Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.1969
Revised: May 9, 2024
Accepted: May 28, 2024
Published online: July 27, 2024
Processing time: 126 Days and 23.2 Hours
This editorial discusses the article “Analysis of the impact of immunotherapy efficacy and safety in patients with gastric cancer and liver metastasis” published in the latest edition of the World Journal of Gastrointestinal Surgery. Immunotherapy has achieved outstanding success in tumor treatment. However, the presence of liver metastasis (LM) restrains the efficacy of immunotherapy in various tumors, including lung cancer, colorectal cancer, renal cell carcinoma, melanoma, and gastric cancer. A decrease in CD8+ T cells and nature killer cells, along with an increase in macrophages and regulatory T cells, was observed in the microenvironment of LM, leading to immunotherapy resistance. More studies are necessary to determine the best strategy for enhancing the effectiveness of immunotherapy in patients with LM.
Core Tip: The liver is one of the most common sites for tumor metastasis. This editorial reviews the impact of liver metastasis on immunotherapy effectiveness and the possible mechanisms, with an aim to provide new clues for clinical treatment in liver metastatic patients receiving immunotherapy.
- Citation: Fu Z, Wang MW, Liu YH, Jiao Y. Impact of immunotherapy on liver metastasis. World J Gastrointest Surg 2024; 16(7): 1969-1972
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/1969.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.1969
Immunotherapy, including immune checkpoint inhibitors, aiming to enhance the anti-tumor response of the body’s own immune system, has achieved remarkable success in treating various tumors[1]. However, despite the significant advances in immunotherapy, only a small subset of patients can benefit from it, while the majority of patients encounter the problem of drug resistance[2]. Up to now, a variety of mechanisms behind resistance to immunotherapy have been explored, and some clinical features are correlated with immune resistance.
Metastasis is a crucial characteristic of malignant tumors, and is also the primary cause of mortality in cancer patients[3]. The liver is one of the most frequent sites of metastasis in various tumors, including lung cancer, colon cancer, and melanoma[4]. Several studies have investigated the influences of liver metastasis (LM) on immunotherapy, and one of them is an article titled “Analysis of the impact of immunotherapy efficacy and safety in patients with gastric cancer and liver metastasis” which was published in the latest issue of the World Journal of Gastrointestinal Surgery.
The efficacy of immunotherapy on advanced cancers with LM has been reported. In non-small cell lung cancer (NSCLC), an anti-programmed death ligand 1 (anti-PD-L1) monoclonal antibody, atezolizumab, could improve overall survival (OS) of patients with LM compared with standard therapy[5]. However, in several studies, patients with LM have a poorer prognosis compared to those without.
Studies have demonstrated that LM was an independent unfavorable prognostic factor in different tumors. In Komiya et al’s[6] study on stage IV NSCLC, OS of patients with LM was significantly worse than that of patients without LM, which indicated that LM was a poor prognostic factor for immunotherapy in advanced lung cancer. Similarly, colorectal cancer patients without LM had better clinical response and superior progression-free survival (PFS) compared to patients with LM when treated with programmed death-1 or PD-L1 targeting therapy[7]. In research of renal cell carcinoma and melanoma, Kaplan-Meier analysis also presented longer OS and PFS in non-LM patients receiving systemic therapy[8,9]. In the latest issue of the World Journal of Gastrointestinal Surgery, Liu et al[10] found that immunotherapy was less effective in patients with advanced gastric cancer and LM, which expanded the findings to gastric cancer.
Although the precise mechanism on how LM weakens the benefits of immunotherapy has not been clearly defined, accumulating evidence has suggested that the tumor microenvironment (TME) played a critical role[11]. The TME is a complex system that mainly contains tumor cells, stromal cells, infiltrating immune cells, and the extracellular matrix[12]. Among them, infiltrating immune cells are strongly associated with tumor progression and immunotherapy responses.
T cells have been recognized as the center of tumor immunology. T cells can be divided into different subtypes, including cytotoxic T cells (CTLs), T helper (Th) cells, and regulatory T cells (Tregs)[13]. CD8+ CTLs are the major effector cells in the TME. CTLs could directly kill target cells via the interaction with Fas/Fas ligands, or indirectly via cytotoxic molecules such as perforin and granzymes[14]. Th cells are CD4+ T cells, and can be divided into Th1 and Th2 according to different cytokines secreted. Th1 cells secrete proinflammatory cytokines like interferon-gamma to play an antitumor role, while Th2 cells produce elevated levels of anti-inflammatory cytokines like interleukin-4 in order to suppress immune responses and facilitate tumor growth and metastasis[15]. Tregs are important immunosuppressive cells in the TME, exerting immunosuppressive effects through direct contact and secretion of inhibitory cytokines, thereby suppressing antigen presenting process and pro-inflammatory cell activation, and ultimately promoting tumor progression[16].
Nature killer (NK) cells and macrophages belong to the innate immune system. NK cells, as a class of cytotoxic lymphocytes, eliminate cancer cells in an MHC-independent manner[17]. Macrophages are phagocytic cells with diverse phenotypes and functions. M1 macrophages release inflammatory mediators that facilitate inflammatory reactions to enhance the anti-tumor immune responses, while M2 macrophages exhibit elevated expression of anti-inflammatory and growth factors which inhibit the function of CTLs and promote tumor growth and metastasis[18]. Tumor associated macrophages (TAMs) typically demonstrate an M2 phenotype and facilitate tumor progression[19].
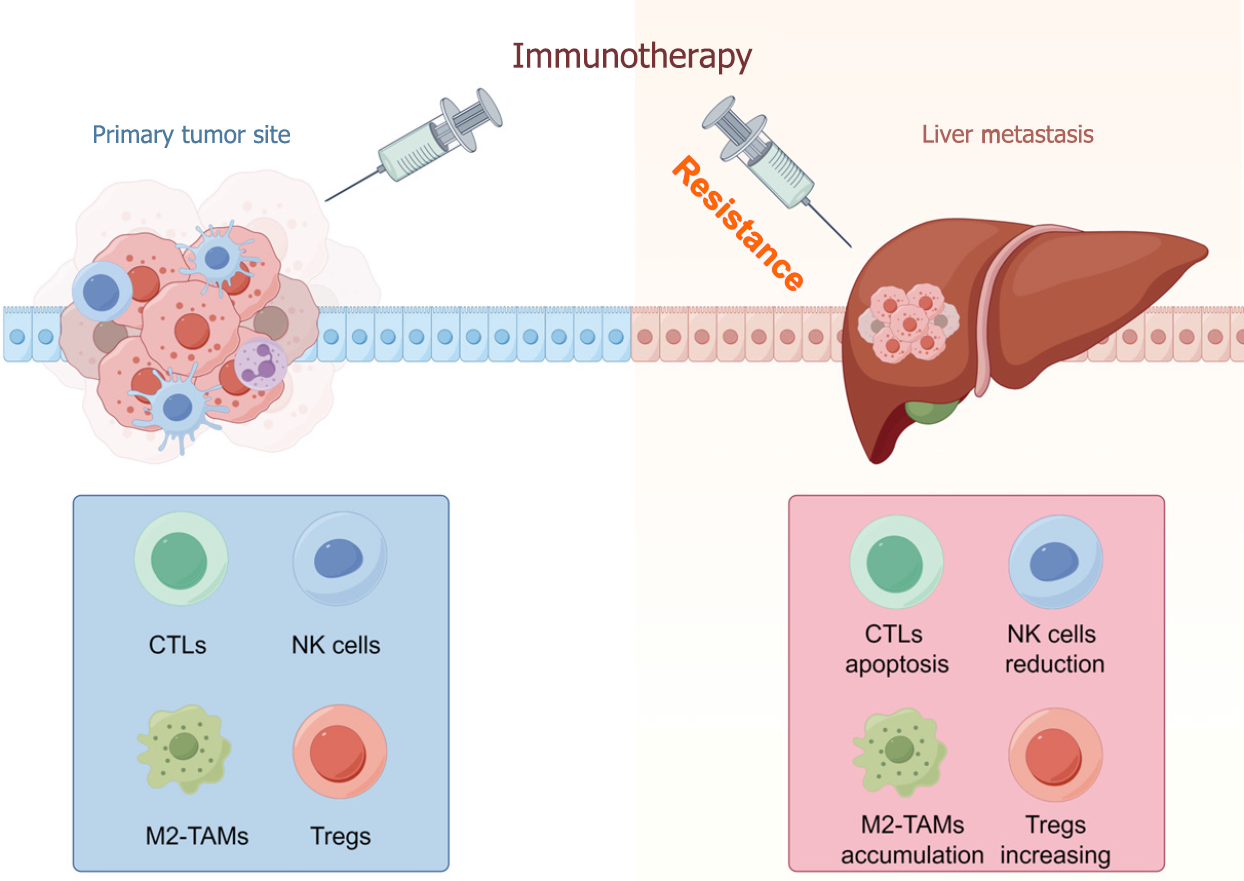
The inhibitory TME of LM is characterized by the absence of effective cells and massive infiltration of inhibitory immune components (Figure 1). RNA-seq analysis showed the decreased immune cell infiltration, especially CD8+ T cells in LM from more than 16 cancer types[20]. Jakubowska et al[21] using histopathological examination confirmed the reduction of tumor infiltrating lymphocytes in LM tissues compared with primary colorectal cancer. Besides, the percentage of NK cells was also reduced in mice with LM[22]. TAMs and Tregs, as important inhibitory cells, both were increased significantly in the LM microenvironment[23,24]. In addition, Yu et al[25] found that increased macrophages by LM expressing high levels of FasL, could recruit and induce the apoptosis of CD8+ T cells, leading to antigen specific T cell diminish in subcutaneous tumor, lymph nodes, and peripheral blood. The hepatic TME suppresses immune response, which in turn facilitates tumor progression and immunotherapy resistance[26].
In conclusion, the presence of LM is a poor prognostic factor in multiple tumors treated with immunotherapy. LM provides an immunosuppressive microenvironment with effect cell reduction and inhibitory cell activation, resulting in immunotherapy resistance. Further studies on the TME are needed to find the optimal combination strategy to improve the efficacy of immunotherapy.
| 1. | Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1361] [Cited by in RCA: 1790] [Article Influence: 358.0] [Reference Citation Analysis (0)] |
| 2. | Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 600] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 3. | Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 4. | Park JH, Kim JH. Pathologic differential diagnosis of metastatic carcinoma in the liver. Clin Mol Hepatol. 2019;25:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Yin WJ, Ma SC, Dong ZY, Xu M, Mao W. Efficacy and Treatment Strategies in Advanced Cancers with Liver Metastasis Receiving Atezolizumab Therapy. Cancer Manag Res. 2021;13:4541-4551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Komiya T, Takamori S, Shimokawa M. Impact of Liver Metastasis on First-Line Immunotherapy in Stage IV Non-Small Cell Lung Cancer. World J Oncol. 2023;14:234-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Wang C, Sandhu J, Ouyang C, Ye J, Lee PP, Fakih M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw Open. 2021;4:e2118416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Kim SH, Kim JK, Park EY, Joo J, Lee KH, Seo HK, Joung JY, Chung J. Liver metastasis and Heng risk are prognostic factors in patients with non-nephrectomized synchronous metastatic renal cell carcinoma treated with systemic therapy. PLoS One. 2019;14:e0211105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Wang X, Ji Q, Yan X, Lian B, Si L, Chi Z, Sheng X, Kong Y, Mao L, Bai X, Tang B, Li S, Zhou L, Cui C, Guo J. The Impact of Liver Metastasis on Anti-PD-1 Monoclonal Antibody Monotherapy in Advanced Melanoma: Analysis of Five Clinical Studies. Front Oncol. 2020;10:546604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Liu K, Wu CX, Liang H, Wang T, Zhang JY, Wang XT. Analysis of the impact of immunotherapy efficacy and safety in patients with gastric cancer and liver metastasis. World J Gastrointest Surg. 2024;16:700-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Vesely MD, Zhang T, Chen L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu Rev Immunol. 2022;40:45-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 86.7] [Reference Citation Analysis (6)] |
| 12. | Belli C, Trapani D, Viale G, D'Amico P, Duso BA, Della Vigna P, Orsi F, Curigliano G. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 13. | Todryk S, Jozwik A, de Havilland J, Hester J. Emerging Cellular Therapies: T Cells and Beyond. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234:8509-8521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 1129] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 15. | Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22:3855-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |
| 16. | Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 17. | Guillerey C. NK Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Zhang J, Zhou X, Hao H. Macrophage phenotype-switching in cancer. Eur J Pharmacol. 2022;931:175229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, Khezri Z, Majidpoor J, Abouzaripour M, Habibi M, Kashani IR, Mortezaee K. Macrophage polarity in cancer: A review. J Cell Biochem. 2019;120:2756-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 362] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 20. | Gao Y, Chen S, Wang H, Wu C, An R, Li G, Yang M, Zhou Y, Xie X, Yu H, Zhang J. Liver metastases across cancer types sharing tumor environment immunotolerance can impede immune response therapy and immune monitoring. J Adv Res. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Jakubowska K, Koda M, Kisielewski W, Kańczuga-Koda L, Famulski W. Tumor-infiltrating lymphocytes in primary tumors of colorectal cancer and their metastases. Exp Ther Med. 2019;18:4904-4912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Liu S, Liu Y, Bhatt D, Estrada J, Belmontes B, Ren X, Canon J, Ouyang W. Liver Metastasis Modulate Responses of Suppressive Macrophages and Exhausted T Cells to Immunotherapy Revealed by Single Cell Sequencing. Adv Genet (Hoboken). 2022;3:2200002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Huang X, Chen Z, Zhang N, Zhu C, Lin X, Yu J, Lan P, Wan Y. Increase in CD4(+)FOXP3(+) regulatory T cell number and upregulation of the HGF/c-Met signaling pathway during the liver metastasis of colorectal cancer. Oncol Lett. 2020;20:2113-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, Daud A, Bluestone JA. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 25. | Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, Chopra Z, El Naqa I, Zhou J, Bian Y, Jiang L, Tezel A, Skvarce J, Achar RK, Sitto M, Rosen BS, Su F, Narayanan SP, Cao X, Wei S, Szeliga W, Vatan L, Mayo C, Morgan MA, Schonewolf CA, Cuneo K, Kryczek I, Ma VT, Lao CD, Lawrence TS, Ramnath N, Wen F, Chinnaiyan AM, Cieslik M, Alva A, Zou W. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 660] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 26. | Santhakumar C, Gane EJ, Liu K, McCaughan GW. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. 2020;14:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |