Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1618
Revised: April 11, 2024
Accepted: April 23, 2024
Published online: June 27, 2024
Processing time: 163 Days and 18.9 Hours
Patients with resectable gastric adenocarcinoma accompanied by vascular cancer thrombus (RGAVCT) have a poor prognosis, with a 5-year survival rate ranging from 18.42%-53.57%. These patients need a reasonable postoperative treatment plan to improve their prognosis.
To determine the most effective postoperative chemotherapy regimen for patients with RGAVCT.
We retrospectively collected the clinicopathological data of 530 patients who un
In all, 530 eligible individuals with RGAVCT were enrolled in this study. The median overall survival (OS) of patients with RGAVCT was 24 months, and the survival rates were 80.2%, 62.5%, and 42.3% at 12, 24, and 59 months, respectively. Preoperative complications, tumor size, T stage, and postoperative chemotherapy were identified as independent factors that influenced OS in patients with RGAVCT according to the Cox multivariate analysis model. A Kaplan-Meier analysis revealed that chemotherapy had no effect on OS of patients with stage I or II RGAVCT; however, chemotherapy did have an effect on OS of stage III patients. Stage III patients who were treated with chemotherapy consisting of dual- or triple-agent regimens had better survival than those treated with single-agent regimens, and no significant difference was observed in the survival of patients treated with chemo
For patients with stage III RGAVCT, a dual-agent regimen of postoperative chemotherapy should be recom
Core Tip: In patients with resectable gastric adenocarcinoma accompanied by vascular cancer thrombus (RGAVCT), postoperative chemotherapy has an independent effect on overall survival and may even improve survival. Patients with stage I and II RGAVCT should not receive postoperative chemotherapy, and low-toxicity single-agent therapy is advised even in the presence of high-risk variables. For patients with stage III RGAVCT, a dual-agent regimen of postoperative chemotherapy should be recommended rather than a triple-agent treatment, as the latter is associated with increased risks.
- Citation: Yang ZF, Dong ZX, Dai CJ, Fu LZ, Yu HM, Wang YS. Correlation between postoperative chemotherapy regimen and survival in patients with resectable gastric adenocarcinoma accompanied with vascular cancer thrombus. World J Gastrointest Surg 2024; 16(6): 1618-1628
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1618.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1618
According to the 2020 Tumor Report[1], gastric cancer (GC) ranked fourth among all cancers according to the number of new cases, with approximately 1.1 million cases per year. GC ranked third in terms of mortality rate, with approximately 760000 deaths per year. GC is an important life-threatening disease and one of the most common malignant tumors worldwide. China ranks second and third in terms of new cases and deaths related to GC, respectively[2]. Studies by Zhang[3] and the Asian Cancer Research Group[4] marked significant advancements in the molecular typing of GC, thereby leading to enhanced medical treatment strategies. For stage III GC, the 5-year survival rate following surgery is 34.8%-54.6%[5], and radical surgical resection remains the preferred course of treatment. When tumor cells infiltrate the interior of lymphatic or vascular vessels, which are composed of endothelial cells, the condition is referred to as lym
Clinicopathological data were retrospectively collected from 530 patients (107 women and 423 men) who underwent radical surgery for GC at Shanxi Cancer Hospital between January 2017 and January 2022 and who were pathologically diagnosed with stomach adenocarcinoma with a vascular cancer embolus. Patient ages ranged from 28-83 years (median, 63 years). This study was approved by the Clinical Research Ethics Committee of Shanxi Cancer Hospital (approval number: KY2023010). During their first visit to the hospital, all patients provided written informed consent for the co
Inclusion and exclusion criteria: The inclusion criteria were as follows: (1) Radical resection of GC at Shanxi Cancer Hospital; (2) postoperative pathological confirmation of gastric adenocarcinoma or adenocarcinoma of the gastroesophageal junction; (3) postoperative pathological evidence of vascular cancer thrombus; (4) clinical stage I, II, or III disease; and (5) availability of complete clinicopathological data. The exclusion criteria were as follows: (1) Presence of other tumors; (2) nonradical resection, such as surgery with positive margins or palliative surgery; and (3) incomplete or una
Staging and Ki-67 positivity: Pathological and histological staging was performed according to the 2019 version of the World Health Organization Classification of Tumors of the Digestive System. Ki-67 positivity < 30% was considered low expression, while Ki-67 positivity ≥ 30% was considered high expression.
Follow-up: Regular gastroenterology outpatient reviews, hospitalization, and telephone interviews were used for patient survival follow-up. The deadline for follow-up was March 2023. Overall survival (OS) was defined as the time from the date of surgery to the date of death or the end of follow-up.
Clinical information: Patient information, including sex, age at the time of surgery, medical history, family history, preoperative complications, surgical procedures, and postoperative chemotherapy, was collected.
Pathological information: The pathology report included information regarding the location and size of the tumor, the degree of tumor differentiation, the Lauren classification, LBVI, neural invasion, the depth of invasion, the total number of cleared lymph nodes, and immunohistochemistry findings.
Research participants and chemotherapy regimens: To ensure the authenticity and reliability of our results, we collected information on patients who received postoperative adjuvant chemotherapy at our hospital.
SPSS (version 25.0; IBM, Armonk, NY) and R 4.3.0 (R Foundation, Vienna, Austria) were used for the statistical analysis. The rank-sum test was used to evaluate the skewed distributions, which are expressed as the mean and standard de
The 530 patients with RGAVCT who met the inclusion criteria ranged in age from 28–83 (median, 63) years (Table 1). A 4:1 male-to-female ratio was noted, with 423 men and 107 women. A total of 9.2% of patients had a family history of tumors, and 86.4% of patients had no preoperative complications. For those patients who did experience preoperative complications, 7.7%, 1.9%, 1.1%, and 2.8% experienced gastrointestinal obstruction, gastrointestinal hemorrhage, other complications (such as perforation, gastric retention, and anemia), and multiple complications, respectively. Open sur
| Characteristic | n (%) | χ2 | P value |
| Age, yr | |||
| < 60 | 211 (39.8) | 5.943 | 0.015 |
| ≥ 60 | 319 (60.2) | ||
| Sex | |||
| Male | 423 (79.8) | 7.533 | 0.006 |
| Female | 107 (20.2) | ||
| Past history | |||
| No | 289 (54.5) | 3.76 | 0.052 |
| Yes | 241 (45.5) | ||
| Family history | |||
| No | 479 (90.4) | 4.711 | 0.030 |
| Yes | 51 (9.6) | ||
| Preoperative complications | |||
| No | 458 (86.4) | 1.982 | 0.037 |
| Digestive tract obstruction | 41 (7.7) | ||
| Alimentary tract hemorrhage | 10 (1.9) | ||
| Others1 | 6 (1.1) | ||
| ≥ 2 complications | 15 (2.8) | ||
| Surgical method | |||
| Open abdominal | 328 (61.9) | 1.853 | 0.396 |
| Laparoscopy | 179 (33.8) | ||
| Joint thoracoabdominal | 23 (4.3) | ||
| Chemotherapy | |||
| No | 136 (25.7) | 63.834 | <0.001 |
| Yes | 394 (74.3) | ||
| Tumor site | |||
| Proximal | 286 (54) | 2.217 | 0.330 |
| Distal | 144 (27.2) | ||
| Body | 100 (18.9) | ||
| Differentiation | |||
| Moderate | 54 (10.2) | 5.742 | 0.057 |
| Moderate-poor | 188 (35.5) | ||
| Poor | 288 (54.3) | ||
| Lauren classification | |||
| Diffused type | 182 (34.3) | 11.859 | 0.008 |
| Intestinal type | 62 (11.7) | ||
| Mixed type | 252 (47.5) | ||
| NA | 34 (6.4) | ||
| Neural invasion | |||
| Absence | 247 (46.6) | 5.899 | 0.015 |
| Presence | 283 (53.4) | ||
| Tumor size, cm | |||
| < 5 | 231 (43.6) | 8.976 | 0.003 |
| ≥ 5 | 299 (56.4) | ||
| HER2 expression | |||
| Negative | 458 (86.4) | 0.495 | 0.482 |
| Positive | 72 (13.6) | ||
| MMR status | |||
| dMMR | 14 (2.6) | 0.229 | 0.633 |
| pMMR | 516 (97.4) | ||
| Ki-67 expression | |||
| Low | 8 (1.5) | 6.019 | 0.014 |
| High | 522 (98.5) | ||
| T stage | |||
| 2 | 44 (8.5) | 25.459 | < 0.001 |
| 3 | 374 (70.6) | ||
| 4a | 93 (17.5) | ||
| 4b | 18 (3.4) | ||
| N stage | |||
| 0 | 37 (7) | 42.8 | < 0.001 |
| 1 | 104 (19.6) | ||
| 2 | 137 (25.8) | ||
| 3a | 158 (29.8) | ||
| 3b | 94 (17.7) | ||
| Stage | |||
| IB | 10 (1.9) | 62.765 | < 0.001 |
| IIA | 43 (8.1) | ||
| IIB | 80 (15.1) | ||
| IIIA | 132 (24.9) | ||
| IIIB | 171 (32.3) | ||
| ШC | 94 (17.7) |
According to the Union for International Cancer Control TNM staging system (8th edition), 10 patients (1.9%) were categorized as stage IB, 43 (8.1%) as stage IIA, 80 (15.1%) as stage IIB, 132 (24.9%) as stage IIIA, 171 (32.3%) as stage IIIB, and 94 (17.7%) as stage IIIC. The RGAVCT tumor sites were mainly distributed in the proximal (54%), distal (27.2%), and gastric bodies (18.9%). Tumors were predominantly poorly differentiated (54.3%), intermediate-poorly differentiated (35.5%), and moderately differentiated (10.2%); none were well differentiated.
The Lauren classification was predominantly mixed (47.5%) or diffused (34.3%), while the intestinal type was less frequent (11.7%). Neural invasion was observed in 53.4% of the patients. Tumors > 5 cm were found in 56.4% of the patients. Immunohistochemistry indicated Her-2 positivity (3+ or 2+ fluorescent in situ hybridization positivity) in ap
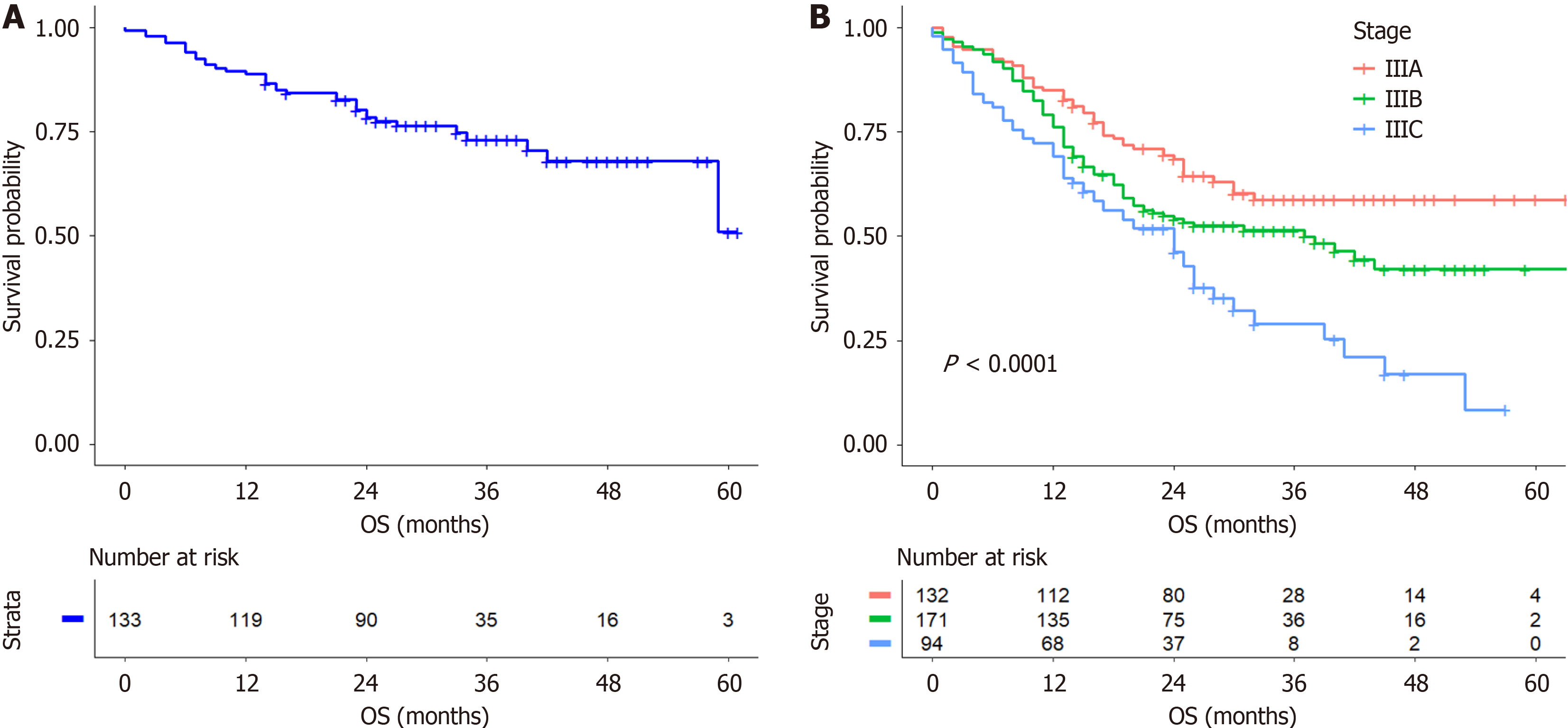
The median OS (mOS) of patients with RGAVCT was 24 months, with survival rates of 80.2%, 62.5%, 54.8%, and 42.3% at 12, 24, 37, and 59 months, respectively. The postoperative chemotherapy group had an mOS of 25 months, and the survival rates were 86.8%, 71.3%, 64.2%, and 52.8% at 12, 24, 37, and 53 months, respectively. The mOS was 15 months in the group that did not receive chemotherapy, and the survival rates were 61%, 38.1%, 28.5%, and 19% at 12, 24, 34, and 59 months, respectively. Patients with stage I and II cancer had an mOS of 27 months, with survival rates of 89.5%, 80%, 74.6%, and 52.2% at 12, 24, 34, and 59 months, respectively (Figure 1A). The mOS of patients with stage III cancer was 23 months, with survival rates of 77.8%, 57.8%, 49.5%, and 40.1% at 12, 24, 37, and 53 months, respectively. The best survival rate was observed in patients with stage IIIA cancer, followed by those with stage IIIB cancer, while the worst survival rate was observed in patients with stage IIIC cancer (Figure 1B).
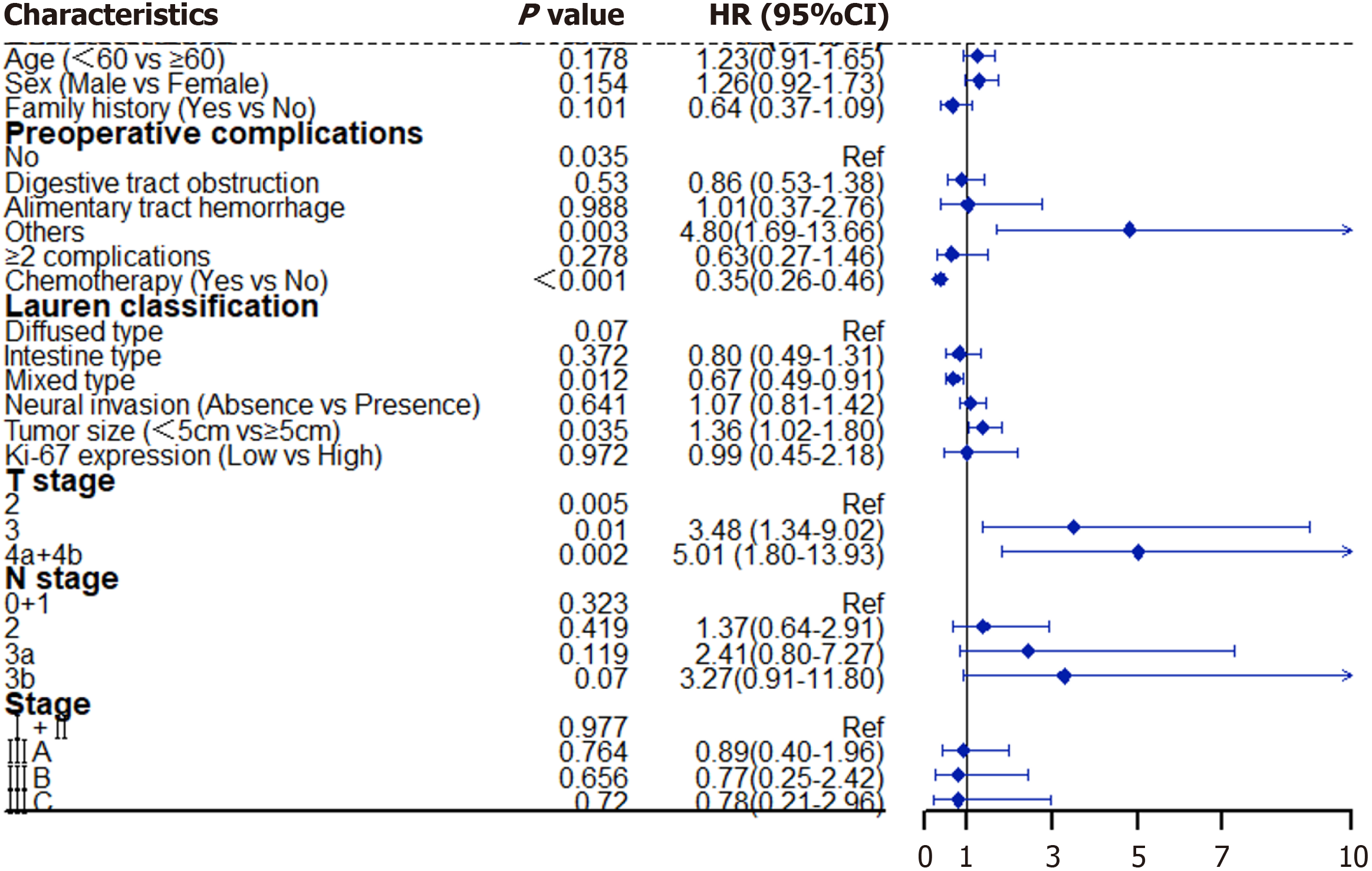
The univariate analysis by Mantel-Cox regression revealed significant differences (P < 0.05) in age, sex, family history, preoperative complications, postoperative chemotherapy, Lauren classification, neural invasion status, tumor size, Ki-67 expression, T and N stage, and clinical stage, and these factors were significantly correlated with the OS of patients with RGAVCT. A multivariate Cox analysis of the significant influencing factors revealed that preoperative complications (P = 0.035), postoperative chemotherapy [P < 0.001; hazard ratio (HR) = 0.35; 95% confidence interval (CI) = 0.26-0.46], tumor size (P = 0.035; HR = 1.36; 95%CI = 1.02-1.80), and T stage (P = 0.005) were independent factors that affected OS (Figure 2).
Of the 394 patients with RGAVCT who were administered postoperative adjuvant chemotherapy, 323 received che
The univariate analysis revealed no significant difference between postoperative chemotherapy and survival in pa
The Cox multivariate analysis revealed that preoperative complications (P = 0.021), neural invasion (P = 0.02; HR = 2.47; 95%CI = 1.16-5.28), and Ki-67 expression (P = 0.007; HR = 0.05; 95%CI = 0.01-0.44) were independent factors found to influence the survival of patients with stage I and II RGAVCT (Table 2). However, postoperative chemotherapy did not affect the OS of high-risk patients with stage I and II RGAVCT (P = 0.653).
| Characteristic | N | Univariate analysis | Multivariate analysis | ||
| χ2 | P value | HR (95%CI) | P value | ||
| Preoperative complications | |||||
| No | 115 | 27.724 | < 0.001 | 1 | 0.021 |
| Digestive tract obstruction | 4 | 2.15 (0.50-9.32) | 0.304 | ||
| Alimentary tract hemorrhage | 1 | 2.90 (0.38-22.41) | 0.307 | ||
| Others1 | 3 | 7.07 (1.91-26.15) | 0.003 | ||
| Neural invasion | |||||
| Absence | 73 | 10.014 | 0.002 | 1 | |
| Presence | 50 | 2.47 (1.16-5.28) | 0.02 | ||
| Ki-67 expression | |||||
| Low | 1 | 5.58 | 0.018 | 1 | |
| High | 122 | 0.05 (0.01-0.44) | 0.007 | ||
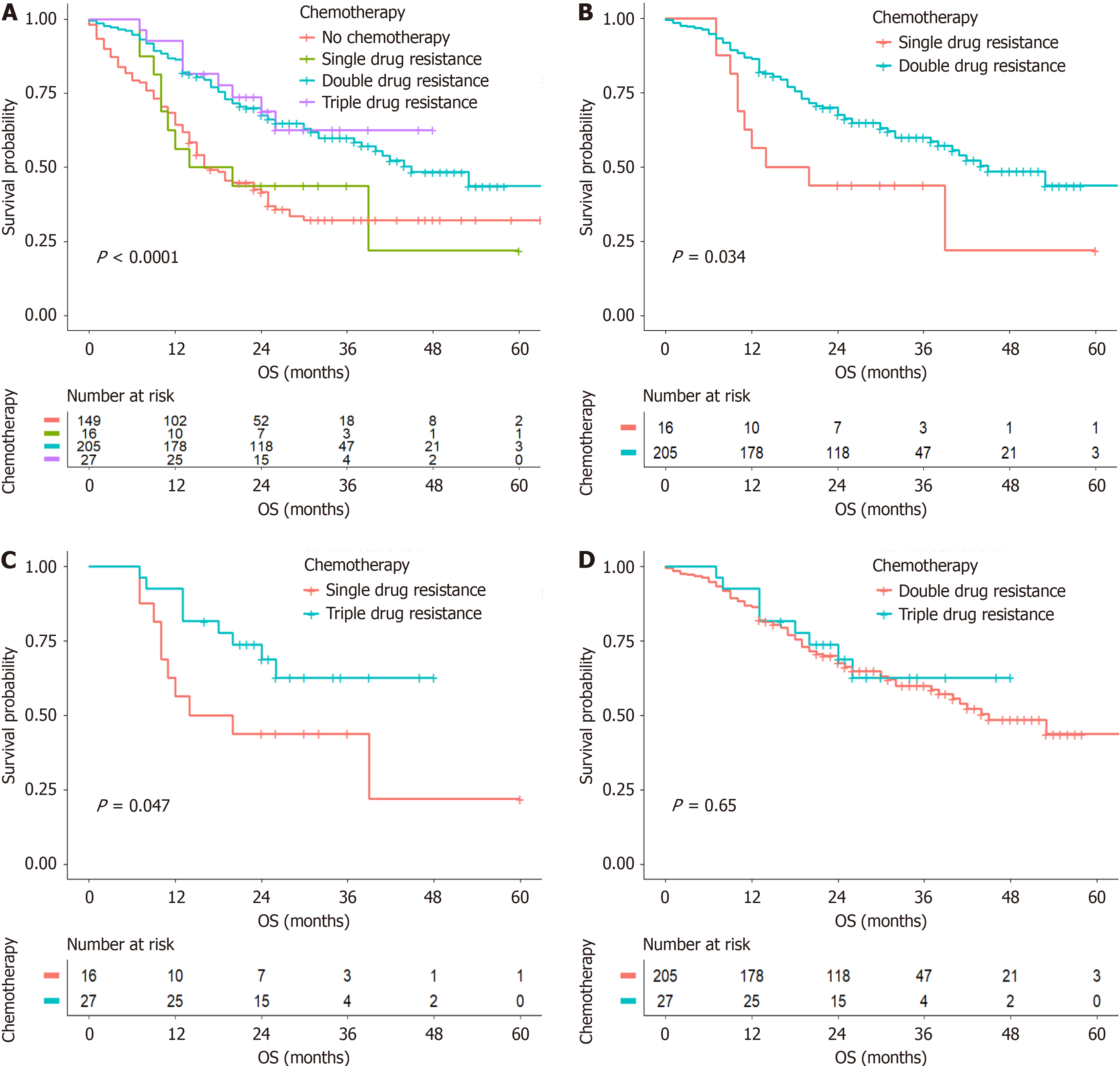
The Kaplan-Meier analysis revealed that postoperative chemotherapy affected the OS of patients with stage III RGAVCT (P < 0.001) (Figure 3A). Furthermore, compared with patients treated with a single-agent regimen, individuals who were treated with chemotherapy consisting of dual- or triple-agent regimens had higher survival rates (P = 0.047 and P = 0.034) (Figure 3B and C), and no significant difference was observed between the survival of patients treated with dual-agent regimens and that of those treated with triple-agent regimens (P = 0.646) (Figure 3D).
The aim of this study was to determine the most effective postoperative chemotherapy regimen for patients with RG
GC is a life-threatening disease and is one of the primary causes of cancer-related death worldwide. The introduction of novel anticancer medications, neoadjuvant radiation, adjuvant chemotherapy, and late-stage palliative care are among the numerous advancements in the systemic treatment of GC. These measures have significantly increased the survival rates of patients with GC. However, high postoperative recurrence and metastasis rates adversely affect the survival of patients with GC. The postoperative clinicopathological features of GC are also the primary prognostic factors associated with this disease. In a study by Chen et al[8], the depth of tumor infiltration, extent of lymph node metastasis, extent of distant metastasis, and pathological score were used to predict the prognosis and OS of patients with GC. The impact of vascular cancer embolism on the prognosis of malignant tumors has received considerable attention because of detailed research on tumor prognostic variables and the concept of micrometastasis[9,10]. Vascular cancer emboli are tumor cells that form aggregates with fibrin clots, coexist with erythrocytes, infiltrate the endothelial cell space arrangement of the surrounding tissue in the absence of erythrocytes, or invade the smooth muscle cell space arrangement[11,12].
Torre et al[13] reported a 2:1 male-to-female ratio in the global incidence of GC in 2012, while Sung et al[1] reported that the global incidence of GC in 2020 was approximately 7.2% for men and 4.4% for women. These studies show that the number of men with GC is greater than that of women. The male-to-female ratio of patients with RGAVCT in this study was 4:1, which was much higher than the global male-to-female ratio of GC patients. Consequently, vascular cancer em
Regarding the baseline characteristics of patients with RGAVCT, our study revealed that the type of surgery (open, laparoscopic, or combined thoracoabdominal) had no effect on OS. Moreover, we found that surgeons involved in clinical assessments selected the appropriate surgical approach based on the specific conditions of the patients. In more than half (53.4%) of the patients with RGAVCT and concomitant neural invasion, we found that vascular cancer embolus and neural invasion were likely to occur simultaneously. Among the patients with RGAVCT who were analyzed, 10 had stage IB RGAVCT, 123 had stage II RGAVCT, and 397 had stage III RGAVCT, which accounted for 74.9% of all patients. This indicates that vascular cancer embolisms occurred more often in patients with advanced GC. The median survival time (mOS = 23 months) and survival rates at 12 months (77.8%), 24 months (57.8%), 34 months (49.5%), and 53 months (40.1%) of patients with stage III RGAVCT were significantly lower than those of patients with stages I and II RGAVCT (mOS = 27 months; 12 months, 89.5%; 59 months, 52.2%).
The risk factors for GC include many immutable variables, such as age[14], sex, and race/ethnicity. Additionally, some modifiable risk factors, such as Helicobacter pylori infection[15], smoking[16], and high nitrate and nitrite diets, have also been identified. Several known hereditary cancer syndromes are associated with GC, including hereditary diffuse GC (CDH1) syndrome, the most strongly associated syndrome, which occurs in approximately 80% of patients[17]. The mul
In this study, the mOS was 25 months in the chemotherapy group, which was greater than that in the nonchemotherapy group (15 months), which indicates that chemotherapy could prolong the survival of patients with RGAVCT. Several studies[19-21] have suggested that postoperative chemotherapy can improve the prognosis of patients with GC. However, postoperative chemotherapy is the only intervening factor among the independent factors that affect the OS of patients with RGAVCT, and a rational postoperative chemotherapy regimen can further improve the prognosis of these patients. A clinical consensus[22] has been established that postoperative adjuvant therapy should be recommended for patients who undergo D2 radical surgery and who do not receive preoperative treatment for postoperative pathological stage II and III progressive GC. In this study, 530 patients were evaluated based on clinical stage, and we found that postoperative chemotherapy did not affect the OS of patients with stage I and II RGAVCT. We further investigated whe
We explored the effect of postoperative chemotherapy on the OS of patients with stage III RGAVCT (P < 0.001) and discovered a substantial difference in survival between patients who received postoperative chemotherapy and those who did not. The recent JACCRO GC-07 study[23], which investigated chemotherapy regimens and clinical outcomes, showed that the continuation of an oral S-1 monotherapy regimen (DS sequential S-1) after six cycles of postoperative docetaxel combined with S-1 improved the survival of patients with stage III GC compared with S-1 alone (3-year re
Postoperative chemotherapy has an independent effect on OS in patients with RGAVCT and can increase survival. However, chemotherapy administered after surgery had little effect on the OS of patients with stage I and II RGAVCT. Moreover, triple-agent treatment is associated with more adverse events than other forms of treatment. Therefore, we recommend that patients with stage II RGAVCT receive dual-agent chemotherapy. The clinical implications and future scope are clear.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64104] [Article Influence: 16026.0] [Reference Citation Analysis (174)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1770] [Article Influence: 442.5] [Reference Citation Analysis (1)] |
| 3. | Zhang W. TCGA divides gastric cancer into four molecular subtypes: implications for individualized therapeutics. Chin J Cancer. 2014;33:469-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1569] [Article Influence: 156.9] [Reference Citation Analysis (0)] |
| 5. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 6. | Zhao Y, Wang X, Huang Y, Zhou X, Zhang D. Conversion of immunohistochemical markers and breast density are associated with pathological response and prognosis in very young breast cancer patients who fail to achieve a pathological complete response after neoadjuvant chemotherapy. Cancer Manag Res. 2019;11:5677-5690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Kooby DA, Suriawinata A, Klimstra DS, Brennan MF, Karpeh MS. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828-35; discussion 835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Chen D, Fu M, Chi L, Lin L, Cheng J, Xue W, Long C, Jiang W, Dong X, Sui J, Lin D, Lu J, Zhuo S, Liu S, Li G, Chen G, Yan J. Prognostic and predictive value of a pathomics signature in gastric cancer. Nat Commun. 2022;13:6903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Huang Q, Fang C, Shi J, Sun Q, Wu H, Gold JS, Weber HC, Guan W, Zhang Y, Yu C, Zou X, Mashimo H. Differences in Clinicopathology of Early Gastric Carcinoma between Proximal and Distal Location in 438 Chinese Patients. Sci Rep. 2015;5:13439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular Invasion and Perineural Invasion Negatively Impact Overall Survival for Stage II Adenocarcinoma of the Colon. Dis Colon Rectum. 2019;62:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 11. | del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, García-Muñiz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21353] [Article Influence: 2135.3] [Reference Citation Analysis (3)] |
| 14. | Camorlinga-Ponce M, Flores-Luna L, Lazcano-Ponce E, Herrero R, Bernal-Sahagún F, Abdo-Francis JM, Aguirre-García J, Muñoz N, Torres J. Age and severity of mucosal lesions influence the performance of serologic markers in Helicobacter pylori-associated gastroduodenal pathologies. Cancer Epidemiol Biomarkers Prev. 2008;17:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 16. | González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, Tumino R, Panico S, Berglund G, Simán H, Nyrén O, Agren A, Martinez C, Dorronsoro M, Barricarte A, Tormo MJ, Quiros JR, Allen N, Bingham S, Day N, Miller A, Nagel G, Boeing H, Overvad K, Tjonneland A, Bueno-De-Mesquita HB, Boshuizen HC, Peeters P, Numans M, Clavel-Chapelon F, Helen I, Agapitos E, Lund E, Fahey M, Saracci R, Kaaks R, Riboli E. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2003;107:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PD, Huntsman DG. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 18. | Zhao L, Han W, Niu P, Lu Y, Zhang F, Jiao F, Zhou X, Wang W, Luan X, He M, Guan Q, Li Y, Nie Y, Wu K, Zhao D, Chen Y. Using nomogram, decision tree, and deep learning models to predict lymph node metastasis in patients with early gastric cancer: a multi-cohort study. Am J Cancer Res. 2023;13:204-215. [PubMed] |
| 19. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 938] [Article Influence: 312.7] [Reference Citation Analysis (0)] |
| 20. | Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 21. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1068] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 22. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 453] [Article Influence: 113.3] [Reference Citation Analysis (1)] |
| 23. | Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 24. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 773] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 25. | Zhu XD, Huang MZ, Wang YS, Feng WJ, Chen ZY, He YF, Zhang XW, Liu X, Wang CC, Zhang W, Ying JE, Wu J, Yang L, Qin YR, Luo JF, Zhao XY, Li WH, Zhang Z, Qiu LX, Geng QR, Zou JL, Zhang JY, Zheng H, Song XF, Wu SS, Zhang CY, Gong Z, Liu QQ, Wang XF, Xu Q, Wang Q, Ji JM, Zhao J, Guo WJ. XELOX doublet regimen versus EOX triplet regimen as first-line treatment for advanced gastric cancer: An open-labeled, multicenter, randomized, prospective phase III trial (EXELOX). Cancer Commun (Lond). 2022;42:314-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |