Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1734
Revised: May 8, 2024
Accepted: May 11, 2024
Published online: June 27, 2024
Processing time: 87 Days and 17.4 Hours
Conventional five-port laparoscopic surgery, the current standard treatment for colorectal carcinoma (CRC), has many disadvantages.
To assess the influence of reduced-port laparoscopic surgery (RPLS) on perioperative indicators, postoperative recovery, and serum inflammation indexes in patients with CRC.
The study included 115 patients with CRC admitted between December 2019 and May 2023, 52 of whom underwent conventional five-port laparoscopic surgery (control group) and 63 of whom underwent RPLS (research group). Comparative analyses were performed on the following dimensions: Perioperative indicators [operation time (OT), incision length, intraoperative blood loss (IBL), and rate of conversion to laparotomy], postoperative recovery (first postoperative exhaust, bowel movement and oral food intake, and bowel sound recovery time), serum inflammation indexes [high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6)], postoperative complications (anastomotic leakage, incisional infection, bleeding, ileus), and therapeutic efficacy.
The two groups had comparable OTs and IBL volumes. However, the research group had a smaller incision length; lower rates of conversion to laparotomy and postoperative total complication; and shorter time of first postoperative exhaust, bowel movement, oral food intake, and bowel sound recovery; all of which were significant. Furthermore, hs-CRP, IL-6, and TNF-α levels in the research group were significantly lower than the baseline and those of the control group, and the total effective rate was higher.
RPLS exhibited significant therapeutic efficacy in CRC, resulting in a shorter incision length and a lower conversion rate to laparotomy, while also promoting postoperative recovery, effectively inhibiting the inflammatory response, and reducing the risk of postoperative complications.
Core Tip: Colorectal carcinoma (CRC) is a fatal but preventable gastrointestinal malignancy, with surgical treatment being the standard of care. However, conventional laparoscopic surgery has obvious disadvantages. This study compared reduced-port laparoscopic surgery (RPLS) and conventional laparoscopic surgery and confirmed that the former had more advantages than the latter in CRC based on perioperative indicators, postoperative recovery, serum inflammatory responses, postoperative complications, and therapeutic efficacy. RPLS not only reduced the incision length and the rate of conversion to laparotomy but also promoted postoperative recovery, effectively inhibited the inflammatory response, and reduced the risk of postoperative complications.
- Citation: Wu HB, Liu DF, Liu YL, Wang XF, Cao YP. Influence of reduced-port laparoscopic surgery on perioperative indicators, postoperative recovery, and serum inflammation in patients with colorectal carcinoma. World J Gastrointest Surg 2024; 16(6): 1734-1741
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1734.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1734
Colorectal carcinoma (CRC) is a fatal but preventable gastrointestinal malignancy and is the third most common cancer worldwide[1]. Smoking, high body fat, lack of exercise, and unhealthy eating habits, among others, may contribute to the occurrence of CRC[2]. Nearly 1.8 million new CRC cases and approximately 900000 associated deaths are reported every year, with a 5-year survival rate < 20% in patients with metastatic CRC[3,4]. Surgical treatment, the standard treatment for CRC, aims to resect the primary tumor and surrounding lymph nodes[5,6]. However, conventional laparoscopic surgery involves a five-port procedure, which not only affects the postoperative aesthetics of patients but also carries the risk of blood vessel and nerve damage in the abdominal wall and complication by trocar site hernia[7,8]. Therefore, optimizing surgical procedures based on conventional laparoscopic surgery could significantly improve the surgical outcomes and postoperative recovery of patients.
Reduced-port laparoscopic surgery (RPLS) implements an auxiliary incision combined with a single-port protocol. Port reduction (final two ports) is achieved by combining two auxiliary operation ports and one observation port in the auxiliary incision and then operating alongside the main operation port to achieve laparoscopic radical resection of CRC[9,10]. This surgical modality is minimally invasive and less labor-intensive[11]. Inaki[12] found that RPLS can be performed for bariatric surgery and sleeve gastrectomy for the resection of benign gastric submucosal tumors, providing better cosmetic effects and even achieving a permanent cure. Borodulin et al[13] demonstrated that the application of RPLS in bilateral salpingectomy provides good cosmetic outcomes while ensuring safety and feasibility. RPLS for patients with CRC has also been reported in previous studies as an alternative to conventional multi-port laparoscopic colectomy[14].
This study hypothesized that RPLS has superior clinical advantages over conventional five-port laparoscopic surgery in the treatment of CRC, which is hereby verified and reported in detail.
One hundred and fifteen patients with CRC admitted to The First Affiliated Hospital of Ningbo University from December 2019 to May 2023 were selected as the study subjects. The control group (n = 52) underwent conventional five-port laparoscopic surgery, while the research group (n = 63) underwent RPLS. The two case groups were clinically comparable with no significant differences in baseline data (P > 0.05).
Inclusion criteria: All patients included in this study were clinically diagnosed with CRC[15] and met the indications for surgical treatment; and had complete clinical data, normal cognitive and communication abilities, and no wa
Exclusion criteria: Minors, elderly patients aged > 80 years, and pregnant or lactating women were ineligible for this study. Also excluded were patients who had recently received chemoradiotherapy; patients with heart/lung/kidney dysfunction, coagulation dysfunction, autoimmune system defects, or other malignant tumors; and those suffering from serious gastrointestinal dysfunction, disorders, or other diseases before surgery that might affect the evaluation of gastrointestinal function.
The research group underwent RPLS. The protocol involved performing an auxiliary incision, and a single port was adopted. The conventional two 5-mm auxiliary operation ports and 10-mm observation port were combined in the auxiliary incision of about 5 cm in length and used together with a 12-mm main operation port for the procedure. After general anesthesia, the patient was placed in a lithotomy position with the head low and feet high. A longitudinal incision approximately 5 cm long was made under the umbilicus into the abdomen, and a disposable retractor was placed in it and secured. After cutting the fingertips of a surgical glove, a 5-mm trocar was inserted into the thumb and little finger as the operating ports, and a 10-mm trocar was inserted into the middle finger as the observation port. A CO2 pneumoperitoneum with an abdominal pressure of 12 mmHg was established, and a 12-mm trocar was inserted as the main operating port and positioned at the location of the CRC. Finally, laparoscopic radical resection of CRC was performed by removing the cancerous bowel segment, anastomosing the intestinal end, and dissecting the regional lymph nodes.
The control group underwent routine five-port laparoscopic surgery. Anesthesia and posture were the same as those in the research group. A pneumoperitoneum was established, the cancerous intestinal segment was removed at the tumor site, the severed intestinal end was anastomosed, and the regional lymph nodes were removed. Finally, indwelling drainage and conventional suture were performed to complete the surgery.
Perioperative indicators: The operation time (OT), incision length, intraoperative blood loss (IBL), and rate of conversion to laparotomy were measured and recorded.
Postoperative recovery: The first postoperative exhaust, bowel movement, and oral food intake, as well as bowel sound recovery, were monitored and documented.
Serum inflammation indexes: Early in the morning, 3 mL of venous blood was collected from the patient with an empty stomach and stored in test tubes for several minutes. After centrifugation, the serum was separated, and the supernatant was collected into EP tubes and refrigerated at −20°C for testing. Enzyme-linked immunosorbent assay was performed to quantify high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6).
Incidence of postoperative complications: Adverse events, such as anastomotic leakage (AL), incision infection, bleeding, and ileus, and their incidence rates were determined and recorded.
Efficacy: Assessment A marked response refers to the significant relief of gastrointestinal symptoms, such as diarrhea, constipation, and rectal bleeding, following treatment; complete removal of cancer lesions on imaging examination; and the absence of postoperative complications or adverse effects. A response is defined as the effective symptom control, complete resection of the cancer on imaging examination, and the presence of mild but controllable complications, such as infection and bleeding, after surgery. Non-response indicates no considerable improvement of symptoms, residual tumor resection by imaging examination, and serious postoperative complications and adverse events. The overall response rate (ORR) is the percentage of the sum of marked response and response cases to the total number of cases.
In this study, both the measurement data (expressed as mean ± SE) and counting data (expressed as number of cases and percentage) were imported into SPSS22.0 software package for statistical analyses. The chi-square test (χ2) was used to compare counting data, and the independent sample t-test was used to compare measurement data between groups. In all tests, P < 0.05 indicated statistical significance.
The comparison of baseline data revealed no significant inter-group differences in terms of sex, age, body mass index, surgical site, and tumor-nodes-metastasis staging (P > 0.05; Table 1).
| Factors | Control group (n = 52) | Research group (n = 63) | χ2/t | P value |
| Sex | 0.151 | 0.697 | ||
| Male | 27 (51.92) | 35 (55.56) | ||
| Female | 25 (48.08) | 28 (44.44) | ||
| Age (yr) | 54.10 ± 9.56 | 55.02 ± 10.97 | 0.474 | 0.636 |
| Body mass index (kg/m2) | 23.73 ± 2.58 | 24.05 ± 3.04 | 0.601 | 0.549 |
| Surgical site | 1.901 | 0.387 | ||
| Left colon | 18 (34.62) | 15 (23.81) | ||
| Right colon | 20 (38.46) | 31 (49.21) | ||
| Rectum | 14 (26.92) | 17 (26.98) | ||
| TNM stage | 0.813 | 0.666 | ||
| II | 19 (36.54) | 20 (31.75) | ||
| III | 18 (34.62) | 27 (42.86) | ||
| IV | 15 (28.85) | 16 (25.40) |
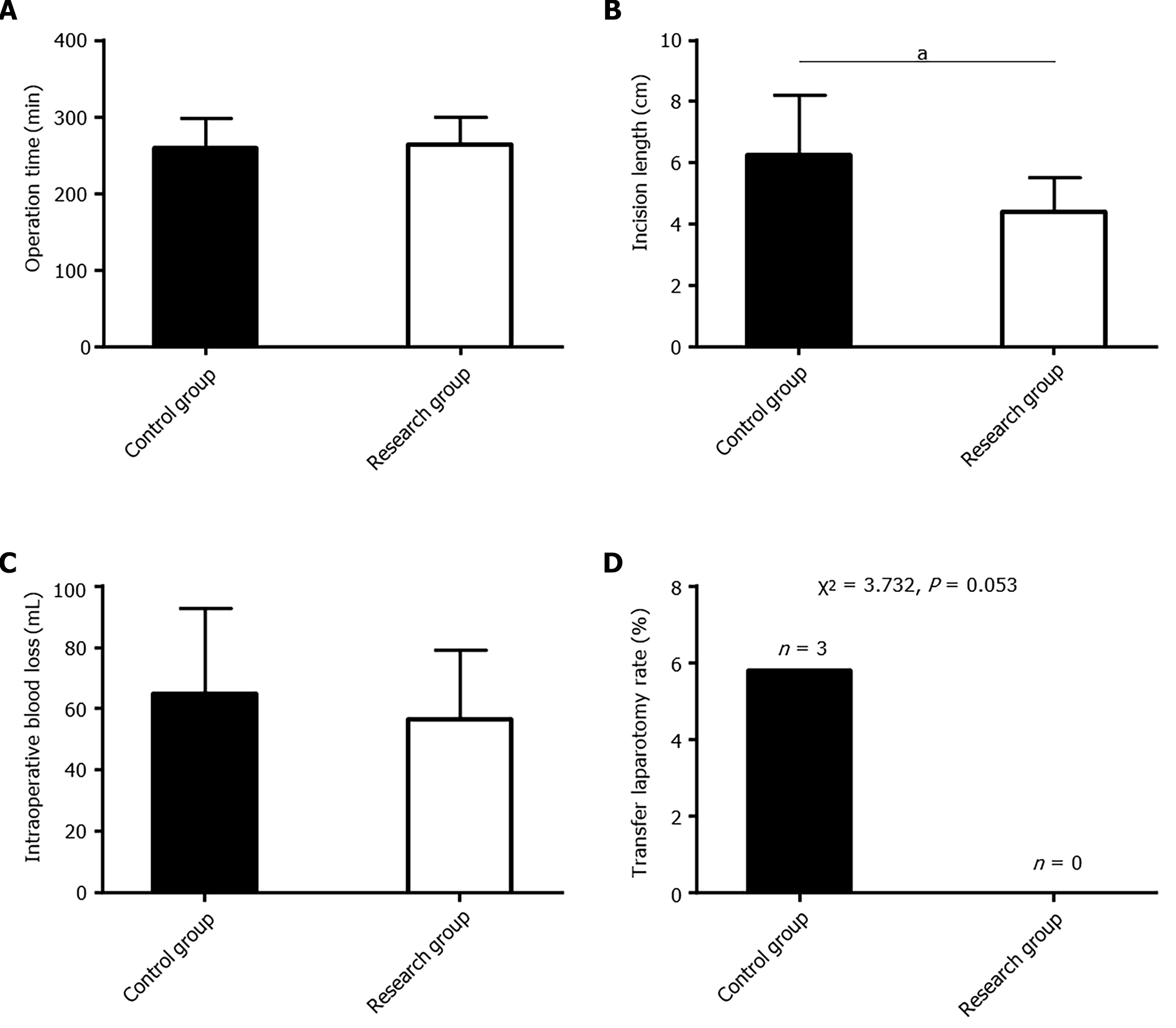
The perioperative indicators analyzed included OT, incision length, IBL, and rate of conversion to laparotomy. OT, IBL, and rate of conversion to laparotomy were comparable between the two groups (P > 0.05); however, the research group had a significantly shorter incision length (P < 0.05; Figure 1).
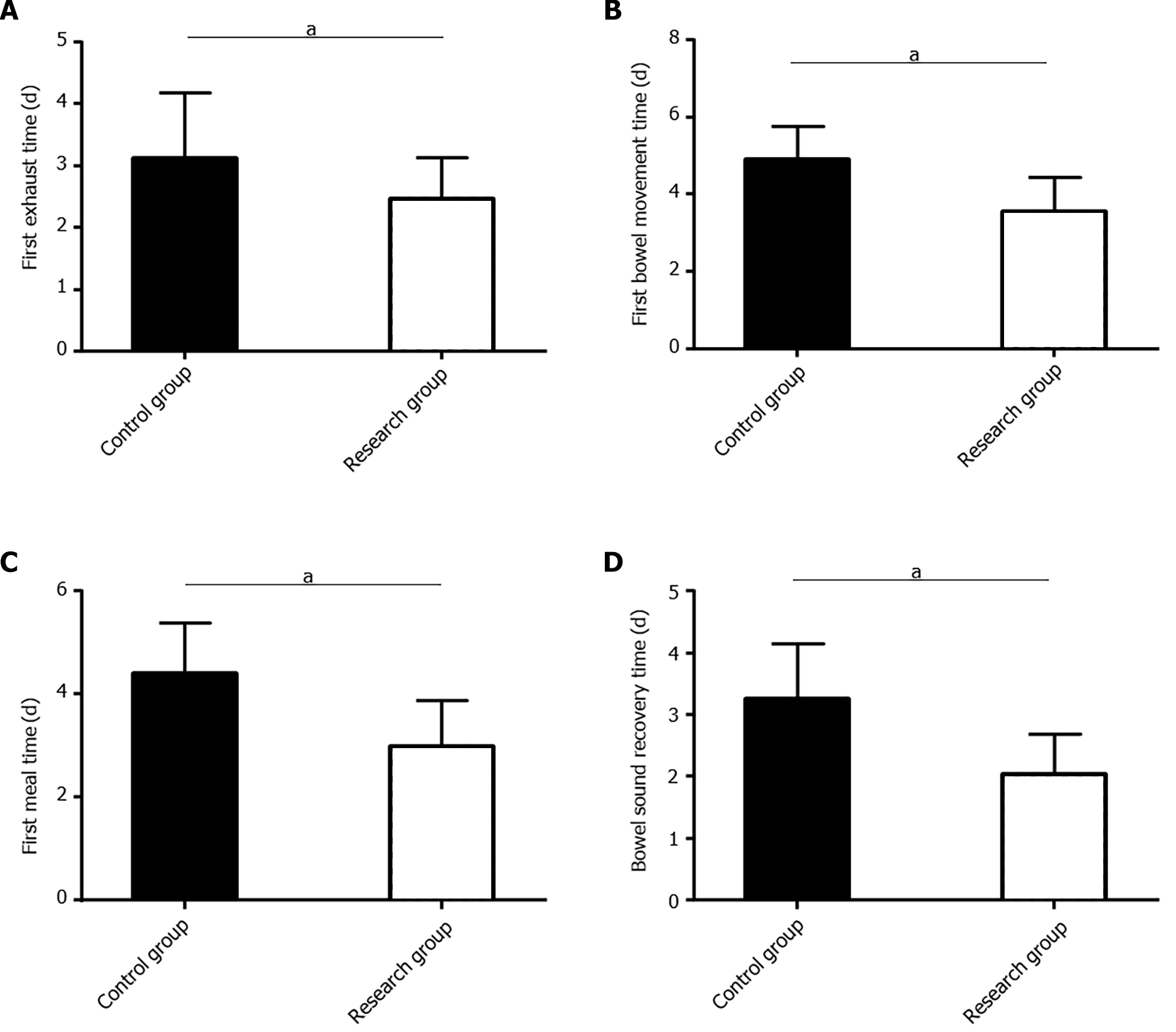
The postoperative recovery of patients was determined mainly by postoperative anal exhaust, bowel movement, oral food intake, and bowel sound recovery. The times to the first postoperative exhaust, bowel movement, and oral intake were markedly shorter and time to bowel sound recovery was faster in the research group than in the control group (P < 0.05; Figure 2).
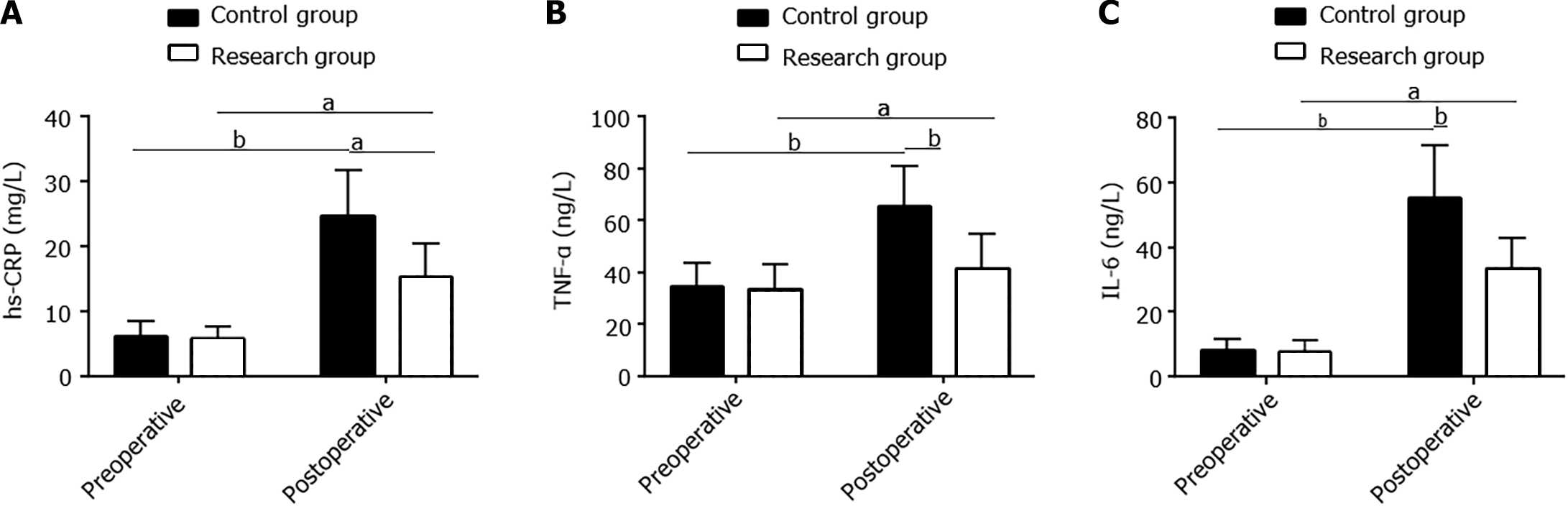
Serum hs-CRP, TNF-α, and IL-6 levels were assessed pre- and postoperatively. No significant inter-group differences were observed in these indexes preoperatively (P > 0.05). However, all of them increased significantly in both groups postoperatively (P < 0.05). However, the levels in the research group were lower than those in the control group (P < 0.05; Figure 3).
In terms of complications, the occurrences of AL, incision infection, bleeding, and ileus were evaluated. The incidence of total postoperative complications was markedly lower in the research group than in the control group (P < 0.05; Table 2).
| Factors | Control group (n = 52) | Research group (n = 63) | χ2 | P value |
| Anastomotic leakage | 3 (5.77) | 0 (0.00) | ||
| Incision infection | 10 (19.23) | 3 (4.76) | ||
| Bleeding | 1 (1.92) | 2 (3.17) | ||
| Ileus | 2 (3.85) | 4 (6.35) | ||
| Total | 16 (30.77) | 9 (14.29) | 4.549 | 0.033 |
The ORR in the research group was 90.48%, which was significantly higher than the 75.00% of the control group (P < 0.05; Table 3).
| Factors | Control group (n = 52) | Research group (n = 63) | χ2 | P value |
| Marked response | 18 (34.62) | 35 (55.56) | ||
| Response | 21 (40.38) | 22 (34.92) | ||
| Non-response | 13 (25.00) | 6 (9.52) | ||
| Overall response rate | 39 (75.00) | 57 (90.48) | 4.947 | 0.026 |
Although surgery is the major treatment option for CRC, the procedure can lead to prolonged hospitalization, surgical infection, and postoperative ileus. The popularization of laparoscopic surgery has uncovered a more effective way of addressing these problems[16,17]. This study hypothesized that, compared with conventional five-port laparoscopic surgery, RPLS has a more prominent clinical effect in CRC treatment, which is hereby verified and reported.
In patients with CRC, RPLS has the same effects as five-port laparoscopic surgery in shortening OT, reducing intraoperative bleeding, and lowering the rate of conversion to laparotomy. However, RPLS more effective in shortening the incision length, as demonstrated by the evaluation of perioperative indicators. In terms of postoperative recovery, the research group exhibited significant advantages over the control group with a shorter time to first exhaust, bowel movement, oral food intake, and bowel sound recovery after surgery, indicating that RPLS can accelerate postoperative recovery. This could be attributed to the shorter incision length and less damage to gastrointestinal function in RPLS, ensuring the effective recovery of gastrointestinal function postoperatively[18,19]. Similarly, a meta-analysis indicated a shorter hospital stay in patients with gastric cancer who underwent RPLS compared with those who underwent conventional laparoscopic surgery, further demonstrating that RPLS accelerates patient recovery[20]. Similar to our results, Wu et al[21] found that RPLS has significant advantages over multi-port laparoscopic surgery in reducing surgical incision length, alleviating postoperative pain, shortening the time to first postoperative anal exhaust, and promoting early ambulation in elderly patients with upper rectal cancer.
hs-CRP, TNF-α, and IL-6 are closely correlated with physical trauma-induced inflammatory responses in patients following RPLS[22]. In the present study, postoperative hs-CRP, TNF-α, and IL-6 Levels in the research group increased significantly but were still significantly lower than those in the control group, indicating that RPLS can significantly inhibit surgery-related inflammatory reactions in patients with CRC. In addition, the research group had a lower in-cidence of total postoperative complications, such as AL, incision infection, bleeding, and ileus, than the control group (14.29% vs 30.77%, respectively). Thus, RPLS has a lower risk of postoperative adverse events. The higher safety rate of RPLS may also be attributed to the shorter incision length, indicating relatively less damage to the patient’s body structure and function, thereby ensuring fewer postoperative complications[23,24]. Finally, the research group showed a significantly higher ORR than the control group (90.48% vs 75.00%, respectively), indicating greater therapeutic efficacy obtained with RPLS. Kim et al[25] reported that RPLS for gastric cancer is not only safe and feasible but also has a shorter learning curve, similar to our findings. This is partly because RPLS has no effect on the surgical field, surgical area judgment, and tumor resection, thereby confirming the safety and clinical efficacy of the procedure[26].
The current study has several limitations. First, the sample size was small, and the effect of potentially confounding factors could not be ruled out, which would limit the generalizability of the research results. Second quality of life and stress indicators were not analyzed, which would have further verified the clinical advantages of RPLS. Finally, prognostic outcomes were not assessed, which would have offered insights into the long-term impact of RPLS.
In summary, RPLS offers more advantages in the treatment of CRC than five-port laparoscopic surgery, as reflected by a shorter incision length, faster recovery, regulation of the inflammatory response, and higher rates of postoperative safety and efficacy.
| 1. | Mitsala A, Tsalikidis C, Pitiakoudis M, Simopoulos C, Tsaroucha AK. Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era. Curr Oncol. 2021;28:1581-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 2. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1120] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 3. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1425] [Article Influence: 356.3] [Reference Citation Analysis (0)] |
| 4. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (2)] |
| 5. | Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, Stein B, Charlebois P, Ghitulescu G, Morin N, Jagoe T, Scheede-Bergdahl C, Minnella EM, Fiore JF Jr. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020;155:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 333] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 6. | Salibasic M, Pusina S, Bicakcic E, Pasic A, Gavric I, Kulovic E, Rovcanin A, Beslija S. Colorectal Cancer Surgical Treatment, our Experience. Med Arch. 2019;73:412-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Yang JW, Yan SY, Lu Y, Han JG, Pei W, Zhao JJ, Li ZK, Zhou H, Yang NN, Wang LQ, Yang YC, Liu CZ. Electroacupuncture vs Sham Electroacupuncture in the Treatment of Postoperative Ileus After Laparoscopic Surgery for Colorectal Cancer: A Multicenter, Randomized Clinical Trial. JAMA Surg. 2023;158:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 8. | Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, Li F, Liu B, Pan Z, Liu J, Du K, Zhou X, Li C, Zhang A, Pu Z, Li Y, Feng B, Tong W. Transanal Drainage Tube Use for Preventing Anastomotic Leakage After Laparoscopic Low Anterior Resection in Patients With Rectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2021;156:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Seo HS, Kim S, Song KY, Lee HH. Feasibility and Potential of Reduced Port Surgery for Total Gastrectomy With Overlap Esophagojejunal Anastomosis Method. J Gastric Cancer. 2023;23:487-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 10. | Nath SK. Reduced Port Laparoscopic Cholecystectomy: Single and a Half Incision Lap Chole. Indian J Surg. 2016;78:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Han HJ, Kang CM. Reduced port minimally invasive distal pancreatectomy: single-port laparoscopic versus robotic single-site plus one-port distal pancreatectomy. Surg Endosc. 2019;33:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg. 2015;8:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Borodulin O, Stockwell E, Howard D. Cosmetic Surgery-Use of 3mm Ports and Reduced-Port Techniques for Gynecologic Surgery. Surg Technol Int. 2020;36:153-156. [PubMed] |
| 14. | Hirano Y, Hiranuma C, Hattori M, Douden K, Yamaguchi S. Single-incision or Single-incision Plus One-Port Laparoscopic Surgery for Colorectal Cancer. Surg Technol Int. 2020;36:132-135. [PubMed] |
| 15. | Zehnbauer B, Temple-Smolkin R, Monzon FA. Guidelines for Colorectal Cancer Testing: Evidence-Based Practice Recommendations. J Mol Diagn. 2017;19:183-186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Kang CY, Chaudhry OO, Halabi WJ, Nguyen V, Carmichael JC, Stamos MJ, Mills S. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am J Surg. 2012;204:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, McMurry TL, Goudreau BJ, Umapathi BA, Kron IL, Sawyer RG, Hedrick TL. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220:430-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 306] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 18. | Wei C, Xiao J, Teng WH, Liao LH, Zang WD. [Application of single incision plus one port laparoscopic surgery in radical right hemicolon cancer surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:54-61. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Wang S, Ling T, Zhao E, Cao H. The surgical treatment of gastric cancer in the era of minimally invasive surgery. Minerva Chir. 2017;72:334-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Alarcón I, Yang T, Balla A, Morales-Conde S. Single/reduced port surgery vs. conventional laparoscopic gastrectomy: systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Wu H, Zheng Z, Xu L, Wu Y, Guan Z, Li W, Chen G. Short- And medium-term outcomes of reduced-port laparoscopic surgery in elderly patients with upper rectal cancer: A retrospective cohort study. Cancer Med. 2020;9:5320-5326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Milone M, Desiderio A, Velotti N, Manigrasso M, Vertaldi S, Bracale U, D'Ambra M, Servillo G, De Simone G, De Palma FDE, Perruolo G, Raciti GA, Miele C, Beguinot F, De Palma GD. Surgical stress and metabolic response after totally laparoscopic right colectomy. Sci Rep. 2021;11:9652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Ishii Y, Ochiai H, Sako H, Watanabe M. Long-term oncological outcome of reduced-port laparoscopic surgery (single-incision plus one port) as a technical option for rectal cancer. Asian J Endosc Surg. 2023;16:687-694. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Fan Y, Wu SD, Kong J, Su Y, Tian Y, Yu H. Feasibility and safety of single-incision laparoscopic splenectomy: a systematic review. J Surg Res. 2014;186:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Kim HG, Kim DY, Jeong O. Transition from Conventional to Reduced-Port Laparoscopic Gastrectomy to Treat Gastric Carcinoma: a Single Surgeon's Experience from a Small-Volume Center. J Gastric Cancer. 2018;18:172-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Watras AJ, Kim JJ, Ke J, Liu H, Greenberg JA, Heise CP, Hu YH, Jiang H. Large-Field-of-View Visualization with Small Blind Spots Utilizing Tilted Micro-Camera Array for Laparoscopic Surgery. Micromachines (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |