healthy newborns (11.12 ± 3.52 mg/L; t = 6.625, P < 0.001). Similarly, serum PCT levels markedly increased in the NEC group (4.22 ± 1.56 ng/mL) compared with healthy newborns (2.35 ± 1.15 ng/mL; t = 6.754, P < 0.001). Furthermore, serum HMGB1 Levels were significantly higher in the NEC group (68.93 ± 10.03 ng/mL) than in healthy newborns (54.54 ± 10.28 ng/mL; t = 7.012, P < 0.001). These findings underscore the potential diagnostic value of serum SAA, PCT, and HMGB1 as valuable biomarkers for the identification of NEC in newborns, highlighting their utility in clinical practice.
| Parameter | Healthy newborns (n = 50) | NEC group (n = 48) | t value | P value |
| Serum SAA (mg/L) | 11.12 ± 3.52 | 15.67 ± 3.28 | 6.625 | < 0.001 |
| Serum PCT (ng/mL) | 2.35 ± 1.15 | 4.22 ± 1.56 | 6.754 | < 0.001 |
| Serum HMGB1 (ng/mL) | 54.54 ± 10.28 | 68.93 ± 10.03 | 7.012 | < 0.001 |
The correlation analysis demonstrated a strong positive correlation between serum SAA levels and the presence of NEC in newborns (r = 0.56, P < 0.001), indicating that the high SAA levels were associated with the condition (Table 4). Similarly, serum PCT levels exhibited a strong positive correlation with NEC (r = 0.57, P < 0.001). Furthermore, serum HMGB1 Levels showed a robust positive correlation with NEC (r = 0.582, P < 0.001), suggesting a potential association between elevated HMGB1 Levels and the presence of the disease. These findings underscore the potential utility of serum SAA, PCT, and HMGB1 as indicative biomarkers for the diagnosis of NEC in newborns, thus holding promise for improving clinical management and outcomes in affected neonates.
| Parameter | R | R2 | P value |
| Serum SAA (mg/L) | 0.560 | 0.313 | < 0.001 |
| Serum PCT (ng/mL) | 0.570 | 0.325 | < 0.001 |
| Serum HMGB1 (ng/mL) | 0.582 | 0.338 | < 0.001 |
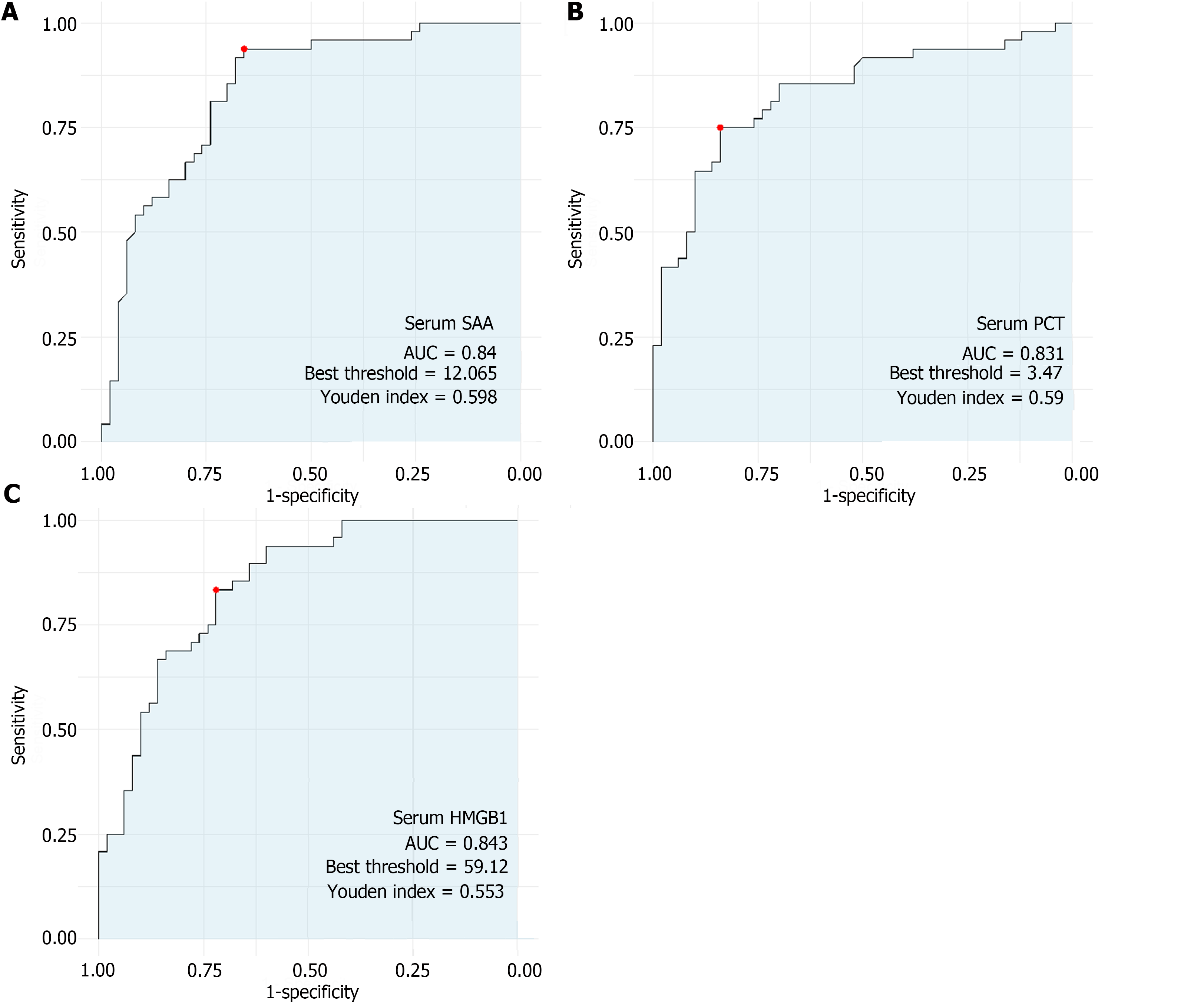
The predictive value analysis revealed promising sensitivity and specificity for serum SAA, PCT, and HMGB1 as potential diagnostic markers for NEC in newborns (Figure 1). Serum SAA exhibited a high sensitivity (0.938) and moderate specificity (0.660), yielding an AUC of 0.840. Similarly, serum PCT demonstrated a good sensitivity (0.750) and specificity (0.840), with an AUC of 0.831, which indicated its potential as a diagnostic indicator for the condition. Furthermore, serum HMGB1 presented a strong sensitivity (0.833), moderate specificity (0.720), and AUC of 0.843, further corroborating its value as a biomarker for the diagnosis of NEC in newborns. These results suggest the potential utility of serum SAA, PCT, and HMGB1 in clinical practice for the early and accurate identification of NEC, thus aiding in timely interventions and improving outcomes for affected neonates.
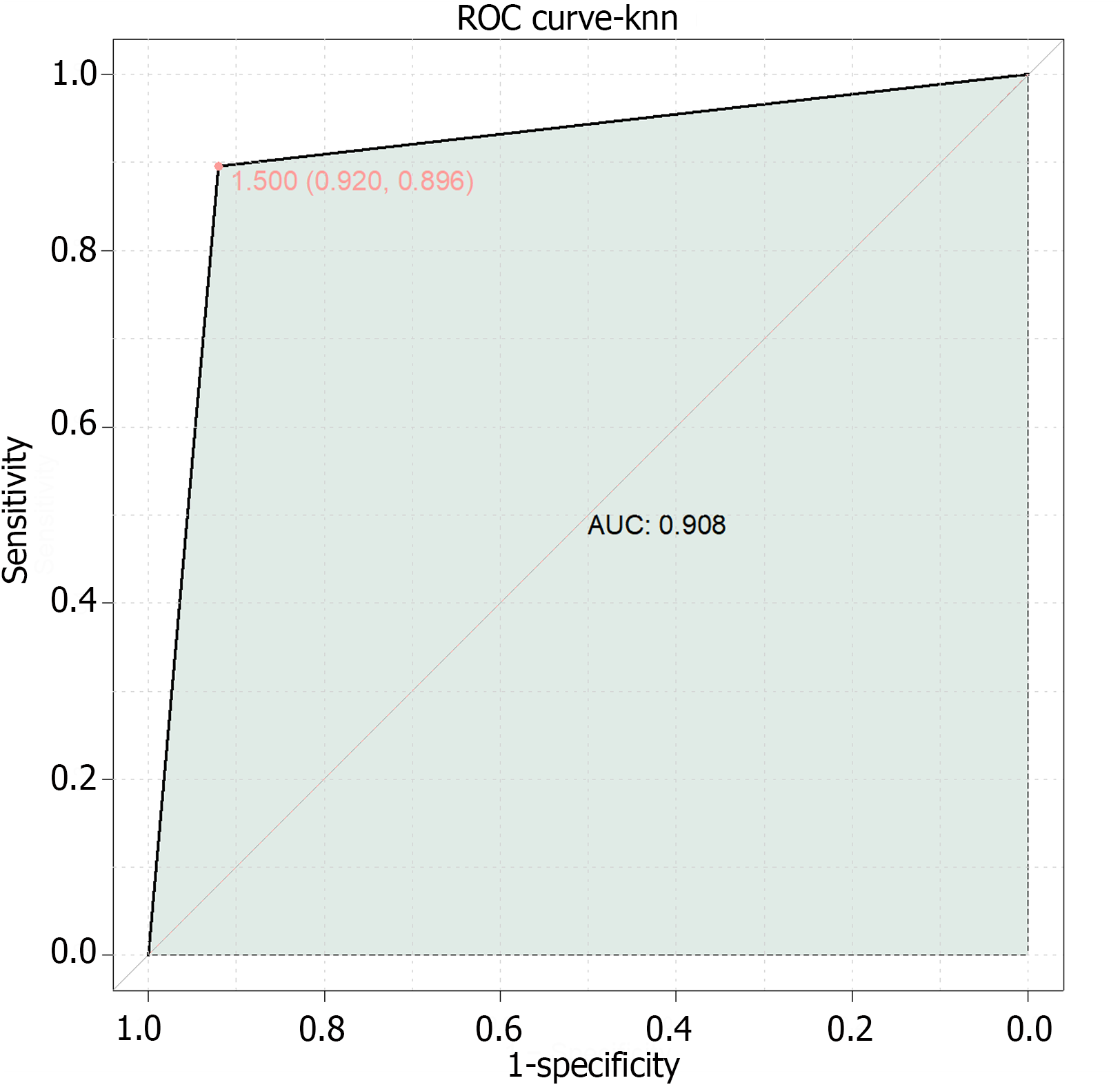
Finally, this study combined serum SAA, PCT, and HMGB1 for the development of a composite model for the prediction of NEC in newborns (Figure 2). The results demonstrated an AUC value of 0.908, indicating that the combined model of serum SAA, PCT, and HMGB1 has high predictive value for the diagnosis of NEC in newborns.
NEC remains an important cause of morbidity and mortality in premature and low-birth-weight neonates, and early and accurate diagnostic methods for improving outcomes are urgently needed[16,17]. In this study, we retrospectively analysed the diagnostic potential of SAA, PCT, and HMGB1 as biomarkers for NEC in newborns. The findings revealed significant differences in the levels of these biomarkers between healthy newborns and those diagnosed with NEC, highlighting their potential diagnostic value.
The clinical manifestations of NEC are diverse, ranging from nonspecific signs, such as feeding intolerance and abdominal distension, to severe systemic complications, highlighting the challenge of early diagnosis[18]. The lack of specific and sensitive biomarkers for NEC has contributed to delays in initiating appropriate treatment, increasing the risk of disease progression and associated complications[19]. Consequently, identifying biomarkers that can aid in the early diagnosis and risk stratification of NEC is crucial for clinical management and outcomes in affected neonates.
SAA, an acute-phase protein, has been identified as a potential biomarker for NEC because of its rapid elevation in response to proinflammatory cytokines[20]. The present study demonstrated significantly elevated levels of serum SAA in newborns diagnosed with NEC compared with healthy controls. These findings align with the known role of SAA as a marker of acute-phase inflammation and tissue injury[21], suggesting its potential utility in early NEC diagnosis. The strong positive correlation between serum SAA levels and the presence of NEC further supports its role as a diagnostic biomarker.
PCT, a precursor of calcitonin, has been extensively studied for its diagnostic and prognostic value in various infectious conditions[22,23]. In the context of NEC, elevated PCT levels were significantly associated with the condition, indicating its potential as a diagnostic marker[24]. The study’s findings of increased serum PCT levels in newborns diagnosed with NEC are consistent with the understanding of PCT as a marker of bacterial infections and systemic inflammation[25]. The presence of strong positive correlations between serum PCT levels and NEC further underscores its potential diagnostic relevance in this context.
HMGB1 protein has emerged as a key mediator of inflammation and tissue injury in various pathological conditions[26,27]. The study revealed significantly higher serum HMGB1 Levels in newborns with NEC compared to healthy controls, suggesting its potential as a diagnostic biomarker for the condition. The robust positive correlation between serum HMGB1 Levels and the presence of NEC supports its potential diagnostic value in identifying NEC in newborns. These findings align with the known role of HMGB1 in inflammation and immune regulation[28], indicating its utility as a diagnostic marker in the context of NEC.
The results of the ROC analysis further underscore the potential diagnostic value of these biomarkers. Serum SAA, PCT, and HMGB1 exhibited promising sensitivities and specificities, with AUC values suggesting their potential utility as diagnostic markers for NEC. Notably, the combined predictive model of serum SAA, PCT, and HMGB1 demonstrated an extremely high AUC value, indicating the synergistic potential of these biomarkers in improving the accuracy of NEC diagnosis in newborns.
The results of this study have significant implications for clinical practice and future research. The identification of serum SAA, PCT, and HMGB1 as potential biomarkers for NEC offers a promising avenue for the development of diagnostic tools that can aid in the early detection and risk stratification of NEC in newborns. By harnessing the diagnostic value of these biomarkers, clinicians may be equipped to initiate timely interventions, thereby improving outcomes for neonates affected by NEC.
Future research endeavours should focus on prospective validation studies to further establish the diagnostic accuracy of serum SAA, PCT, and HMGB1 in NEC. Longitudinal studies could provide insights into the kinetics of these biomarkers throughout the course of NEC, shedding light on their potential prognostic value and utility in monitoring disease progression and therapeutic responses. Additionally, the integration of these biomarkers into multiplex diagnostic platforms may enhance their clinical applicability and utility in routine practice.
This study provides compelling evidence supporting the diagnostic value of serum SAA, PCT, and HMGB1 in the context of NEC in newborns. The findings underscore the potential of compounds as biomarkers for early detection, risk stratification, and clinical management of this critical condition, ultimately improving outcomes for affected neonates.
| 1. | Bellodas Sanchez J, Kadrofske M. Necrotizing enterocolitis. Neurogastroenterol Motil. 2019;31:e13569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Duchon J, Barbian ME, Denning PW. Necrotizing Enterocolitis. Clin Perinatol. 2021;48:229-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Neu J. Necrotizing Enterocolitis: The Future. Neonatology. 2020;117:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Bosco A, Piu C, Picciau ME, Pintus R, Fanos V, Dessì A. Metabolomics in NEC: An Updated Review. Metabolites. 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 5. | Kim JH. Role of Abdominal US in Diagnosis of NEC. Clin Perinatol. 2019;46:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Meister AL, Doheny KK, Travagli RA. Necrotizing enterocolitis: It's not all in the gut. Exp Biol Med (Maywood). 2020;245:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Bethell GS, Hall NJ. Recent advances in our understanding of NEC diagnosis, prognosis and surgical approach. Front Pediatr. 2023;11:1229850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Hackam DJ, Sodhi CP. Bench to bedside - new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol. 2022;19:468-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 9. | Webb NR. High-Density Lipoproteins and Serum Amyloid A (SAA). Curr Atheroscler Rep. 2021;23:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Sack GH Jr. Serum Amyloid A (SAA) Proteins. Subcell Biochem. 2020;94:421-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Horns H, Draenert R, Nistal M. [Procalcitonin]. MMW Fortschr Med. 2021;163:54-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Mobed A, Darvishi M, Tahavvori A, Alipourfard I, Kohansal F, Ghazi F, Alivirdiloo V. Nanobiosensors for procalcitonin (PCT) analysis. J Clin Lab Anal. 2024;38:e25006. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Chen R, Kang R, Tang D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 2022;54:91-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 451] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 14. | Yang H, Wang H, Andersson U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 15. | Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI, Carbone M, Yang H. HMGB1 as a therapeutic target in disease. J Cell Physiol. 2021;236:3406-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 16. | Golubkova A, Hunter CJ. Updates and recommendations on the surgical management of NEC. Semin Perinatol. 2023;47:151698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Klerk DH, van Avezaath LK, Loeffen EAH, Hulscher JBF, Kooi EMW. Fetal-neonatal exposure to antibiotics and NEC development: A systematic review and meta-analysis. Front Pediatr. 2022;10:1102884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Lapidaire W, Lucas A, Clayden JD, Clark C, Fewtrell MS. Human milk feeding and cognitive outcome in preterm infants: the role of infection and NEC reduction. Pediatr Res. 2022;91:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Garg PM, Paschal JL, Ansari MAY, Block D, Inagaki K, Weitkamp JH. Clinical impact of NEC-associated sepsis on outcomes in preterm infants. Pediatr Res. 2022;92:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Coufal S, Kokesova A, Tlaskalova-Hogenova H, Frybova B, Snajdauf J, Rygl M, Kverka M. Urinary I-FABP, L-FABP, TFF-3, and SAA Can Diagnose and Predict the Disease Course in Necrotizing Enterocolitis at the Early Stage of Disease. J Immunol Res. 2020;2020:3074313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Wang Q, Jin K, Su X, Liu J. Predictive value of serum markers in the operation evaluation of neonatal necrotizing enterocolitis. Transl Pediatr. 2023;12:897-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Cai N, Liao W, Chen Z, Tao M, Chen S. The Mean Platelet Volume Combined with Procalcitonin as an Early Accessible Marker Helps to Predict the Severity of Necrotizing Enterocolitis in Preterm Infants. Int J Gen Med. 2022;15:3789-3795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Zhang C, Zhao X, Zhu Z, Wang K, Moon BF, Zhang B, Sadat SN, Guo J, Bao J, Zhang D, Zhang X. Evaluation of white matter microstructural alterations in premature infants with necrotizing enterocolitis. Quant Imaging Med Surg. 2023;13:6412-6423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Elfarargy MS, El Farargy MS, Atef MM, El-Deeb OS, Elsharaby RM, Elhady HAE. Early Biomarkers in Neonatal Necrotizing Enterocolitis: A Pilot Study. J Popul Ther Clin Pharmacol. 2019;26:e1-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Liebe H, Lewis S, Loerke C, Golubkova A, Leiva T, Stewart K, Sarwar Z, Gin A, Porter M, Chaaban H, Hunter CJ. A Retrospective Case Control Study Examining Procalcitonin as a Biomarker for Necrotizing Enterocolitis. Surg Infect (Larchmt). 2023;24:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Huo R, Liu H, Chen J, Sheng H, Miao L. Serum HMGB1 level is correlated with serum I-FABP level in neonatal patients with necrotizing enterocolitis. BMC Pediatr. 2021;21:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Cao H, Guo D. Association of High-Mobility Group Box 1 (HMGB1) Gene Polymorphisms with Susceptibility and Better Survival Prognosis in Chinese Han Neonatal Necrotizing Enterocolitis. Med Sci Monit. 2021;27:e930015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Yu R, Jiang S, Tao Y, Li P, Yin J, Zhou Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J Cell Physiol. 2019;234:13431-13438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |