Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1717
Revised: May 21, 2024
Accepted: May 24, 2024
Published online: June 27, 2024
Processing time: 116 Days and 22.3 Hours
Laparoscopic-assisted radical gastrectomy (LARG) is the standard treatment for early-stage gastric carcinoma (GC). However, the negative impact of this proce
To investigate the influence of pressure-controlled ventilation volume-guaranteed (PCV-VG) and volume-controlled ventilation (VCV) on blood gas analysis and pulmonary ventilation in patients undergoing LARG for GC based on the lung ultrasound score (LUS).
The study included 103 patients with GC undergoing LARG from May 2020 to May 2023, with 52 cases undergoing PCV-VG (research group) and 51 cases undergoing VCV (control group). LUS were recorded at the time of entering the operating room (T0), 20 minutes after anesthesia with endotracheal intubation (T1), 30 minutes after artificial pneumoperitoneum (PP) establishment (T2), and 15 minutes after endotracheal tube removal (T5). For blood gas analysis, arterial partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2) were observed. Peak airway pressure (Ppeak), plateau pressure (Pplat), mean airway pressure (Pmean), and dynamic pulmonary compliance (Cdyn) were recorded at T1 and T2, 1 hour after PP establishment (T3), and at the end of the operation (T4). Postoperative pulmonary complications (PPCs) were recorded. Pre- and postoperative serum interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) were measured by enzyme-linked immunosorbent assay.
Compared with those at T0, the whole, anterior, lateral, posterior, upper, lower, left, and right lung LUS of the research group were significantly reduced at T1, T2, and T5; in the control group, the LUS of the whole and partial lung regions (posterior, lower, and right lung) decreased significantly at T2, while at T5, the LUS of the whole and some regions (lateral, lower, and left lung) increased significantly. In comparison with the control group, the whole and regional LUS of the research group were reduced at T1, T2, and T5, with an increase in PaO2, decrease in PaCO2, reduction in Ppeak at T1 to T4, increase in Pmean and Cdyn, and decrease in Pplat at T4, all significant. The research group showed a significantly lower incidence of PPCs than the control group within 3 days postoperatively. Postoperative IL-1β, IL-6, and TNF-α significantly increased in both groups, with even higher levels in the control group.
LUS can indicate intraoperative non-uniformity and postural changes in pulmonary ventilation under PCV-VG and VCV. Under the lung protective ventilation strategy, the PCV-VG mode more significantly improved intraoperative lung ventilation in patients undergoing LARG for GC and reduced lung injury-related cytokine production, thereby alleviating lung injury.
Core Tip: This study mainly analyzed the effects of pressure-controlled ventilation volume-guaranteed (PCV-VG) and volume-controlled ventilation (VCV) on blood gas analysis and pulmonary ventilation in patients with gastric carcinoma (GC) undergoing laparoscopic-assisted radical gastrectomy (LARG) based on the lung ultrasound score (LUS). We performed validation analyses by evaluating the peak airway pressure (Ppeak), plateau pressure (Pplat), mean airway pressure (Pmean), dynamic pulmonary compliance (Cdyn), occurrence of postoperative pulmonary complications (PPCs), and levels of serum interleukin (IL)-1β, IL-6, and tumor necrosis factor-α before and after surgery. We confirmed that LUS can indicate non-uniformity and postural changes in lung ventilation under the two ventilation modes. However, PCV-VG is superior to VCV in significantly alleviating lung injury and inflammatory responses in patients undergoing LARG for GC, improving lung ventilation, and exerting a protective effect against PPCs. Thus, PCV-VG is a practical ventilation option in clinical practice.
- Citation: Tan J, Bao CM, Chen XY. Lung ultrasound score evaluation of the effect of pressure-controlled ventilation volume-guaranteed on patients undergoing laparoscopic-assisted radical gastrectomy. World J Gastrointest Surg 2024; 16(6): 1717-1725
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1717.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1717
Gastric carcinoma (GC) is the third leading cause of death from tumors worldwide and the fifth most prevalent malignancy. It often occurs in the elderly and is characterized by a progressive decline in physical function[1,2]. Risk factors include dietary factors, such as high salt, oil, and sugar intake; unhealthy lifestyle habits like smoking and alcohol consumption; and Helicobacter pylori infection, all of which contribute to an increased risk of developing GC[3]. Over 1 million new GC cases are diagnosed every year and nearly 800000 deaths, with a 5-year survival of 36.3%[4]. This is because of the low cure rate and high treatment difficulty due to the nonspecific manifestations in early GC cases the loss of the best time for surgery in advanced ones[5]. Laparoscopic-assisted radical gastrectomy (LARG), the standard treatment for early-stage GC, is advantageous over conventional open gastrectomy in minimal invasiveness, good visualization, lesser intraoperative bleeding, and faster postoperative recovery[6,7]. However, anesthesia, mechanical synchronization, and other factors involved in the procedure may induce various negative effects on the patient’s respiratory function and cause adverse events, such as abnormal blood gas analysis indexes, uneven pulmonary ventilation, and postoperative pulmonary complications (PPCs)[8,9].
Therefore, the optimized management of LARG would be highly beneficial to avoid such adverse events and improve the efficacy and safety of patients. This paper evaluated the influence of pressure-controlled ventilation volume-guaranteed (PCV-VG) on blood gas analysis indexes and pulmonary ventilation in patients undergoing LARG for GC and compared it with volume-controlled ventilation (VCV) as the control. A comparative evaluation was also conducted based on the lung ultrasound score (LUS), which has not been analyzed by other investigators[10]. VCV is a ventilation mode that applies a uniform flow rate, which may increase the peak airway pressure (Ppeak) in the alveoli, leading to lung loss in patients[11]. In contrast, as a more advanced dual-control ventilation mode, PCV-VG delivers pressure-controlled gas, with the pressure control level automatically adjusted according to factors, such as the patient’s lung resistance and compliance, resulting in a lower Ppeak and higher dynamic pulmonary compliance (Cdyn). These features may help prevent lung pressure-related losses[12,13]. The LUS is a non-invasive and radiation-free lung scoring standard used to evaluate pulmonary ventilation[14]. This study evaluated the clinical effect of PCV-VG in LARG based on the LUS and is hereby reported in detail.
The participants were 103 patients undergoing LARG for GC at the Lishui District People’s Hospital from May 2020 to May 2023. The research group (n = 52) underwent PCV-VG, whereas the control group (n = 51) underwent VCV.
The inclusion criteria were as follows: Patients that presented GC symptoms and met the diagnostic criteria for GC; no extensive infiltration of surrounding tissues and organs or distant metastasis; underwent LARG under general anesthesia; and complete case records.
The exclusion criteria were the following: Severe dysfunction of the heart, lung, kidneys, etc.; psychological illness or mental disturbance; pulmonary bullae, asthma, or moderate to severe ventilation dysfunction; and neuromuscular diseases, lung surgery history, or preoperative anemia (hemoglobin ≤ 70 g/L).
All patients were conventionally monitored for blood pressure, electrocardiography, and SpO2 after entering the operating room. A radial artery puncture needle was inserted under local anesthesia with 1 mL of 2% lidocaine for invasive mean arterial pressure (MAP) monitoring. The anesthesia induction scheme consisted of midazolam (0.02 mg/kg), sufentanil (0.5 μg/kg), etomidate (0.2 mg/kg), and rocuronium bromide (0.6 mg/kg). Catheterization was performed in the right internal jugular deep vein. After establishing an artificial pneumoperitoneum (PP), the patient was placed in a dorsal elevated position at 30°. Sevoflurane (1%–2%), remifentanil (0.1–0.3 μg/kg/min), dexmedetomidine (0.2 μg/kg/h), and rocuronium bromide (5–6 μg/kg/min) were used for anesthesia maintenance, with the dosage adjusted depending on the anesthesia depth. The bispectral index was maintained at 40–60. The intraoperative fluctuation amplitude of MAP was controlled to not exceed 20% of the base value. If the MAP decreased > 20% of the base value after rehydration the
After endotracheal intubation, the patients in the research and control groups were placed under PCV-VG and VCV, respectively. Protective ventilation strategies were adopted in both groups: inhaled oxygen concentration: 60%; fresh gas velocity: 1.5 L/min; tidal volume: 8 mL/kg; respiratory rate (RR): 12 breaths/min; inspiratory/expiratory: 1:2; PETCO2 (adjusted by respiratory rate): 30–35 mmHg; and pressure: 35 cmH2O.
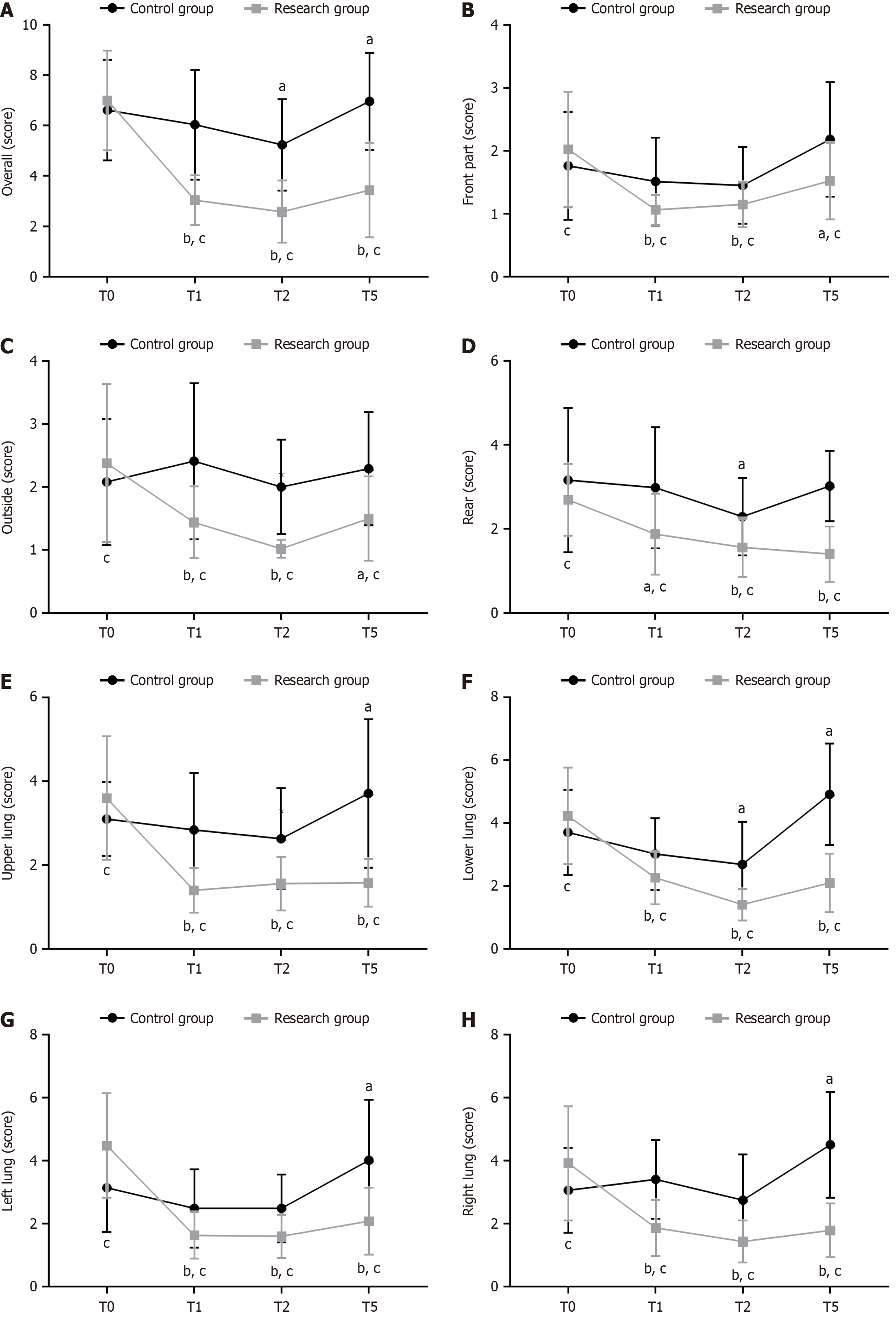
A portable ultrasound machine was used to record the LUS of patients at the time of entering the operating room (T0), 20 min after anesthesia with endotracheal intubation (T1), 30 min after establishing artificial PP (T2), and 15 min after endotracheal tube removal (T5). The LUS was divided into 12 pulmonary zones: the chest wall was divided into anterior, lateral, and posterior sides based on the anterior and posterior axillary lines. Each part was then divided into upper and lower sections to make up 12 evaluable lung regions. The possible total score of the 12 Lung regions was 0–36 points, with a higher score indicating greater lung ventilation injury.
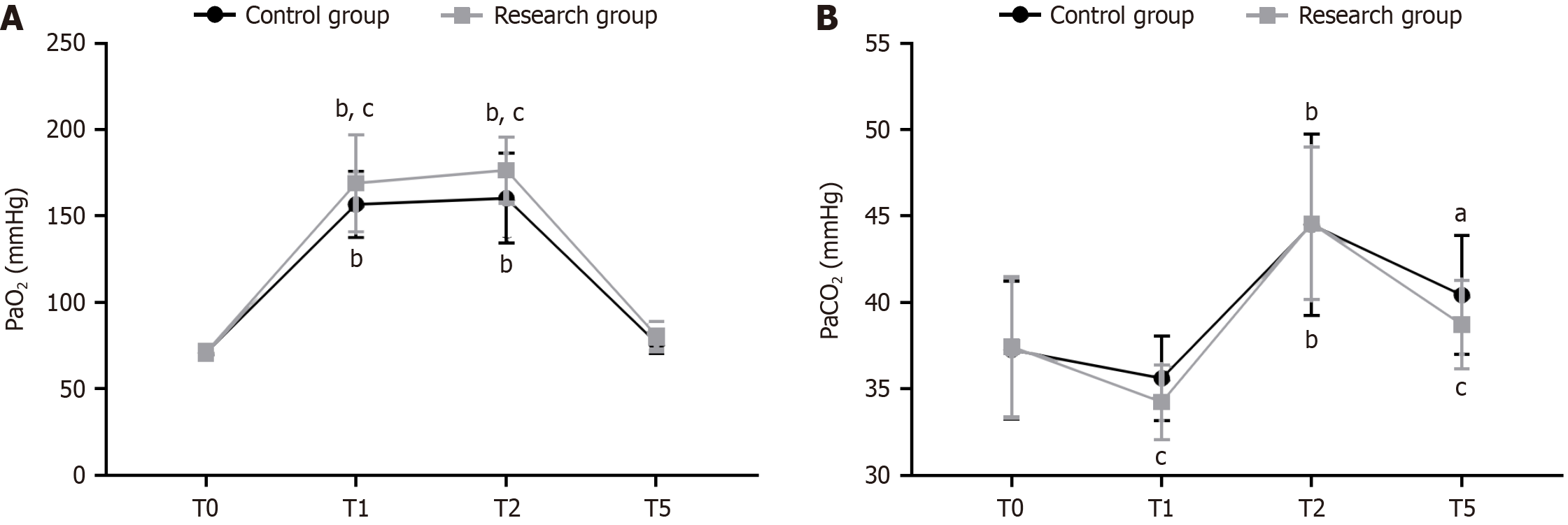
Arterial blood was collected at T0, T1, T2, and T5, and blood gas analysis was performed to record the arterial partial pressure of oxygen (PaO2) and partial pressure of carbon dioxide (PaCO2).
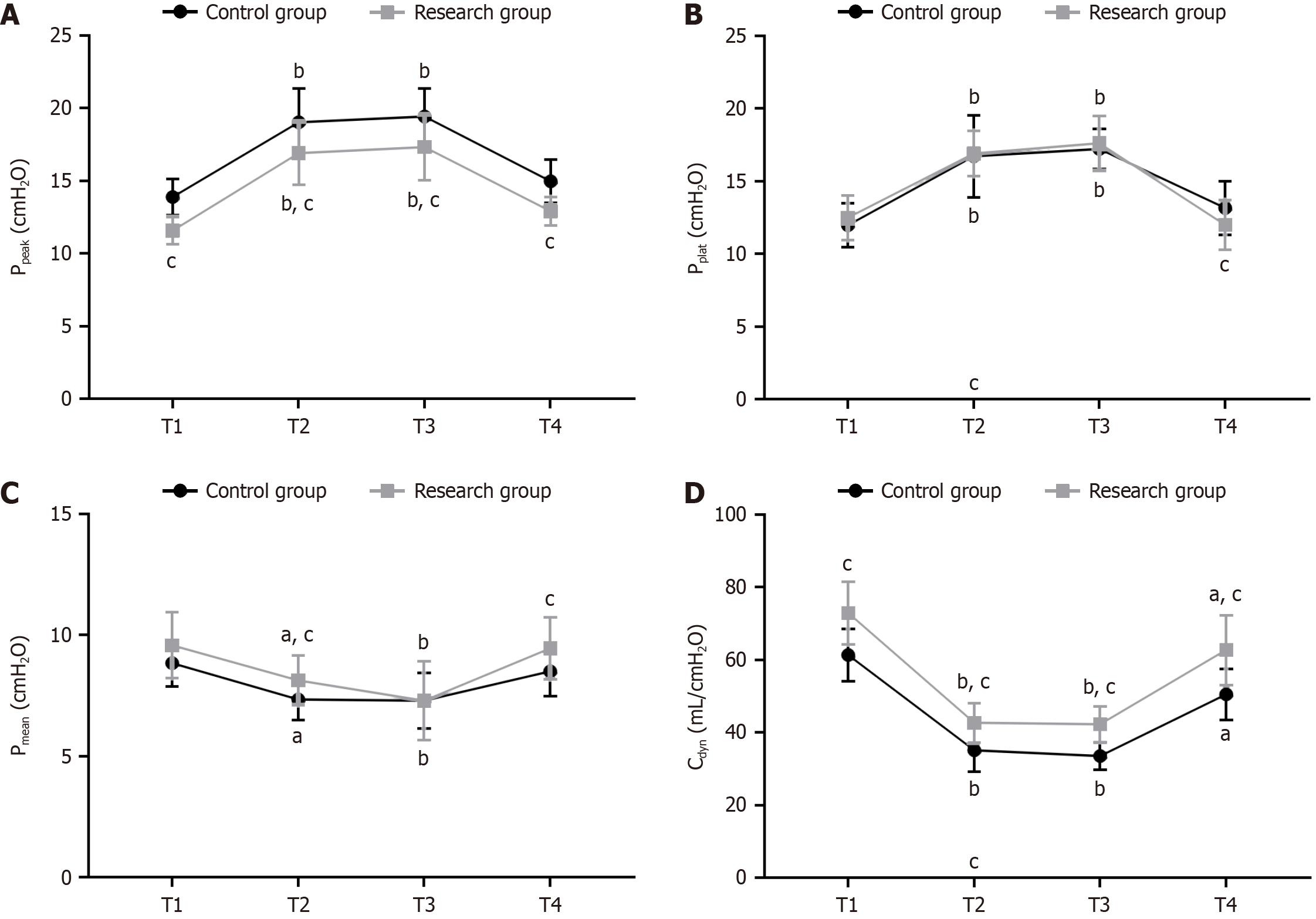
The Ppeak, platform pressure (Pplat), mean airway pressure (Pmean), and Cdyn were monitored and recorded at T1, T2, T3 (1 h after PP establishment), and T4 (at the end of surgery).
For PPCs, incidence of adverse events, such as pneumonia, hypoxemia, bronchospasm, atelectasis, and pneumothorax, were recorded and the total incidence calculated.
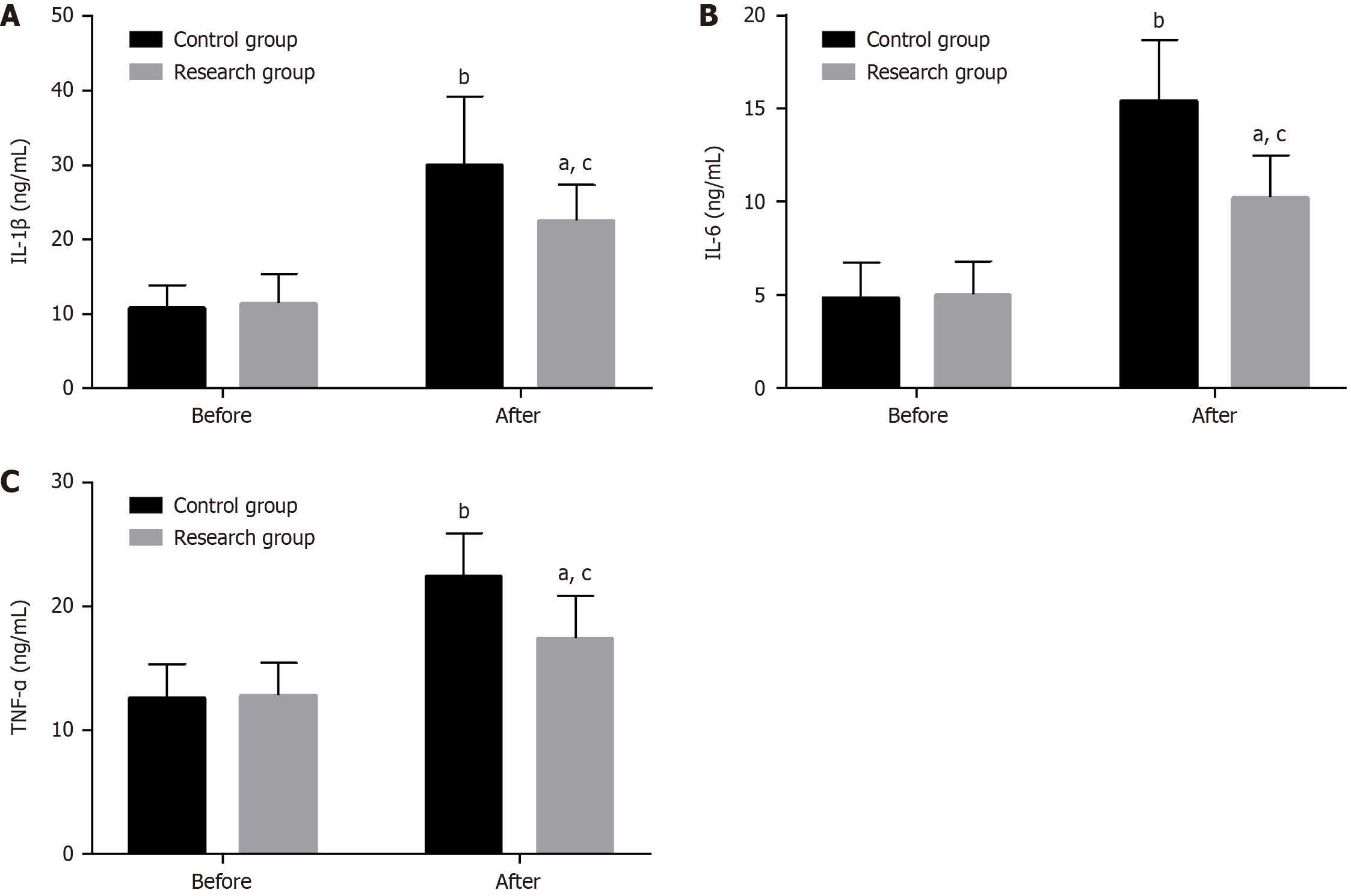
Venous blood (3 mL) was drawn on an empty stomach pre- and postoperatively and centrifuged to obtain serum for measuring interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) levels by enzyme-linked immunosorbent assay.
Continuous variables (e.g., age, body mass index, PP duration, LUS, PaO2, and PaCO2) were expressed mean ± SE. To identify statistical significance (threshold: P < 0.05), the independent sample t-test was performed for inter-group comparisons of continuous variables and paired t test for intra-group comparisons before and after treatment. Categorical variables [sex, America Society of Anesthesiologist (ASA) grade, etc.) were expressed as rate (percentage) and compared between groups using the χ2 test. The collected experimental data were analyzed using SPSS20.0.
The two patient cohorts were similar in sex, age, body mass index, PP duration, ASA grade, and other general data (P > 0.05; Table 1).
| Indicators | Control group (n = 51) | Research group (n = 52) | χ2/t | P value |
| Sex (male/female) | 25/26 | 24/28 | 0.085 | 0.771 |
| Age (years) | 58.78 ± 8.01 | 61.13 ± 7.40 | 1.547 | 0.125 |
| Body mass index (kg/m2) | 21.49 ± 2.40 | 22.12 ± 3.01 | 1.173 | 0.244 |
| Duration of pneumoperitoneum (minutes) | 66.25 ± 12.89 | 64.63 ± 13.6 | 0.620 | 0.537 |
| ASA grade (II/III) | 28/23 | 25/27 | 0.480 | 0.488 |
We compared the LUS of the different lung regions between the two patient groups. Compared with those at T0, the whole, anterior, lateral, posterior, upper, lower, left, and right lung LUS of the research group were significantly lower at T1, T2, and T5 (P < 0.05). In the control group, the whole, posterior, and lower lung region LUS decreased significantly at T2 (P < 0.05), whereas the whole, upper, lower, left, and right lung LUS increased at T5 (P < 0.05). At T0, the two groups exhibited marked differences in anterior, lateral, posterior, upper, lower, left, and right lung region LUS (P < 0.05). At T1, T2, and T5, the research group had significantly lower LUS for the whole lung and all lung regions than the control group (P < 0.05; Figure 1).
The blood gas analysis indexes at each time point were comparatively analyzed. The two groups showed similar PaO2 and PaCO2 at T0 (P > 0.05) and a significant increase in the two indexes at T1 and T2 (P < 0.05). PaO2 and PaCO2 were significantly lower at T5 but did not differ significantly with T0 Levels (P > 0.05). PaO2 and PaCO2 at T1 and T2 were higher in the research group than in the control group (P < 0.05). PaCO2 initially decreased in both groups at T1, increased significantly at T2 (P < 0.05), and decreased significantly at T5 to a level that was still higher than that at T0 (P < 0.05). Significant inter-group differences was also observed in PaCO2 at T1 and T5 (P < 0.05) (Figure 2).
The two groups differed significantly in the Ppeak and Cdyn at T1 (P < 0.05). Ppeak and Pplat increased significantly in both groups at T2 and T3 compared at T1, while at T4, these two indicators in reduced significantly to levels that did not differ significantly from those at T1 (P > 0.05). The research group had lower Ppeak at T2, T3, and T4 and Pplat at T4 than the control group (P < 0.05). Compared with T1, the Pmean and Cdyn in both groups decreased significantly at T2 and T3. At T4, the Pmean of both groups increased to levels comparable with those at T1 (P > 0.05). The Cdyn of both groups also rose significantly but remained significantly lower than that at T1 (P < 0.05). The research group showed higher Pmean at T2 and T4 and higher Cdyn at T2, T3, and T4 compared with the control group (P < 0.05; Figure 3).
The most common PPCs in the research group was hypoxemia, followed by atelectasis, bronchospasm, and pneumothorax. The most common PPC in the control group patients was hypoxemia, followed by atelectasis, pneumonia, and bronchospasm and pneumothorax. The incidence of PPCs was markedly lower in the research group than in the control group (P < 0.05; Table 2).
| Indicators | Control group (n = 51) | Research group (n = 52) | χ2 | P value |
| Pneumonia | 3 (5.88) | 0 (0.00) | ||
| Hypoxemia | 7 (13.73) | 4 (7.69) | ||
| Bronchospasm | 2 (3.92) | 2 (3.85) | ||
| Atelectasis | 5 (9.80) | 3 (5.77) | ||
| Pneumothorax | 2 (3.92) | 1 (1.92) | ||
| Total | 19 (37.25) | 10 (19.23) | 4.135 | 0.042 |
Preoperative IL-1β, IL-6, and TNF-α did not differ significantly between the groups (P > 0.05). However, all of them increased significantly in both patient cohorts postoperatively (P < 0.05), with a more significant increase in the control group (P < 0.05; Figure 4).
Although the treatment of GC has been continuously optimized and the incidence rate reduced, LARG remains the best choice for the treatment of non-metastatic GC[15]. The advantages of LARG have been established, but this procedure still carries a risk of postoperative adverse events[16]; thus, further optimization of the management of this procedure is required.
The whole, anterior, lateral, posterior, upper, lower, left, and right lung LUS decreased significantly in the research group at T1, T2, and T5, whereas the control group only showed significantly lower whole, posterior, and lower lung LUS at T2. Moreover, the research group had evidently lower LUS of the whole lung and all regional lung regions than the control group at T1, T2, and T5. These data indicate that, compared with VCV, PCV-VG can achieve uniform ventilation by expanding trapped and unventilated alveoli, thereby more effectively reducing lung ventilation damage. The LUS results in the control group indicate optimal functional residual capacity due to the patient’s dorsal elevated position after VCV intervention and artificial PP establishment, in addition to the minimal pulmonary vascular resistance at this time, which ultimately resulted in more blood flow in the lower lung and the basolateral region, thereby improving the lung ventilation and the ventilation/blood flow ratio in these regions[17,18]. The lower and left lung LUS were the highest in the control group at T5, indicating that the LUS can indicate overall and regional ventilation changes perioperatively under both ventilation modes. PCV-VG, a ventilation modality optimized based on PCV and VCV, prevents venti
Blood gas analysis revealed that the research group had significantly higher levels of PaO2 at T1 and T2 and signi
This study has several limitations. First, the study was conducted at a single center, which may limit the generalizability of the results. Second, the retrospective study design may introduce selection bias and confounding variables. Third, the factors influencing the occurrence of PPCs in patients was not performed. Future studies need to address these three aspects to generate more robust results.
This study demonstrated that LUS can indicate non-uniformity and postural changes in lung ventilation under the two ventilation modes. Compared with VCV, PCV-VG intervention more significantly alleviated lung injury, improved lung ventilation, and reduced inflammation in patients undergoing LARG for GC, while exerting protective activity against PPCs, making it the more practical ventilation option in clinical practice.
| 1. | Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Fang W, Hu H, Jia L, Zhang J, Huang C, Hu S. Survival disparities among non-elderly American adults with locally advanced gastric cancer undergoing gastrectomy by health insurance status. Am J Med Sci. 2022;364:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Putthanachote N, Promthet S, Hurst C, Suwanrungruang K, Chopjitt P, Wiangnon S, Chen SL, Yen AM, Chen TH. The XRCC 1 DNA repair gene modifies the environmental risk of stomach cancer: a hospital-based matched case-control study. BMC Cancer. 2017;17:680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 5. | Ilson DH. Advances in the treatment of gastric cancer: 2019. Curr Opin Gastroenterol. 2019;35:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Sugimoto M, Kinoshita T, Shibasaki H, Kato Y, Gotohda N, Takahashi S, Konishi M. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc. 2013;27:4291-4296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Sun L, Zhao B, Huang Y, Lu H, Luo R, Huang B. Feasibility of laparoscopy gastrectomy for gastric cancer in the patients with high body mass index: A systematic review and meta-analysis. Asian J Surg. 2020;43:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 528] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 9. | Yajima K, Kanda T, Tanaka R, Sato Y, Ishikawa T, Kosugi S, Honda T, Hatakeyama K. Reexpansion Pulmonary Edema following Laparoscopy-Assisted Distal Gastrectomy for a Patient with Early Gastric Cancer: A Case Report. Case Rep Surg. 2012;2012:863163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Ghodraty MR, Pournajafian AR, Tavoosian SD, Khatibi A, Safari S, Motlagh SD, Abhari MB, Shafighnia S, Porhomayon J, Nader ND. A clinical trial of volume- versus pressure-controlled intraoperative ventilation during laparoscopic bariatric surgeries. Surg Obes Relat Dis. 2021;17:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Schick V, Dusse F, Eckardt R, Kerkhoff S, Commotio S, Hinkelbein J, Mathes A. Comparison of Volume-Guaranteed or -Targeted, Pressure-Controlled Ventilation with Volume-Controlled Ventilation during Elective Surgery: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 12. | Kim YS, Won YJ, Lee DK, Lim BG, Kim H, Lee IO, Yun JH, Kong MH. Lung ultrasound score-based perioperative assessment of pressure-controlled ventilation-volume guaranteed or volume-controlled ventilation in geriatrics: a prospective randomized controlled trial. Clin Interv Aging. 2019;14:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Li Bassi G, Comaru T, Martí D, Xiol EA, Chiurazzi C, Travierso C, Carbonara M, Ranzani OT, Amaro R, Frigola G, Fuster C, Saco MA, Zanella A, De Rosa F, Rigol M, Fernandez L, Luque N, Ramirez J, Blasi F, Suen J, Fraser J, Torres A. Recruitment manoeuvres dislodge mucus towards the distal airways in an experimental model of severe pneumonia. Br J Anaesth. 2019;122:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Wang F, Wang C, Shi J, Shan Y, Miao H, Sun T, Zhou Y, Zhang Y. Lung ultrasound score assessing the pulmonary edema in pediatric acute respiratory distress syndrome received continuous hemofiltration therapy: a prospective observational study. BMC Pulm Med. 2021;21:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Feng F, Tian Y, Zang Y, Sun L, Hong L, Yang J, Guo M, Lian X, Fan D, Zhang H. Low forced vital capacity predicts poor prognosis in gastric cancer patients. Oncotarget. 2017;8:28897-28905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Lou S, Yin X, Wang Y, Zhang Y, Xue Y. Laparoscopic versus open gastrectomy for gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Int J Surg. 2022;102:106678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 17. | Ordies S, Orlitova M, Heigl T, Sacreas A, Van Herck A, Kaes J, Saez B, Vanstapel A, Ceulemans L, Vanaudenaerde BM, Vos R, Verschakelen J, Verleden GM, Verleden SE, Van Raemdonck DE, Neyrinck AP. Flow-controlled ventilation during EVLP improves oxygenation and preserves alveolar recruitment. Intensive Care Med Exp. 2020;8:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Peng Z, Xia J, Yin N, Xue H. The effects of volume-controlled ventilation versus pressure-controlled ventilation on hemodynamic and respiratory parameters in patients undergoing lumbar spine fusion surgery: a randomized controlled trial. Ann Palliat Med. 2021;10:9553-9563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Jaju R, Jaju PB, Dubey M, Mohammad S, Bhargava AK. Comparison of volume controlled ventilation and pressure controlled ventilation in patients undergoing robot-assisted pelvic surgeries: An open-label trial. Indian J Anaesth. 2017;61:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Assad OM, El Sayed AA, Khalil MA. Comparison of volume-controlled ventilation and pressure-controlled ventilation volume guaranteed during laparoscopic surgery in Trendelenburg position. J Clin Anesth. 2016;34:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Brandly JE, Midon M, Douglas HF, Hopster K. Flow-controlled expiration reduces positive end-expiratory pressure requirement in dorsally recumbent, anesthetized horses. Front Vet Sci. 2023;10:1135452. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Toker MK, Altıparmak B, Uysal Aİ, Demirbilek SG. [Comparison of pressure-controlled volume-guaranteed ventilation and volume-controlled ventilation in obese patients during gynecologic laparoscopic surgery in the Trendelenburg position]. Braz J Anesthesiol. 2019;69:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Grübler MR, Wigger O, Berger D, Blöchlinger S. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly. 2017;147:w14491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Turan Civraz AZ, Saracoglu A, Saracoglu KT. Evaluation of the Effect of Pressure-Controlled Ventilation-Volume Guaranteed Mode vs. Volume-Controlled Ventilation Mode on Atelectasis in Patients Undergoing Laparoscopic Surgery: A Randomized Controlled Clinical Trial. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Koo BW, Oh AY, Ryu JH, Lee YJ, Han JW, Nam SW, Park DJ, Seo KS. Effects of deep neuromuscular blockade on the stress response during laparoscopic gastrectomy Randomized controlled trials. Sci Rep. 2019;9:12411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Shao RG, Xie QW, Pan LH, Lin F, Qin K, Ming SP, Li JJ, Du XK. Necrostatin-1 attenuates Caspase-1-dependent pyroptosis induced by the RIPK1/ZBP1 pathway in ventilator-induced lung injury. Cytokine. 2022;157:155950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |