Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1756
Revised: April 29, 2024
Accepted: May 23, 2024
Published online: June 27, 2024
Processing time: 109 Days and 23.3 Hours
The recurrence rate of liver cancer after surgery is high. Radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) is an effective treatment for liver cancer; however, its efficacy in recurrent liver cancer remains unclear.
To investigate the clinical effect of TACE combined with RFA in the treatment of recurrent liver cancer.
Ninety patients with recurrent liver cancer were divided into 2 groups according to treatment plan: Control (RFA alone); and experimental [TACE combined with RFA (TACE + RFA)]. The incidence of increased alanine aminotransferase levels, complications, and other indices were compared between the two groups before and after the procedures.
One month after the procedures, the short-term efficacy rate and Karnofsky Performance Status scores of the experimental group were significantly higher than those of the control group (P < 0.05). Alpha-fetoprotein (AFP) and total bilirubin levels were lower than those in the control group (P < 0.05); The overall response rate was 82.22% and 66.67% in the experimental and control groups, respectively; The disease control rate was 93.33% and 82.22% in the experimental and control groups, respectively, the differences are statistically significant (P < 0.05). And there were no statistical differences in complications between the two groups (P > 0.05).
TACE + RFA was effective for the treatment of recurrent liver cancer and significantly reduced AFP levels and improved various indices of liver function.
Core Tip: The postoperative recurrence rate of liver cancer is high. Although radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) is an effective treatment for liver cancer, its efficacy in recurrent liver cancer remains unclear. Results of the present study revealed that TACE combined with RFA was effective for the treatment of recurrent liver cancer and could significantly reduce alpha-fetoprotein levels and improve various indices of liver function.
- Citation: Guo JY, Zhao LL, Cai HJ, Zeng H, Mei WD. Radiofrequency ablation combined with transcatheter arterial chemoembolization for recurrent liver cancer. World J Gastrointest Surg 2024; 16(6): 1756-1764
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1756.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1756
Primary liver cancer (PLC) is the sixth most common cancer and third leading cause of cancer-related death globally[1]. In 2020, approximately 900000 cases of PLC were newly diagnosed worldwide, and approximately 830000 related deaths were reported[2]. China accounted for approximately 410000 new cases (45.3%) and 390000 deaths (47.1%), ranking first globally[3]. Hepatocellular carcinoma, also known as liver cancer, is the most common pathological type of PLC, accounting for approximately 90% of PLC cases in China[4]. Surgery is the preferred treatment for patients with resectable liver cancer. In recent years, the overall 5-year survival rate after liver resection in patients with liver cancer has increased to 60.0%[5]. However, the postoperative recurrence rate of liver cancer is high, with rates of 40%-70% within 5 years after surgery, and most within 1-3 years after surgery[6].
Surgical resection remains the preferred treatment option for recurrent liver cancer; however, due to factors such as location, size, distant metastasis, and multicentric occurrence, only 10.4%-31.0% of patients are suitable candidates[7]. In recent years, radiofrequency ablation (RFA) has been widely used for the treatment of recurrent liver cancer due to its minimally invasive nature, simplicity of operation, broad indications, repeatability, low cost, high patient acceptance, and satisfactory therapeutic effects[8]. However, studies have found that recurrent liver cancer has a unique location and multicentric origin, as well as the characteristics of intrahepatic micrometastasis, which greatly increases the risk for recurrence[9]. Additionally, because RFA can only target locally detectable recurrent lesions, it has poor efficacy for small, undetectable lesions, resulting in a higher risk for recurrence[10]. In recent years, transcatheter arterial chemoembolization (TACE) has been used to selectively embolize recurrent intrahepatic lesions, effectively solving the problems associated with RFA[11-14]. Therefore, some investigators have proposed that the combination of TACE and RFA (TACE + RFA) may further improve clinical efficacy. However, there are currently few such studies, and treatment effects and prognosis still need to be comprehensively evaluated. As such, the present study aimed to evaluate the efficacy of TACE + RFA for the treatment of recurrent liver cancer to provide a reference for clinical treatment.
We retrospectively collected 90 patients diagnosed with recurrent liver cancer who were admitted to the hospital between February 2021 and February 2023. According to the method of random number table, participants were randomly assigned to the TACE + RFA group or the RFA group in a 1:1 ratio: Control [RFA (n = 45)]; and experimental [TACE + RFA (n = 45)].
Inclusion criteria were as follows[15]: Pathologically confirmed liver cancer and a history of curative surgical treatment; postoperative review of computed tomography (CT) or magnetic resonance imaging (MRI) and other relevant imaging examinations revealing recurrent liver cancer; fulfill treatment indications for TACE and RFA; age ≥ 18 years; liver function classified as Child-Pugh grade A or B with no extrahepatic metastasis; and complete case information.
The exclusion criteria were as follows: Complications, such as hepatic encephalopathy, refractory ascites, or large vessel occlusion; tumor metastasis to distant sites; heart or kidney dysfunction; history of substance abuse or organic diseases; pregnancy or lactation; Karnofsky Performance Status (KPS) score < 70; and expected survival < 6 months.
The present study was approved by the research ethics committee of the Second People's Hospital of Zhejiang Province Taizhou Yuhuan city (No. 2023-028), and all patients voluntarily participated.
Patients in the control group underwent RFA[16], which used an radiofrequency generator (RITA 1500X) equipped with a 14-gauge StarBurst TMXL electrode needle (Angio Dynamics, Latham NY, United States). After CT positioning, an appropriate puncture pathway was selected to avoid damaging surrounding structures. All patients received local (2% lidocaine) and intravenous anesthesia (propofol and fentanyl). CT was performed after electrode needle placement to ensure that the needle tip was in the intended position. Needle tract ablation was performed at the end of treatment to prevent bleeding and tumor implantation. For larger tumors, the position of the ablation needle was adjusted multiple times based on the size and shape of the tumor to achieve as much ablation range as possible, exceeding the tumor boundary by 0.5-10 cm, to ensure the elimination of potentially infiltrated tumor portions. Immediate postoperative CT was performed to check for complications such as bleeding, pneumothorax, and/or perforation.
Patients in the experimental group underwent TACE + RFA[17]. RFA was performed 1 week after TACE, and the patients underwent treatment under anesthesia and intravenous anesthesia. The experimental group first underwent TACE using the Seldinger method to puncture the femoral artery, guiding the catheter to the hepatic artery through digital subtraction angiography (DSA), to comprehensively evaluate tumor size, number, blood supply artery, venous patency, and venous fistula, followed by intravascular administration of 500-750 mg of fluorouracil, 50-200 mg of oxaliplatin, and 10-30 mg of irinotecan. The embolic agents included a 38.0% hypertonic iodine solution and gelatin sponge particles at a dose of 5-20 mL. Accurate dosage of the drug was adjusted according to tumor size, quantity, liver function, and iodine oil filling in the tumor. At the conclusion of treatment, another DSA examination was performed to confirm complete tumor vascular embolization. Repeat embolization was performed if necessary. Hemostasis was applied to the puncture site after catheter removal and compression was applied for 12 h on the puncture side. Bed rest was prescribed for 24 h to prevent bleeding and hematoma formation at the puncture site. TACE + RFA was performed in the experimental group and compared with that in the control group. Immediate CT scans were performed postoperatively to rule out complications, such as pneumothorax, bleeding, and perforation. Observations were performed for 2 wk, with routine blood examinations twice per week, and liver and kidney function examinations once per week. Interleukin-11 treatment was administered to patients with blood platelet counts < 25 × 109/L, and granulocyte colony-stimulating factor treatment was administered to patients with a white blood cell count < 2.5 × 109/L or neutrophil count < 1.0 × 109/L.
Postoperative evaluation of short-term efficacy[18]: According to the modified response evaluation criteria in solid tumors (mRECIST), accurately measurable and reproducible target lesions were selected. CT- or MRI-enhanced scans were used to measure the enhanced region of the tumor within the lesion. If all lesions exhibited no enhancement during the arterial phase, it was considered a complete response (CR); if the sum of the diameters of the lesions during the arterial phase decreased by at least 30%, it was considered a partial response (PR); if the sum of the diameters of the lesions increased by at least 20%, it was considered progressive disease (PD); and if neither meets the criteria for PR nor PD, it was considered stable disease (SD). Overall response rate (ORR) was calculated as (CR + PR) cases/total cases × 100%, and the disease control rate (DCR) as calculated as (CR + PR + SD) cases/total cases × 100%.
KPS scores of the two groups before and 1 month after the end of treatment[19]: The KPS score ranges from 0 to 100 and is directly proportional to patient health status.
Liver function indicators (alanine aminotransferase and total bilirubin) and alpha-fetoprotein tumor marker levels before and 1 month after treatment: The incidence of complications within 1 month of treatment was calculated for both groups of patients.
Statistical analysis was performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, United States). Continuous variables are expressed as mean ± SD, and comparisons were performed using the t-test. In order to perform the t tests, the data had been tested for normality using the Kolmogorov simirnov test. Categorical data are expressed as number (percentage) and comparisons were performed using the chi-squared test. Differences with P < 0.05 were considered to be statistically significant.
The TACE + RFA group included 35 males and 10 females, with average age of 57.9 ± 8.3 years. Tumor diameter was ≤ 3 cm in 23 cases and > 3 cm in 22 cases. Alpha-fetoprotein (AFP) level was ≤ 400 ng/mL in 31 cases and > 400 ng/mL in 14 cases. The mean time to recurrence was 1.9 ± 0.8 months. The Child-Pugh liver function classification was class A in 18 patients and class B in 27 cases. Tumor diameter and number were distributed as follows: ≤ 3 cm (n = 23); > 3 cm (n = 22); single tumor (n = 26); and multiple tumors (n = 19). Among these patients, 43 were positive for the hepatitis B surface antigen, 2 were negative, 13 had diabetes, and 15 had hypertension. Seventeen patients had no history of hypertension or diabetes mellitus.
The RFA group included 36 males and 9 females, with a mean age of 60.1 ± 9.6 years. Tumor diameter was ≤ 3 cm in 25 cases and > 3 cm in 20 cases. AFP level was ≤ 400 ng/mL in 32 cases and > 400 ng/mL in 13 cases. The mean time to recurrence was 1.7 ± 0.6 months. The Child-Pugh liver function classification was class A in 16 patients and class B in 29 cases. Tumor diameter and number were distributed as follows: ≤ 3 cm (n = 25); > 3 cm (n = 20); single tumor (n = 24); and multiple tumors (n = 21). Among these patients, 40 were positive for hepatitis B surface antigen, 5 were negative, 11 had diabetes, and 16 had hypertension. Eighteen patients had no history of hypertension or diabetes mellitus. There were no statistical differences in baseline data including age, sex, tumor diameter, AFP level, Child-Pugh classification of liver function, and average time of recurrence (P > 0.05), indicating comparability (Table 1).
| Index | TACE + RFA group (n = 45) | RFA group (n = 45) | χ2/t | P value |
| Age (years) | 57.9 ± 8.3 | 60.1 ± 9.6 | 1.163 | 0.248 |
| Sex | 0.067 | 0.796 | ||
| Male | 35 | 36 | ||
| Female | 10 | 9 | ||
| Recurrence time (months) | 1.9 ± 0.8 | 1.7 ± 0.6 | 1.342 | 0.183 |
| Tumor diameter (cm) | 0.179 | 0.673 | ||
| ≤ 3 | 23 | 25 | ||
| > 3 | 22 | 20 | ||
| Number of lesions | 0.180 | 0.671 | ||
| Single | 26 | 24 | ||
| Multiple | 19 | 21 | ||
| Hepatitis B surface antigen | 0.620 | 0.431 | ||
| Positive | 43 | 40 | ||
| Negative | 2 | 5 | ||
| Child–Pugh class | 0.189 | 0.664 | ||
| A | 18 | 16 | ||
| B | 27 | 29 | ||
| AFP (ng/mL) | 0.053 | 0.818 | ||
| ≤ 400 | 31 | 32 | ||
| > 400 | 14 | 13 | ||
| Complications (n) | 0.228 | 0.893 | ||
| Diabetes | 13 | 11 | ||
| Hypertension | 15 | 16 | ||
| No | 17 | 18 |
One month after treatment, the short-term therapeutic effects in the two groups of patients were compared. The ORR was 82.22% and 66.67% in the experimental and control groups, respectively; this difference was statistically significant (P < 0.05). The DCR was 93.33% and 82.22% in the experimental and control groups, respectively. Once again, this difference was statistically significant (P < 0.05, Table 2).
| Index | TACE + RFA group (n = 45) | RFA group (n = 45) | χ2 | P value |
| CR | 15 | 9 | ||
| PR | 22 | 21 | ||
| SD | 5 | 7 | ||
| PD | 3 | 8 | ||
| ORR | 37 (82.22) | 30 (66.67) | 6.355 | 0.012 |
| DCR | 42 (93.33) | 37 (82.22) | 5.752 | 0.017 |
Before treatment, there were no statistically significant differences in AFP, alanine aminotransferase (ALT), and total bilirubin (TBiL) levels between the two groups (P > 0.05). After treatment, the mean levels of AFP, ALT, and TBiL in both groups significantly decreased compared with before treatment, and the experimental group was significantly lower than the control group, with a statistically significant difference (P < 0.001; Table 3).
| Index | TACE + RFA group (n = 45) | RFA group (n = 45) | t | P value | |
| AFP (ng/L) | Before treatment | 423.35 ± 25.64 | 422.47 ± 24.85 | 0.165 | 0.869 |
| After treatment | 86.21 ± 26.71a | 126.70 ± 38.21a | 5.826 | < 0.001 | |
| ALT (U/L) | Before treatment | 283.61 ± 21.63 | 279.82 ± 20.09 | 0.861 | 0.392 |
| After treatment | 47.34 ± 5.22a | 109.23 ± 25.32a | 16.059 | < 0.001 | |
| TBiL (μmol/L) | Before treatment | 91.37 ± 8.58 | 91.64 ± 8.94 | 0.146 | 0.884 |
| After treatment | 36.26 ± 4.23a | 48.83 ± 4.54a | 13.589 | < 0.001 | |
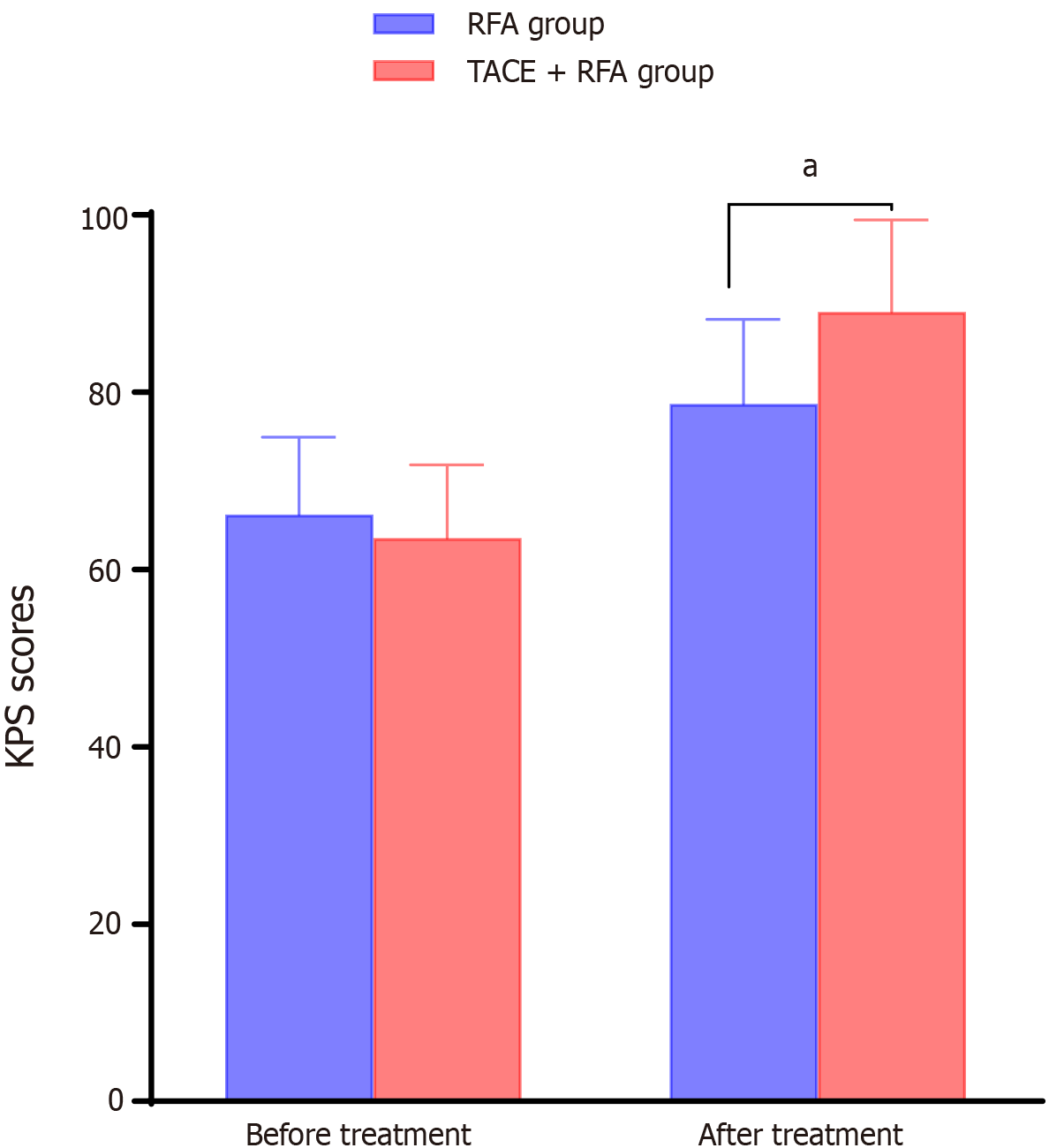
After treatment, the KPS scores of both groups of patients increased compared to those before treatment, and the KPS scores of the experimental group were significantly higher than those of the control group (P < 0.05; Figure 1).
The incidence of complications in the experimental group was 26.67% (12/45), which was higher than that in the control group [17.78% (8/45)], although the difference was not statistically significant (P > 0.05; Table 4).
| Index | TACE + RFA group (n = 45) | RFA group (n = 45) | χ2 | P value |
| Fever | 3 | 2 | ||
| Bleeding | 1 | 1 | ||
| Liver abscess | 1 | 0 | ||
| Nausea and vomiting | 1 | 1 | ||
| Upper gastrointestinal bleeding | 1 | 0 | ||
| Abdominal pain | 1 | 1 | ||
| Abnormal liver function | 4 | 3 | ||
| Total incidence rate | 12 (26.67) | 8 (17.78) | 2.286 | 0.131 |
For patients with liver cancer, surgical resection is the main treatment method, but the recurrence and metastasis rates are high[20,21]. Postoperative recurrence is the main obstacle to long-term survival of patients with liver cancer[22].With the continuous development of minimally invasive surgeries in recent years, TACE and other local treatments have been widely used in clinical practice[23]. However, repeated chemotherapy can worsen liver function damage, which may affect treatment outcomes. With the continuous advancement of medical technology, RFA has gradually become a new treatment option that can be combined with TACE[24]. However, due to the limited ablation range of RFA, there may be a zone of incomplete ablation of small metastatic lesions. Therefore, in clinical practice, TACE is often combined with RFA. A study by Feng[25] found that combined RFA and TACE for recurrent liver cancer resulted in better disease-free survival and overall survival than RFA alone, indicating that combination therapy is more effective in preventing tumor progression and recurrence.
The results of the present study also showed that 1 month after surgery, the ORR in the study group was 82.22%, while in the control group it was 66.67%, with a statistically significant difference (P < 0.05). The DCR in the experimental group was 93.33%, while that in the control group was 82.22%, with a statistically significant difference between the two groups (P < 0.05). This indicated that the short-term clinical efficacy of combination therapy was superior to that of monotherapy[26,27]. These results suggest that TACE + RFA may enhance the body’s absorption and sensitivity to chemotherapeutic drugs. Simultaneously, TACE can occlude the tumor’s blood-supplying artery, significantly reducing the cooling effect of blood on thermal ablation, thereby enhancing ― to some extent ― the tumor-killing effect of RFA and achieving a synergistic effect, resulting in a higher treatment effectiveness rate.
Results of this study also revealed that, after treatment, the levels of liver function indicators (ALT and TBiL) and AFP tumor markers in both groups decreased to some extent. However, liver function indicators (ALT and TBiL) and AFP tumor markers were significantly lower in the experimental group than in the control group (P < 0.05). This is because combination therapy can completely kill tumor cells, prevent invasion or metastasis, and lower the risk for postoperative recurrence. These findings were consistent with those reported by Ouyang et al[28].
In addition, the incidence rates of complications in the 2 groups were compared, and no statistically significant differences were found (P > 0.05). According to a study by Wang et al[29], TACE combined with RFA did not significantly increase the rate of complications in patients with liver cancer. This result is consistent with those reported in the literature. The results of this study also demonstrated that KPS scores for both patient groups before and 4 weeks after treatment were higher than those before treatment, and the KPS scores of the experimental group were significantly higher than those of the control group (P < 0.05). This is consistent with results reported by Bholee et al[30]. Possible explanations for this are as follows. RFA has a stronger destructive effect on deep liver cancer tissues, especially lesions around liver lobules, effectively avoiding residual lesions after TACE treatment. At the same time, RFA can induce secondary destruction of chemotherapy-resistant cancer cells by causing DNA damage, promoting the degradation of chromosomes and absorption of nuclear fragments, thereby improving patient quality of life.
The present study had some limitations, the first of which was its single-center design and small sample size, with inherent risks for selection bias. Second, it had a short follow-up; as such, long-term efficacy needs to be assessed with a larger sample size and multiple centers. A randomized controlled clinical study should be conducted for further verification. Finally, another limitation of this study was that the patients were not further grouped according to clinical stage, which may ― to some extent ― have affected the reliability of the results. In future studies, the sample size will be expanded, multiple control groups will be established, and multicenter comparative studies will be conducted.
In summary, TACE + RFA yielded greater efficacy for the treatment of recurrent liver cancer. TACE + RFA significantly decreased AFP levels and improved various indicators of liver function. This finding should be promoted in future clinical trials.
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2198] [Article Influence: 732.7] [Reference Citation Analysis (1)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15305] [Article Influence: 3061.0] [Reference Citation Analysis (4)] |
| 3. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1792] [Article Influence: 448.0] [Reference Citation Analysis (1)] |
| 4. | Bhatt A, Wu J. Immunotherapy for recurrent hepatocellular carcinoma. World J Gastroenterol. 2023;29:2261-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, Park JW. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol. 2021;74:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 6. | Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Jiang C, Cheng G, Liao M, Huang J. Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol. 2021;19:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Takayama T, Hasegawa K, Izumi N, Kudo M, Shimada M, Yamanaka N, Inomata M, Kaneko S, Nakayama H, Kawaguchi Y, Kashiwabara K, Tateishi R, Shiina S, Koike K, Matsuyama Y, Omata M, Makuuchi M, Kokudo N. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer. 2022;11:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 9. | Shi T, Xu C, Feng Y, Wei Y, Lv H, Zhu Q. Surgical resection versus radiofrequency ablation for early recurrent hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2022;34:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Gou H, Liu S, Zhu G, Peng Y, Li X, Yang X, He K. Effectiveness of radiofrequency ablation versus transarterial chemoembolization for recurrent hepatocellular carcinoma: A meta-analysis. Acta Radiol Open. 2022;11:20584601221085514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Qin Y, Dong P, Ning H, Wang G. Liver resection, radiofrequency ablation, and radiofrequency ablation combined with transcatheter arterial chemoembolization for very-early- and early-stage hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis for comparison of efficacy. Front Oncol. 2022;12:991944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Zhang YJ, Chen MS, Chen Y, Lau WY, Peng Z. Long-term Outcomes of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation as an Initial Treatment for Early-Stage Hepatocellular Carcinoma. JAMA Netw Open. 2021;4:e2126992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Keshavarz P, Raman SS. Comparison of combined transarterial chemoembolization and ablations in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY). 2022;47:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Bureau of Medical Administration, National Health Commission of the People's Republic of China. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 16. | Liu W, Xu H, Ying X, Zhang D, Lai L, Wang L, Tu J, Ji J. Radiofrequency Ablation (RFA) Combined with Transcatheter Arterial Chemoembolization (TACE) for Patients with Medium-to-Large Hepatocellular Carcinoma: A Retrospective Analysis of Long-Term Outcome. Med Sci Monit. 2020;26:e923263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Cucchetti A, Serenari M, Sposito C, Di Sandro S, Mosconi C, Vicentin I, Garanzini E, Mazzaferro V, De Carlis L, Golfieri R, Spreafico C, Vanzulli A, Buscemi V, Ravaioli M, Ercolani G, Pinna AD, Cescon M. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J Hepatol. 2020;73:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Agsalda-Garcia M, Shieh T, Souza R, Kamada N, Loi N, Oda R, Acosta-Maeda T, Choi SY, Lim E, Misra A, Shiramizu B. Raman-Enhanced Spectroscopy (RESpect) Probe for Childhood Non-Hodgkin Lymphoma. SciMed J. 2020;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Zhang X, Bai Y, Xu L, Zhang B, Feng S, Xu L, Zhang H, Xu L, Yang P, Niu T, Zheng S, Liu J. Clinical and morpho-molecular classifiers for prediction of hepatocellular carcinoma prognosis and recurrence after surgical resection. Hepatol Int. 2019;13:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Colasanti M, Berardi G, Mariano G, Ferretti S, Meniconi RL, Guglielmo N, Ettorre GM. Laparoscopic Left Hepatectomy for Hepatocellular Carcinoma Recurrence Following Liver Transplantation. Ann Surg Oncol. 2022;29:2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ouchi K, Sugawara T, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Yamanami H, Nakagawa K. Prediction of recurrence and extratumor spread of hepatocellular carcinoma following resection. J Surg Oncol. 2000;75:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Dong H, Ge D, Qu B, Zhu P, Wu Q, Wang T, Wang J, Li Z. Transarterial chemoembolization with or without multikinase inhibitors for patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2023;13:1139025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Feng Z. Minshan Chen: combination of TACE and RFA can improve the treatment of HCC. Ann Transl Med. 2013;1:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Orimo T, Kamiyama T, Yokoo H, Wakayama K, Shimada S, Einama T, Kamachi H, Taketomi A. Salvage Hepatectomy for Recurrent Hepatocellular Carcinoma after Radiofrequency Ablation and/or Transcatheter Arterial Chemoembolization: A Propensity Score-Matched Analysis. Dig Surg. 2018;35:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Dan Y, Meng W, Li W, Chen Z, Lyu Y, Yu T. Transarterial Chemoembolization Combined With Radiofrequency Ablation Versus Hepatectomy for Hepatocellular Carcinoma: A Meta-Analysis. Front Surg. 2022;9:948355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Ouyang T, Cao Y, Chen L, Zheng C. Comparison of the Efficacy Among Transcatheter Arterial Chemoembolization (TACE)-Radiofrequency Ablation Plus Apatinib, TACE Plus Apatinib, and TACE Alone for Hepatocellular Carcinoma: A Retrospective Study. Cardiovasc Intervent Radiol. 2022;45:780-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 29. | Wang C, Liao Y, Qiu J, Yuan Y, Zhang Y, Li K, Zou R, Wang Y, Zuo D, He W, Zheng Y, Li B, Yuan Y. Transcatheter arterial chemoembolization alone or combined with ablation for recurrent intermediate-stage hepatocellular carcinoma: a propensity score matching study. J Cancer Res Clin Oncol. 2020;146:2669-2680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Bholee AK, Peng K, Zhou Z, Chen J, Xu L, Zhang Y, Chen M. Radiofrequency ablation combined with transarterial chemoembolization versus hepatectomy for patients with hepatocellular carcinoma within Milan criteria: a retrospective case-control study. Clin Transl Oncol. 2017;19:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |