Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1871
Revised: April 19, 2024
Accepted: April 29, 2024
Published online: June 27, 2024
Processing time: 146 Days and 13.4 Hours
The development of laparoscopic technology has provided a new choice for surgery of gastric cancer (GC), but the advantages and disadvantages of laparoscopic total gastrectomy (LTG) and laparoscopic-assisted total gastrectomy (LATG) in treatment effect and safety are still controversial. The purpose of this study is to compare the efficacy and safety of the two methods in the treatment of GC, and to provide a basis for clinical decision-making.
To compare the efficacy of totally LTG (TLTG) and LATG in the context of radical gastrectomy for GC. Additionally, we investigated the safety and feasibility of the total laparoscopic esophagojejunostomy technique.
Literature on comparative studies of the above two surgical methods for GC (TLTG group and LATG group) published before September 2022 were searched in the PubMed, Web of Science, Wanfang Database, CNKI, and other Chinese and English databases. In addition, the following search keywords were used: Gastric cancer, total gastrectomy, total laparoscopy, laparoscopy-assisted, esophagojejunal anastomosis, gastric/stomach cancer, total gastrectomy, totally/completely laparoscopic, laparoscopic assisted/laparoscopy assisted/laparoscopically assisted, and esophagojejunostomy/esophagojejunal anastomosis. Review Manager 5.3 software was used for the meta-analysis after two researchers independently screened the literature, extracted the data, and evaluated the risk of bias in the included studies.
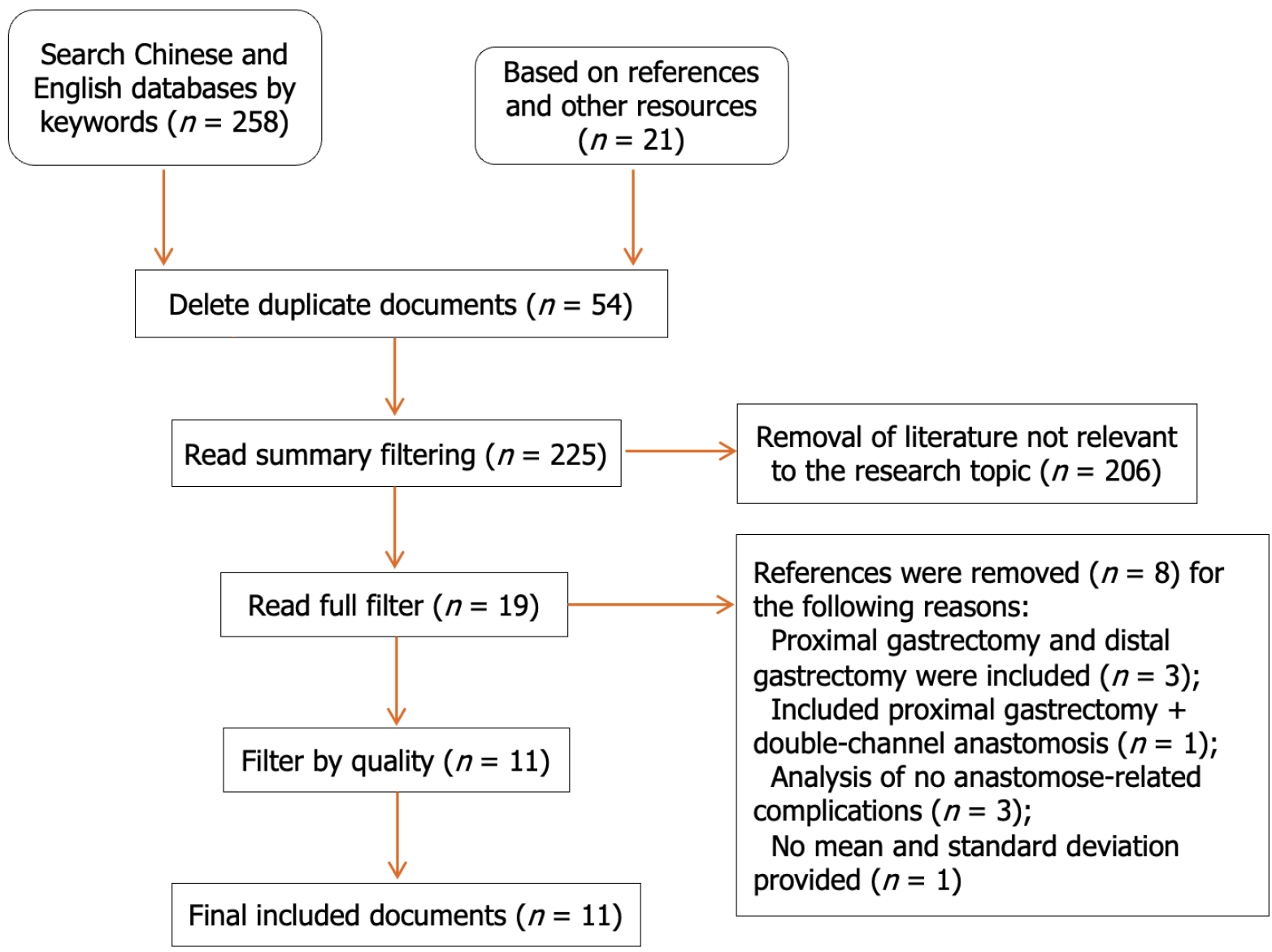
After layer-by-layer screening, 258 pieces of literature were recovered, and 11 of those pieces were eventually included. This resulted in a sample size of 2421 instances, with 1115 cases falling into the TLTG group and 1306 cases into the LATG group. Age or sex differences between the two groups were not statistically significant, according to the meta-analysis, however the average body mass index of the TLTG group was considerably higher than that of the LATG group (P = 0.01). Compared with those in the LATG group, the incision length in the TLTG group was significantly shorter (P < 0.001), the amount of intraoperative blood loss was significantly lower (P = 0.003), the number of lymph nodes removed was significantly greater (P = 0.04), and the time of first postoperative feeding and postoperative hospitalization were also significantly shorter (P = 0.03 and 0.02, respectively). There were no significant differences in tumor size, length of proximal incisal margin, total operation time, anastomotic time, postoperative pain score, postoperative anal exhaust time, postoperative anastomosis-related complications (including anastomotic fistula, anastomotic stenosis, and anastomotic hemorrhage), or overall postoperative complication rate (P > 0.05).
TLTG and esophagojejunostomy are safe and feasible. Compared with LATG, TLTG has the advantages of less trauma, less bleeding, easier access to lymph nodes, and faster postoperative recovery, and TLTG is also suitable for obese patients.
Core Tip: This study used a systematic review and meta-analysis to determine how well and safely laparoscopic total gastrectomy and laparoscopically assisted total gastrectomy can treat gastric cancer (GC). Clinical trial data from relevant literature were collected and analyzed to evaluate the differences between the two surgical methods in terms of surgical effect, postoperative complications, and postoperative quality of life. Through the systematic synthesis of the results, an objective evaluation of the advantages and disadvantages of these two surgical methods is provided, which provides a scientific basis for clinicians to optimize the treatment of GC patients.
- Citation: Li L, Liu DY, Leng J, Tao XM, Wu HQ, Zhu YP. Comparison efficacy and safety of total laparoscopic gastrectomy and laparoscopically assisted total gastrectomy in treatment of gastric cancer. World J Gastrointest Surg 2024; 16(6): 1871-1882
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1871.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1871
Surgery has been used for more advanced cases of gastric cancer (GC) as well as earlier cases of the disease[1]. Additionally, surgical methods have changed from laparoscopic-assisted radical gastrectomy to total laparoscopic radical gastrectomy[2]. However, reconstructing the digestive tract through total laparoscopic surgery is difficult. This is especially true for totally laparoscopic total gastrectomy (TLTG) and esophagojejunostomy, which require advanced endoscopic techniques and are not sure how well they work. There is also debate about whether they are safe and possible[3].
This study will conduct a meta-analysis of published comparative studies on TLTG and laparoscopic-assisted total gastrectomy (LATG) in the treatment of GC, aiming to observe the difference in efficacy of the two surgical methods in the treatment of GC and discuss the safety and feasibility of TLTG for GC and esophagojejunostomy. The aim is to obtain the best evidence to guide clinical practice.
The subject of this study was a controlled clinical study of TLTG and LATG for GC published before September 2023. The search databases used were PubMed, Web of Science, Wanfang Database, CNKI, and other Chinese and English databases. Keywords in the Chinese search were GC, total gastrectomy, total laparoscopy, and laparoscopy-assisted esophagojejunostomy. The key words in English were gastric/stomach cancer, total gastrectomy, totally/completely laparoscopic, laparoscopic assisted/laparoscopic assisted/laparoscopic assisted, and esophagojejunostomy/esophagojejunal anastomosis. The literature was reviewed by hand.
All patients were confirmed by pathological examination to have GC; (2) all studies involved the comparison of the efficacy of TLTG and LATG in GC; (3) accurate and important clinical data should be provided, especially the incidence of postoperative anastomosis-related complications; (4) original statistical data should be provided, such as the mean and standard deviation of continuous variables and specific values of binary variables; and (5) for the literature of the same unit, recently published literature with higher quality statistics should be selected.
(1) Patients with cancers other than those of the stomach; (2) patients who underwent distal gastrectomy, proximal gastrectomy, or palliative total gastrectomy; (3) patients who received neoadjuvant chemotherapy before surgery; and (4) patients whose clinical data, such as the rate of anastomosis-related complications, were not available.
Using a unified data collection table, two system evaluators independently extracted the following data from the included literature: first author, data source (country), publication time, number of cases, patient age, sex, body mass index (BMI), length of surgical incision, total surgical time, anastomosis time, intraoperative blood loss, number of lymph node removals, tumor size, length of proximal incisal margin, postoperative pain score (visual analog scale), postoperative anal exhaust time, postoperative eating time, postoperative hospital stay, postoperative surgery overall complication rate, postoperative anastomosis-related complication rate, and other indicators. Disagreements were resolved after discussion with a third researcher.
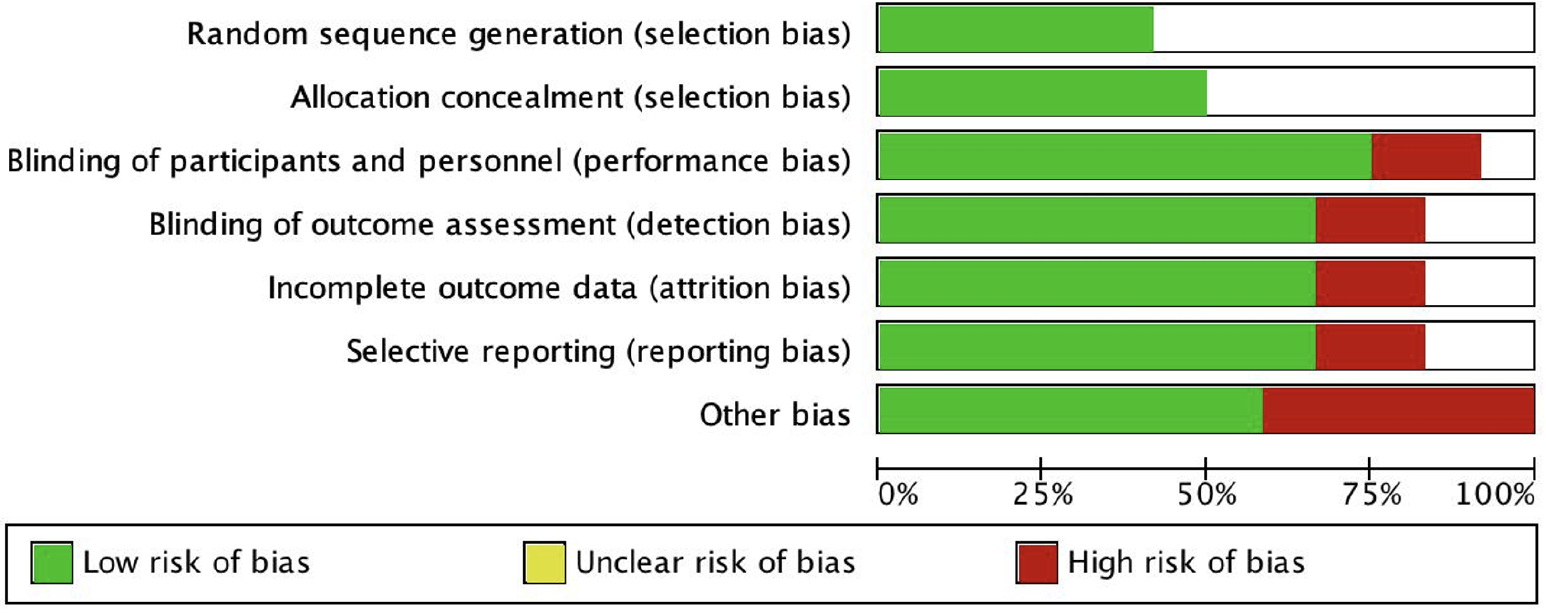
The Newcastle-Ottawa Scale (NOS) was used to score the quality of the included studies. The evaluation content included three main aspects: research selection, research comparison, and interesting research results. The maximum possible score was 9 points, and an overall score > 6 was considered to indicate high-quality research.
The Cochrane Collaboration provided RevMan 5.3 software for statistical analysis. The odds ratio of binary data was calculated as the combined statistic, and the weighted mean difference (WMD) of continuous variable data was calculated as the combined statistic. The results were expressed with a 95%CI, and the test level was α = 0.05. The incidence of postoperative anastomosis-related complications was measured by a funnel plot to determine publication bias. A heterogeneity test was conducted for all included studies. An I2 ≤ 50% indicated that there was no significant heterogeneity among all studies. A fixed effects model (F model) was used for combined analysis; otherwise, a random effects model (R model) was used. To avoid the influence of different surgical operators and surgical methods on the results, the R model was adopted for the analysis of clinical data related to surgery.
Based on the inclusion and exclusion criteria (Figure 1), 11 papers[4-14] about clinical control studies of TLTG and LATG in GC were ultimately included. These were all nonrandomized controlled studies. There were 2421 clinical cases, including 1115 in the TLTG group and 1306 in the LATG group, mainly from China, South Korea, and Japan. According to the NOS quality score, nine studies[4-12] were considered to be of high quality, all of which compared the two groups by age and sex. Seven of them[4-6,8-11] also compared the body mass indices of the two groups, and the results showed that there was no significant difference in age or sex between the two groups (P = 0.60 and 0.61, respectively), while the average BMI of the TLTG group was significantly greater than that of the LATG group (P = 0.01). The basic information and quality evaluation of the included studies are shown in Table 1.
| Ref. | Countries | Sample size | Age (yr, mean ± SD) | Body mass index (kg/m²), mean ± SD) | NOS score | |||
| TLTG group | LATG group | TLTG group | LATG group | TLTG group | LATG group | |||
| Kim et al[4], 2013 | South Korea | 90 | 23 | 58.0 ± 10.8 | 56.8 ± 14.2 | 23.2 ± 2.9 | 22.2 ± 1.8 | 7 |
| Kim et al[5], 2016 | South Korea | 27 | 29 | 60.8 ± 9.1 | 59.3 ± 13.1 | 24.0 ± 2.9 | 23.3 ± 3.2 | 7 |
| Chen et al[6] | China | 108 | 145 | 59.4 ± 11.1 | 57.3 ± 12.5 | 23.5 ± 3.5 | 23.1 ± 4.2 | 8 |
| Gong et al[7], 2017 | South Korea | 421 | 266 | 57.78 ± 11.2 | 55.69 ± 11.96 | / | / | 7 |
| Huang et al[8], 2017 | China | 51 | 456 | 55.5 ± 12.1 | 61.6 ± 11.2 | 22.5 ± 13.1 | 22.3 ± 13.5 | 7 |
| Lu et al[9], 2016 | China | 25 | 25 | 59+8.9 | 58.4 ± 7.7 | 22.5 ± 2.5 | 22.9 ± 3.7 | 8 |
| Cui et al[10] | China | 16 | 47 | 61.3 ± 13. | 67.6+13 | 22.8 ± 1.2 | 23.2+1.3 | 8 |
| Hong et al[11], 2017 | China | 183 | 190 | 58 ± 11 | 60 ± 10 | 23 ± 3 | 22 ± 3 | 8 |
| Hua et al[12], 2017 | China | 47 | 47 | 48.51 ± 2.47 | 48.67 ± 2.51 | / | 7 | |
| Xiao et al[13], 2015 | China | 30 | 32 | / | / | / | 6 | |
| Ito et al[14], 2014 | Japan | 117 | 46 | / | / | 6 | ||
The meta-analysis results of this study showed that, compared with those in the LATG group, the length of surgical incisions in the TLTG group was significantly shorter (P < 0.001), the amount of intraoperative blood loss was significantly less (P = 0.003), and the number of lymph nodes removed was significantly greater (P = 0.04). The time of first feeding and hospital stay were also significantly shorter (P = 0.03 and 0.02, respectively), but there were no significant differences in tumor size, length of proximal incisal margin, operation time, postoperative pain score, postoperative anal exhaust time, or postoperative complication rate (P > 0.05). Table 2 summarizes the intraoperative and postoperative information of the 11 studies included in this paper.
| Observation indicators | References | Sample size (examples) | Heterogeneity | Effect model | Comprehensive effect value | 95%CI | P value | |
| TLTG group | LATG group | |||||||
| Operative time | 9 | 664 | 1008 | < 0.001, 83% | R | WMD = 7.62 | -4.35-19.59 | 0.21 |
| Match time | 3 | 165 | 206 | < 0.001, 86% | R | WMD = 6.40 | -2.28-15.08 | 0.15 |
| Tumor size | 7 | 905 | 1134 | 0.02, 61% | R | WMD = -0.29 | -0.65-0.17 | 0.12 |
| Proximal edge distance | 5 | 597 | 512 | < 0.001, 84% | R | WMD = -0.22 | -0.86-0.41 | 0.49 |
| Intraoperative bleeding volume | 9 | 604 | 1017 | < 0.001, 84% | R | WMD = -26.29 | -43.70 to | 0.003 |
| Number of lymph node removals | 9 | 973 | 1235 | 0.02, 57% | R | WMD = 1.78 | 0.06-3.50 | 0.04 |
| Postoperative pain score | ||||||||
| On the first day after surgery (8:00 am) | 2 | 511 | 289 | 0.91, 0% | R | WMD = 0.03 | -0.20-0.27 | 0.78 |
| On the 3rd day after surgery (8:00 am) | 2 | 511 | 289 | 0.46, 0% | R | WMD = 0.08 | -0.13-0.28 | 0.45 |
| Postoperative peak pain | 2 | 511 | 289 | 0.09, 66% | R | WMD = 0.17 | -0.71-1.05 | 0.70 |
| Postoperative anal exhaust time | 8 | 547 | 962 | < 0.001, 89% | R | WMD = -0.22 | -0.56-0.13 | 0.22 |
| Postoperative feeding time | 8 | 547 | 962 | < 0.001, 86% | R | WMD = -0.56 | -1.07 to | 0.03 |
| Postoperative hospitalization time | 9 | 577 | 994 | < 0.001, 93% | R | WMD = -1.53 | -2.83 to | 0.02 |
| The incidence of anastomotic complications | 11 | 1115 | 1306 | 0.74, 0% | R | OR = 0.71 | 0.47-1.06 | 0.09 |
| Anastomotic fistula | 11 | 1115 | 1306 | 0.53, 0% | R | OR = 0.70 | 0.42-1.18 | 0.18 |
| Anastomotic stenosis | 11 | 1115 | 1306 | 0.85, 0% | R | OR = 0.84 | 0.41-1.71 | 0.63 |
| Anastomotic bleeding | 8 | 439 | 817 | 0.93, 0% | R | OR = 0.86 | 0.34-2.15 | 0.74 |
| Overall incidence of complications | 9 | 947 | 804 | 0.81, 0% | R | OR = 0.93 | 0.71-1.21 | 0.58 |
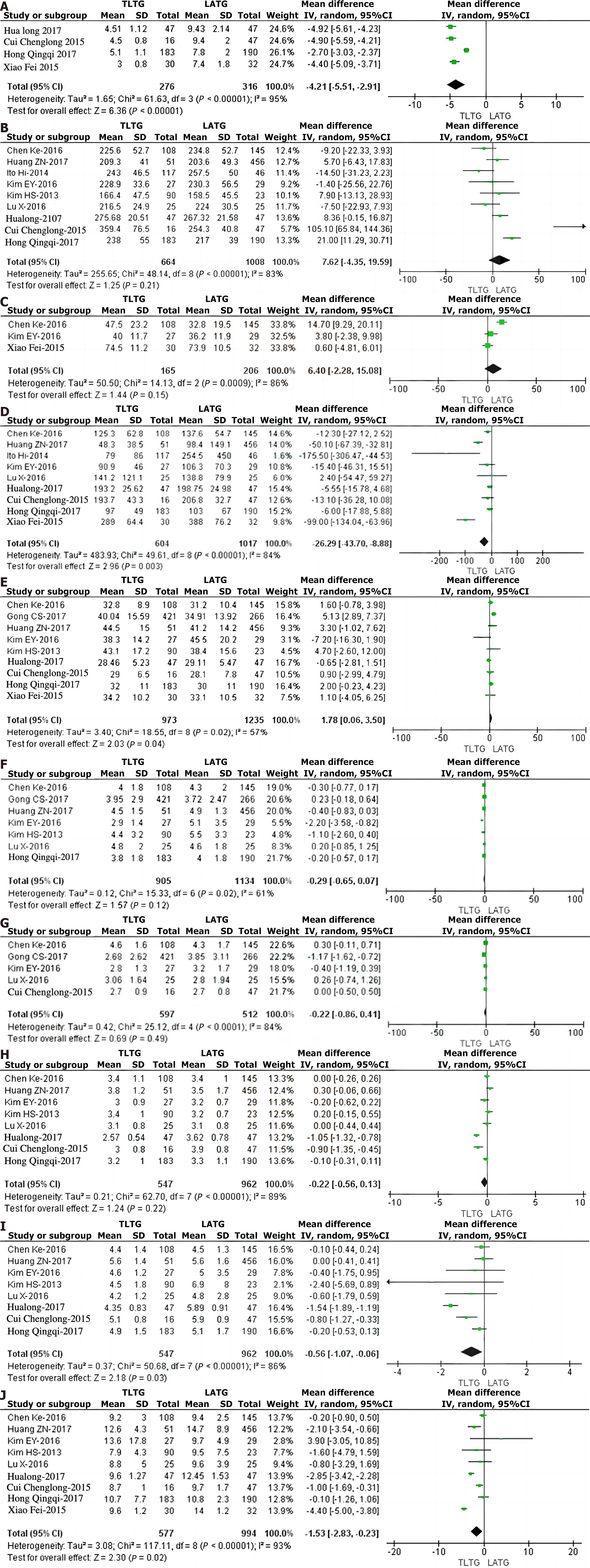
Four of the eleven studies in this paper[10-13] examined the length of surgical incisions. The incisions in the TLTG group were significantly shorter than those in the LATG group (WMD = -4.21, 95%CI: -5.51 to -2.91, P < 0.001; Figure 2A). The total operation time was compared in nine studies[4-6,8-12,14]. There was no statistically significant difference in the total operation time between the two groups (WMD = 7.62, 95%CI: -4.35–19.59, P = 0.21; Figure 2B). Three studies[5,6,13] compared surgical anastomosis time, and the results indicated that there was no statistically significant difference in the anastomosis time between the TLTG group and the LATG group (WMD = 6.40, 95%CI: -2.28-15.08, P = 0.15; Figure 2C). Nine studies[5,6,8-14] compared the amount of blood loss during surgery. Much less blood was lost in the TLTG group than in the LATG group (WMD = -26.29, 95%CI: -43.70 to -8.88, P = 0.003; Figure 2D).
In nine studies[4-8,10-13], the number of lymph nodes removed during surgery was compared. More lymph nodes were removed in the TLTG group than in the LATG group (WMD = 1.78, 95%CI: 0.06-3.50; P = 0.04; Figure 2E). Seven studies[4-9,11] examined the sizes of tumors that had been removed. The results showed that there was no statistically significant difference in the sizes of the tumors between the two groups (WMD = -0.29, 95%CI: -0.65-0.07, P = 0.12; Figure 2F). Five studies[5-7,9,10] compared the length of the proximal incisal margin, and the results indicated that there was no statistically significant difference in the length of the proximal incisal margin between the TLTG group and the LATG group (WMD = -0.22, 95%CI: -0.86-0.41, P = 0.49; Figure 2G).
Two studies[4,7] compared postoperative pain scores, and the results indicated that there was no statistically significant difference in postoperative pain peak values or pain scores at the 1st and 3rd days after surgery between the TLTG group and the LATG group (P = 0.70, P = 0.78, and P = 0.45, respectively). In 8 studies[4-6,8-12], the postoperative anal exhaust time was compared. There was no significant difference between the two groups (WMD = -0.22, 95%CI: -0.56-0.13, P = 0.22; Figure 2H). Eight studies[4-6,8-12] compared the first feeding after surgery. The first feeding in the TLTG group occurred significantly earlier than that in the LATG group (WMD = -0.56, 95%CI: -1.07 to -0.06, P = 0.03; Figure 2I).
A total of nine studies[4-6,8-13] compared the length of hospital stay after surgery. The TLTG group had a significantly shorter length of hospital stay than did the LATG group (WMD = -1.53, 95%CI: -2.83 to -0.23, P = 0.02; Figure 2J). The 11 studies included in this study all compared the incidence of postoperative anastomosis-related complications, and the results indicated that there was no significant difference between the two groups (OR = 0.71, 95%CI: 0.47-1.06, P = 0.09; Figure 3 and Table 2). The incidence of anastomotic fistula in the TLTG and LATG groups was 2.51% and 3.98%, respectively (OR = 0.70, 95%CI: 0.42-1.18, P = 0.18); the incidence of anastomotic stenosis was 1.43% and 1.45%, respectively (OR = 0.84, 95%CI: 0.41-1.71, P = 0.63); and the incidence of anastomotic hemorrhage was 1.59% and 1.22%, respectively (OR = 0.86, 95%CI: 0.34-2.15, P = 0.74; Figure 4). Nine studies[4-7,9-13] examined the overall rate of complications after surgery. The results showed that there was no statistically significant difference between the two groups in this rate (OR = 0.93, 95%CI: 0.71-1.21; P = 0.58). In all the studies included in this study, no surgery-related deaths occurred in either group, and no second surgery was reported.
The incidence of postoperative anastomosis-related complications is an important index for evaluating surgical efficacy, and it is also the focus of this study. Since low-quality research data may affect the overall research results[15], when the two studies of lower quality[13,14] were removed, the incidence data of postoperative anastomosis-related complications from nine high-quality studies[4-12] were pooled and analyzed. The number of complications related to the anastomosis after surgery did not differ significantly between the TLTG and LATG groups (OR = 0.72, 95%CI: 0.47-1.09, P = 0.12), which suggests that the addition of two lower-quality studies did not change the initial results.
This study focused on the safety and feasibility of in vivo esophagojejunostomy. Different anastomosis methods or anastomosis instruments may have affected the final results of the study. Among the 11 studies included in this paper[4-14], 9 items[4,5,7-13] were reconstructed by Roux-en-Y anastomosis. Among them, 8 items[4,5,7-10,12,13] were all esophagojejunostomies using linear staplers. Huang et al[8] reported a new anastomosis method (the isometric retrocut and overlap method of the jejunum). Ito et al[14] used circular staplers for anastomosis, and the other two[6,11] used various anastomosis methods. Therefore, the last four studies[6,8,11,14] were removed from further sensitivity analysis. When the four studies were removed, there was no significant change in the results of any of the studies (Figure 3).
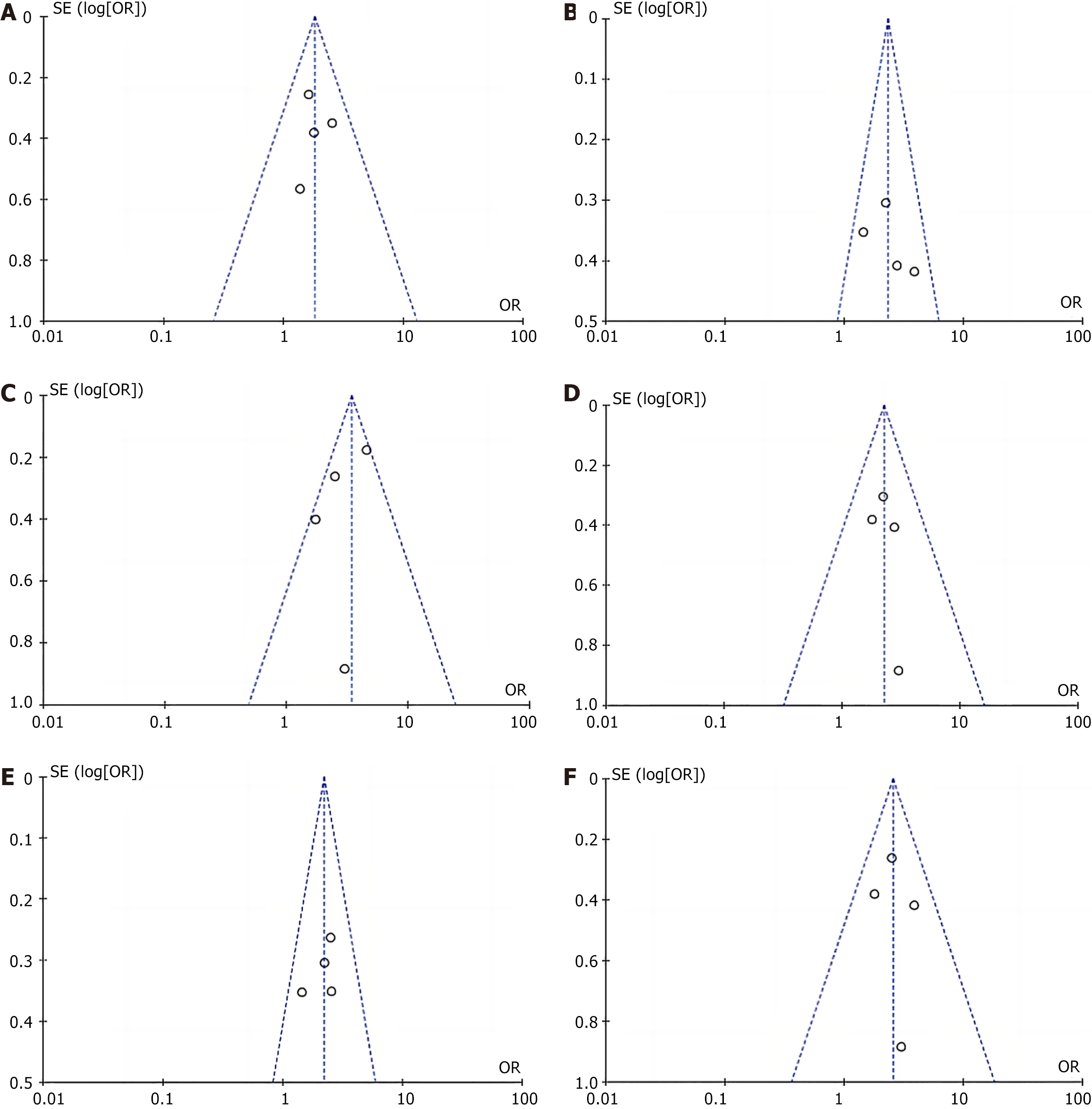
In this paper, funnel plots were drawn for the incidence of postoperative anastomosis-related complications in the TLTG group and the LATG group. The results of the plots showed basic symmetry, suggesting no clear publication bias (Figure 4). Egger linear regression analysis further confirmed that there was no significant publication bias in the included literature (P > 0.05).
Laparoscopic-assisted radical gastrectomy has become the most commonly used surgical method for treating GC[15]. With the improvement of endoscopy technology, the surgical path for treating GC has gradually moved toward full laparoscopy. However, due to the late development of total laparoscopic radical gastrectomy, high technical requirements, and difficulty, most medical units do not regard it as the preferred method for GC surgery[16]. Digestive tract reconstruction is the focus and difficulty of laparoscopic radical gastrectomy for treating GC. In total laparoscopic radical gastrectomy for GC, esophagojejunal anastomosis is very difficult due to its "anatomical particularity" (high anastomosis site, narrow operating space, etc.), and it is also the key to successful surgery. Therefore, the safety and feasibility of TLTG and esophagojejunostomy are of great concern.
There are many ways to reconstruct the digestive tract after laparoscopic total gastrectomy[17]. Roux-en-Y anastomosis, which is currently the main surgical method for reconstruction, can effectively reduce reflux esophagitis and maintain good nutritional status[18]. In previous studies, two methods of in vivo esophagojejunostomy were introduced, including manual suturing and mechanical suturing (linear stapling and circular stapling)[19]. Using linear staplers for reconstruction inside the body during endoscopy can make tension-free anastomosis possible, preventing damage to nearby structures[20]. Unlike circular staplers, linear staplers can be inserted into the abdominal cavity through a cannula hole (Tocar) to complete digestive tract reconstruction without the need for an auxiliary incision. Gong et al[7] suggested that TLTG with a linear stapler was more suitable for endoscopic surgery than LATG with a circular stapler and recommended it for the treatment of upper GC. Nine of the eleven studies in this paper[4,5,7-13] used Roux-en-Y anastomosis to reconstruct the digestive tract. Huang et al[8] reported a new anastomosis method called the isometric retroincision and overlapping jejunum method. Eight studies[4,5,7-10,12,13] used linear staplers to connect the esophagus and jejunum. Ito et al[14] used circular staplers, and two studies[6,11] used more than one method of anastomosis. The results of this meta-analysis showed that the incidence of postoperative anastomosis-related complications (including anastomotic fistula, anastomotic stenosis, and anastomotic hemorrhage) was lower in the TLTG group than in the LATG group (4.39% and 6.20%, respectively), and there was no statistically significant difference between the two groups (P = 0.09). Some studies[6,8,11,14] that used different anastomosis methods or devices were removed from the sensitivity analysis in this paper. The results of these studies did not change significantly. Therefore, in vivo digestive tract reconstruction is safe and feasible. To confirm the effectiveness of different anastomotic methods, further long-term and large-scale randomized controlled trials are needed.
Studies[21-25] have shown that total laparoscopic radical gastrectomy and esophagojejunostomy for GC can increase the duration of surgery and even cause much more blood to be lost during surgery. The results of this meta-analysis showed that there were no statistically significant differences in the total operation time or anastomotic time between the TLTG group and the LATG group, and the intraoperative blood loss in the TLTG group was significantly less than that in the LATG group[26-28]. The reasons for the low blood loss in the TLTG group were as follows: (1) there was no auxiliary abdominal incision in the TLTG, and the intraperitoneal wound was small; (2) TLTG can reduce excessive tissue traction and reduce the risk of bleeding; and (3) laparoscopic surgical techniques may affect intraoperative blood loss, and operators differ between the TLTG and LATG groups. According to relevant studies, TLTG will take much less time after the surgeon learns and practices total laparoscopic distal gastrectomy (residual stomach and duodenal anastomosis). Therefore, this may be related to the operator's proficiency in endoscopic surgery.
The main difference between complete laparoscopic and laparoscopic-assisted radical gastrectomy for GC lies in the different paths of digestive tract reconstruction[29]. The former is completed under laparoscopy (in vivo), while the latter requires an auxiliary incision in vitro. However, for obese patients, total gastrectomy and esophagojejunal anastomosis in vitro with the aid of an abdominal incision are very difficult to perform, and the surgical incision must be prolonged, which ultimately extends the operation time and even increases the surgical risk and postoperative pain of patients[30-33]. The results of this meta-analysis showed that the average BMI of the TLTG group was significantly greater than that of the LATG group, and the length of surgical incision was significantly shorter than that of the LATG group; however, there were no statistically significant differences in the total operation time, anastomotic time, or postoperative pain score between the two groups. In conclusion, complete LGT and esophagojejunostomy in obese patients are still safe, feasible, and even more advantageous.
Most of the studies[34-38] included in this paper only compared the short-term efficacy of TLTG and LATG, and the results showed that the postoperative feeding time and postoperative hospital stay in the TLTG group were significantly shorter than those in the LATG group, while there were no significant differences in the postoperative anal venting time, postoperative anastomosis-related complications, or overall complications between the two groups[39-41]. Therefore, compared with patients in the LATG group, patients in the TLTG group achieved faster postoperative recovery.
This study has several limitations. First, we focused on the safety and feasibility of in vivo esophagojejunostomy, but laparoscopic surgical skills may affect surgical outcomes, and surgical operators differ between the TLTG and LATG groups. Second, most of the studies that were examined did not report or evaluate the long-term effectiveness of total laparoscopic radical gastrectomy for GC. This means that the differences in long-term effectiveness between TLTG and LATG need to be studied further. In addition, all the included studies were retrospective studies and did not include blinded or randomized controlled trials, and the sample size may not be sufficient; therefore, a large-scale randomized care trial study of the two groups is needed in the future.
TLTG under full laparoscopy is technically safe and feasible, and it is equally suitable or even more beneficial for obese patients. Compared with LATG, TLTG has the advantages of less trauma, less bleeding, easier access to lymph nodes, faster postoperative recovery, etc. Total laparoscopic radical gastrectomy is likely to be a future direction for the treatment of GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Dimofte GM, Romania S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Etoh T, Ohyama T, Sakuramoto S, Tsuji T, Lee SW, Yoshida K, Koeda K, Hiki N, Kunisaki C, Tokunaga M, Otsubo D, Takagane A, Misawa K, Kinoshita T, Cho H, Doki Y, Nunobe S, Shiraishi N, Kitano S; Japanese Laparoscopic Surgery Study Group (JLSSG). Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial. JAMA Surg. 2023;158:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 106] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 2. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel) 2024; 12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 3. | Makuuchi R, Terashima M, Terada M, Mizusawa J, Kita R, Tokunaga M, Omori T, Ojima T, Ehara K, Watanabe M, Yanagimoto Y, Nunobe S, Kinoshita T, Ito S, Nishida Y, Hihara J, Boku N, Kurokawa Y, Yoshikawa T; Stomach Cancer Study Group of Japan Clinical Oncology Group. Randomized controlled phase III trial to investigate superiority of robot-assisted gastrectomy over laparoscopic gastrectomy for clinical stage T1-4aN0-3 gastric cancer patients (JCOG1907, MONA LISA study): a study protocol. BMC Cancer. 2023;23:987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 4. | Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy methods for the surgical treatment of early gastric cancer near the gastroesophageal junction. J Laparoendosc Adv Surg Tech A. 2013;23:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Kim EY, Choi HJ, Cho JB, Lee J. Totally Laparoscopic Total Gastrectomy Versus Laparoscopically Assisted Total Gastrectomy for Gastric Cancer. Anticancer Res. 2016;36:1999-2003. [PubMed] |
| 6. | Chen K, Pan Y, Cai JQ, Wu D, Yan JF, Chen DW, Yu HM, Wang XF. Totally laparoscopic versus laparoscopic-assisted total gastrectomy for upper and middle gastric cancer: a single-unit experience of 253 cases with meta-analysis. World J Surg Oncol. 2016;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Gong CS, Kim BS, Kim HS. Comparison of totally laparoscopic total gastrectomy using an endoscopic linear stapler with laparoscopic-assisted total gastrectomy using a circular stapler in patients with gastric cancer: A single-center experience. World J Gastroenterol. 2017;23:8553-8561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Huang ZN, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Lin JL. Digestive tract reconstruction using isoperistaltic jejunum-later-cut overlap method after totally laparoscopic total gastrectomy for gastric cancer: Short-term outcomes and impact on quality of life. World J Gastroenterol. 2017;23:7129-7138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Lu X, Hu Y, Liu H, Mou T, Deng Z, Wang D, Yu J, Li G. Short-term outcomes of intracorporeal esophagojejunostomy using the transorally inserted anvil vs extracorporeal circular anastomosis during laparoscopic total gastrectomy for gastric cancer: a propensity score matching analysis. J Surg Res. 2016;200:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Cui CL, Liang W, Zhu ZQ, YAO HH, Wu Y, Liu L, Guan JJ. Feasibility, safety and short-term efficacy of totally laparoscopic total gastrectomy for upper stomach cancer. Zhongguo Putongwaike Zazhi. 2015;24:1377-1382. |
| 11. | Hong QQ, Wang W, Zhang J, Fan L, Zhu JM, Ji G, Yan S, You J. Clinical efficacies of totally laparoscopic and laparoscopy-assisted radical total gastrectomies in 373 patients: a multicentre retrospective study. Zhonghua Xiaohua Waike Zazhi. 2017;16:822-827. [DOI] [Full Text] |
| 12. | Hua L, Wang CY. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy in patients with upper gastric cancer. Aizheng Jinzhan. 2017;15:186-188,192. [DOI] [Full Text] |
| 13. | Xiao F, Hu B, Song YF, You J. Comparison of clinical efficacy of total gastrectomy assisted by endoscopy and total gastrectomy for gastric cancer. Wuhandax Xuebao (Yixueban). 2015;36:786-791. [DOI] [Full Text] |
| 14. | Ito H, Inoue H, Odaka N, Satodate H, Onimaru M, Ikeda H, Takayanagi D, Nakahara K, Kudo SE. Evaluation of the safety and efficacy of esophagojejunostomy after totally laparoscopic total gastrectomy using a trans-orally inserted anvil: a single-center comparative study. Surg Endosc. 2014;28:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Fan Y, Liu M, Li S, Yu J, Qi X, Tan F, Xu K, Zhang N, Yao Z, Yang H, Zhang C, Xing J, Wang Z, Cui M, Su X. Surgical and oncological efficacy of laparoscopic-assisted total gastrectomy vs open total gastrectomy for gastric cancer by propensity score matching: a retrospective comparative study. J Cancer Res Clin Oncol. 2021;147:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Zhou D, Yu X, Wang Y, Tang F, Yang C, Luo D. Laparoscopically Assisted, Tubular, Stomach Construction for the Radical Resection of Esophageal Cancer. Altern Ther Health Med. 2023;29:200-205. [PubMed] |
| 17. | Wu Q, Wang Y, Peng Q, Bai M, Shang Z, Li L, Tian F, Jing C. Safety and effectiveness of totally laparoscopic total gastrectomy vs laparoscopic-assisted total gastrectomy: a meta-analysis. Int J Surg. 2024;110:1245-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 18. | Illuminati G, D'Urso A, Fiori E, Cerasari S, Nardi P, Lapergola A, Pasqua R, Sorrenti S, Pironi D, Lauro A, D'Andrea V. Laparoscopy-assisted vs open total gastrectomy with D2 Lymphadenectomy for advanced gastric cancer: results of a retrospective, multicenter study. Updates Surg. 2023;75:1645-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 20. | Seo HS, Kim S, Song KY, Lee HH. Feasibility and Potential of Reduced Port Surgery for Total Gastrectomy With Overlap Esophagojejunal Anastomosis Method. J Gastric Cancer. 2023;23:487-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 21. | Motamiez A, Maximous D, Salem AAS, Ahmed BM, Kong SH, Park DJ, Lee HJ, Yang HK. Surgical Outcomes of Laparoscopic-assisted Distal Gastrectomy Versus Totally Laparoscopic Distal Gastrectomy Billroth I for Gastric Cancer. Surg Laparosc Endosc Percutan Tech. 2024;34:80-86. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Xing J, Wang Y, Shan F, Li S, Jia Y, Ying X, Zhang Y, Li Z, Ji J. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol. 2021;47:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Lin GT, Chen JY, Chen QY, Que SJ, Liu ZY, Zhong Q, Wang JB, Lin JX, Lu J, Lin M, Huang ZN, Xie JW, Li P, Huang CM, Zheng CH. Patient-Reported Outcomes of Individuals with Gastric Cancer Undergoing Totally Laparoscopic Versus Laparoscopic-Assisted Total Gastrectomy: A Real-World, Propensity Score-Matching Analysis. Ann Surg Oncol. 2023;30:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 25. | Xu SZ, Cai JC. Laparoscopic-Assisted Natural Orifice Specimen Extraction Gastrectomy Using an Auxiliary Incision-Free Tube for Gastric Cancer. J Surg Res. 2022;270:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 27. | Wu CY, Lin JA, Huang QZ, Xu JH, Zhong WJ, Kang WG, Wang JT, Chen JX, Zheng HD, Ye K. Comparison of short-term and long-term clinical effects of modified overlap anastomosis and conventional incision-assisted anastomosis in laparoscopic total gastrectomy. BMC Surg. 2023;23:306. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Lin J, Liang H, Zheng H, Li S, Liu H, Ge X. CONUT can be a predictor of postoperative complications after laparoscopic-assisted radical gastrectomy for elderly gastric cancer patients. Medicine (Baltimore). 2023;102:e35424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Hoeppner J. [Robot-assisted Total Gastrectomy with D2 Lymphadenectomy and Intracorporal Reconstruction]. Zentralbl Chir. 2022;147:427-429. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Charalabopoulos A, Davakis S, Paraskeva P, Machairas N, Kapelouzou A, Kordzadeh A, Sakarellos P, Vailas M, Baili E, Bakoyiannis C, Felekouras E. Feasibility and Short-Term Outcomes of Three-Dimensional Hand-Sewn Esophago-Jejunal Anastomosis in Completely Laparoscopic Total Gastrectomy for Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Li Z, Ai S, Wang F, Tao L, Sun F, Song P, Shen X, Hu Q, Li X, Liu S, Wang M, Guan W. Comparison of short-term outcomes between robotic-assisted and laparoscopic gastrectomy guided by carbon nanoparticle suspension injection in gastric cancer. World J Surg Oncol. 2022;20:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Du GS, Jiang EL, Qiu Y, Wang WS, Yin JH, Wang S, Li YB, Chen YH, Yang H, Xiao WD. Single-incision plus one-port laparoscopic gastrectomy vs conventional multi-port laparoscopy-assisted gastrectomy for gastric cancer: a retrospective study. Surg Endosc. 2022;36:3298-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Alzahrani K, Park JH, Lee HJ, Park SH, Choi JH, Wang C, Alzahrani F, Suh YS, Kong SH, Park DJ, Yang HK. Short-term Outcomes of Pylorus-Preserving Gastrectomy for Early Gastric Cancer: Comparison Between Extracorporeal and Intracorporeal Gastrogastrostomy. J Gastric Cancer. 2022;22:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 36. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 37. | Zhao RY, Li HH, Zhang KC, Cui H, Deng H, Gao JW, Wei B. Comparison of short-term efficacy between totally laparoscopic gastrectomy and laparoscopic assisted gastrectomy for elderly patients with gastric cancer. World J Gastrointest Surg. 2022;14:950-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Zhong X, Wei M, Ouyang J, Cao W, Cheng Z, Huang Y, Liang Y, Zhao R, Yu W. Efficacy and Safety of Totally Laparoscopic Gastrectomy Compared with Laparoscopic-Assisted Gastrectomy in Gastric Cancer: A Propensity Score-Weighting Analysis. Front Surg. 2022;9:868877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 39. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB. 2024;10:1256. [DOI] [Full Text] |
| 40. | Park SH, Suh YS, Kim TH, Choi YH, Choi JH, Kong SH, Park DJ, Lee HJ, Yang HK. Postoperative morbidity and quality of life between totally laparoscopic total gastrectomy and laparoscopy-assisted total gastrectomy: a propensity-score matched analysis. BMC Cancer. 2021;21:1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Luo G, Xiang Q, Wang X, Li Y, Cao Y, Gong J. Hand-assisted laparoscopic vs laparoscopic-assisted radical gastrectomy in the treatment of advanced distal gastric cancer: final results of a single-center randomized study. J Int Med Res. 2022;50:3000605221109361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |