Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1601
Revised: March 21, 2024
Accepted: April 23, 2024
Published online: June 27, 2024
Processing time: 132 Days and 0.2 Hours
This study was designed to investigate the clinical efficacy and safety of Gamma Knife® combined with transarterial chemoembolization (TACE) and immunotherapy in the treatment of primary liver cancer.
To investigate the clinical efficacy and safety of Gamma Knife® combined with TACE and immune-targeted therapy in the treatment of primary liver cancer.
Clinical data from 51 patients with primary liver cancer admitted to our hospital between May 2018 and October 2022 were retrospectively collected. All patients underwent Gamma Knife® treatment combined with TACE and immunotherapy. The clinical efficacy, changes in liver function, overall survival (OS), and progression-free survival (PFS) of patients with different treatment responses were evaluated, and adverse reactions were recorded.
The last follow-up for this study was conducted on October 31, 2023. Clinical evaluation of the 51 patients with primary liver cancer revealed a partial response (PR) in 27 patients, accounting for 52.94% (27/51); stable disease (SD) in 16 patients, accounting for 31.37% (16/51); and progressive disease (PD) in 8 patients, accounting for 15.69% (8/51). The objective response rate was 52.94%, and the disease control rate was 84.31%. Alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and alpha-fetoprotein isoform levels decreased after treatment compared with pretreatment (all P = 0.000). The median OS was 26 months [95% confidence interval (95%CI): 19.946-32.054] in the PR group and 19 months (95%CI: 14.156-23.125) in the SD + PD group, with a statistically significant difference (P = 0.015). The median PFS was 20 months (95%CI: 18.441-34.559) in the PR group and 12 months (95%CI: 8.745-13.425) in the SD + PD group, with a statistically significant difference (P = 0.002). Common adverse reactions during treatment included nausea and vomiting (39.22%), thrombocytopenia (27.45%), and leukopenia (25.49%), with no treatment-related deaths reported.
Gamma Knife® combined with TACE and immune-targeted therapy is safe and effective in the treatment of primary liver cancer and has a good effect on improving the clinical benefit rate and liver function of patients.
Core Tip: Most patients with primary liver cancer are diagnosed in the middle and late stage, and lose the best opportunity for surgical treatment. Transcatheter arterial chemoembolization (TACE), immune targeted therapy and gamma knife technology are all important methods for clinical treatment of liver cancer. This study mainly discusses the clinical efficacy of gamma knife combined with TACE and immune targeted therapy for primary liver cancer, and provides reference for clinical treatment.
- Citation: Wang GF, Shu CX, Cai XD, Wang HB, Xu JH, Jia YQ. Clinical efficacy of Gamma Knife® combined with transarterial chemoembolization and immunotherapy in the treatment of primary liver cancer. World J Gastrointest Surg 2024; 16(6): 1601-1608
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1601.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1601
Primary liver cancer is the fourth leading cause of cancer-related deaths worldwide, and China is considered a region with a high incidence of primary liver cancer, ranking second among malignant tumors in the country[1]. Due to its insidious onset and lack of obvious clinical symptoms in the early stages, more than 80% of patients are diagnosed at an advanced stage[2], resulting in missed opportunities for optimal surgical treatment and considerable resistance to conventional chemotherapy and radiation therapy. Transarterial chemoembolization (TACE) is a commonly used and effective method for patients with unresectable or advanced liver cancer, and multiple studies have shown favorable outcomes with TACE in the treatment of primary liver cancer[3]. However, some studies have indicated that the local tumor necrosis rate after TACE is only 10% to 20%[4]. It has been suggested that TACE treatment can induce partial ischemia and shrinkage of intrahepatic tumors, reducing tumor burden. At this stage, combination immunotherapy can achieve better clinical results[5]. Immunotherapy utilizes the body’s own immune system to attack and destroy cancer cells, inhibiting tumor growth and improving patient survival. One commonly used method of immunotherapy is the use of checkpoint inhibitors, such as programmed cell death ligand 1 (PD-1) monoclonal antibodies, which block the interaction between PD-1 and its ligands PD-L1 and PD-L2, thus relieving immune suppression, activating T-cell function, generating tumor immune responses, and exerting antitumor effects. They have been widely used in immunotherapy[6]. With the development of stereotactic radiosurgery, Gamma Knife® has been extensively used to treat neurosurgical and somatic tumor diseases, improving treatment precision based on precise tumor localization[7]. In this study, we primarily aimed to explore the clinical efficacy of Gamma Knife® combined with TACE and immunotherapy in the treatment of primary liver cancer, providing a reference for clinical treatment.
This retrospective study was conducted in accordance with the principles of the Helsinki Declaration. The clinical data of 51 patients with primary liver cancer admitted to Yangzhou Friendship Hospital Affiliated with the Medical College of Yangzhou University from May 2018 to October 2022 were retrospectively collected. The inclusion criteria were as follows: Histopathologically or radiologically confirmed primary liver cancer; barcelona clinic liver cancer (BCLC) stage B to C, with an expected survival period of more than 3 months; Child–Pugh class A or B; treatment plan involving Gamma Knife® combined with TACE and immunotherapy; and availability of complete clinical data. This study was approved by the Ethics Committee of Yangzhou Friendship Hospital Affiliated with the Medical College of Yangzhou University, and informed consent was waived.
The exclusion criteria included contraindications for TACE, the presence of other malignant tumors, coagulation disorders, or immune dysfunction, a pathological diagnosis of metastatic liver cancer, poor medication compliance, incomplete case data, and loss to follow-up.
TACE: After local infiltration anesthesia with 2% lidocaine injection, the Seldinger technique was used for percutaneous puncture of the femoral artery. A 5F catheter was inserted into the hepatic artery for angiography to identify the tumor-feeding arteries. Chemotherapeutic drugs, including floxuridine powder (30 mg) and gemcitabine powder (1000 mg/m2), were infused through the catheter, and ethiodized oil was used as the embolic agent. The endpoint for embolization was the absence of tumor staining during angiography. After the procedure, patients underwent repeat TACE treatment every 3 to 4 wk based on the deposition of iodized oil, liver function, alpha-fetoprotein levels, and other laboratory indicators.
Gamma Knife® procedure: Gamma Knife® treatment was typically scheduled between two interventions or after the second intervention. Before treatment began, physicians utilized imaging techniques such as magnetic resonance imaging or computed tomography to determine the location and size of the tumors within the liver. A treatment plan was then formulated, specifying the direction, dosage, and treatment area for the radiation. The specific procedure involved the patient assuming a supine position and being immobilized using a vacuum body mold. The Gamma Knife®, a stereotactic radiosurgery system, was employed to deliver focused radiation in a 360-degree rotational manner, ensuring that the 50% isodose curve acutely covered the target volume. The tumor region was delineated through a dose-volume histogram, aiding in the development of the treatment strategy. The peripheral dose around the tumor ranged from 3 Gy to 5 Gy per session, with a total peripheral dose ranging from 36 Gy to 40 Gy over 2 wk to 3 wk. The shape of the radiation field was meticulously designed based on the visualization of the radiation beams, ensuring that the target tumor area was well within the treatment range while minimizing direct exposure to vital organs and tissues. Routine liver protection therapy was administered throughout the treatment period.
Targeted therapy: (1) For patients with a body weight < 60 kg, oral administration of lenvatinib mesylate capsules was at a dosage of 8 mg once daily. For patients with a body weight ≥ 60 kg, the dosage was 2 mg once daily; (2) oral administration of sorafenib tosylate tablets was at a dosage of 0.4 g twice daily; (3) the oral administration apatinib mesylate tablets dosage was 750 mg once daily. The aforementioned three medications were taken until progressive disease (PD) or the occurrence of intolerable adverse reactions; and (4) the oral administration dosage of regorafenib tablets was 160 mg once daily for 28 d, constituting one cycle. The medication was taken from day 1 to day 2 of each cycle.
Immunosuppressive therapy: (1) Dilly’s regimen: 200 mg, given intravenously on day 1 of each cycle, every 3 wk; and (2) regarding bevacizumab biosimilar (Davotin, both referring to bevacizumab injection), the dosage was 7.5 mg/kg, administered via intravenous infusion once every 3 wk, starting on the first day of each cycle.
(1) Treatment efficacy: The evaluation criteria for treatment efficacy followed the Response Evaluation Criteria in Solid Tumors (RECIST)[8], which categorizes the response as complete response (CR), partial response (PR), stable disease (SD), or PD. The objective response rate was calculated as (number of CRs + number of SD patients) divided by the total number of patients, multiplied by 100%. The disease control rate was calculated as (number of CRs + number of PRs + number of SD patients) divided by the total number of patients, multiplied by 100%; (2) liver function: Liver function changes before and after treatment were assessed; (3) adverse reaction evaluation: Adverse reactions during the treatment period were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE 5.0), which classifies adverse reactions into grades 1 to 5, with grades 3 to 5 indicating severe adverse events; and (4) survival outcomes: Analysis of overall survival (OS) and progression-free survival (PFS) among patients with different treatment efficacies. The final follow-up for this study was conducted until October 31, 2023. OS was defined as the time from diagnosis to either patient death or the last follow-up. PFS was defined as the time from diagnosis to PD or the last follow-up, with PD defined as abnormal serum tumor markers or imaging findings indicating an increase in preexisting lesions or the appearance of new lesions.
Data analysis was performed using SPSS 23.0 software. Normally distributed continuous data are presented as the mean ± SD, and the t test was used for group comparisons. Categorical data are presented as n (%) and were compared using the Chi-square test. K-M survival curves were used to analyze the OS and PFS of patients with different treatment outcomes. A level of P < 0.05 was considered indicative of statistical significance.
Among the 51 patients with primary liver cancer, there were 42 males and 9 females. Seven patients were younger than 60 years, while 44 patients were 60 years old or older. Eleven patients had no viral infection, 34 patients had hepatitis B infection, 4 patients had hepatitis C infection, and 2 patients had coinfection of hepatitis B and hepatitis C. Forty-five patients were classified as Child-Pugh grade A, and six patients were classified as grade B. Among the patients, 11 were in BCLC stage B, and 40 were in stage C. Twenty patients had a single tumor, while 31 had two or more tumors. Nineteen patients had a tumor diameter less than 5 cm, while 32 had a diameter of 5 cm or larger. The baseline characteristics are presented in Table 1.
| Item | Case |
| Gender | |
| Male | 42 (82.35) |
| Female | 9 (17.65) |
| Age | |
| < 60 | 7 (13.73) |
| ≥ 60 | 44 (86.27) |
| Viral infection | |
| No | 11 (21.57) |
| HBV | 34 (66.67) |
| HCV | 4 (7.84) |
| HBV + HCV | 2 (3.92) |
| Child-Pugh grade | |
| A | 45 (88.24) |
| B | 6 (11.76) |
| BCLC | |
| B | 11 (21.57) |
| C | 40 (78.43) |
| Tumor number | |
| 1 | 20 (39.22) |
| ≥ 2 | 31 (60.78) |
| Tumor diameter | |
| < 5 cm | 19 (37.25) |
| ≥ 5 cm | 32 (62.75) |
The last follow-up in this study was conducted until October 31, 2023. The clinical efficacy evaluation of 51 patients with primary liver cancer revealed a PR in 27 patients, accounting for 52.94% (27/51) of the total patients; SD in 16 patients, accounting for 31.37% (16/51); and PD in 8 patients, accounting for 15.69% (8/51). The objective response rate was 52.94%, and the disease control rate was 84.31% (Table 2).
| Item | Case |
| CR | 0 (0) |
| PR | 27 (52.94) |
| SD | 16 (31.37) |
| PD | 8 (15.69) |
| Objective response rate | 27 (52.94) |
| Disease control rate | 43 (84.31) |
After treatment, the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alpha-fetoprotein isoform (AFP-L3) in the patients decreased significantly compared to those before treatment (P < 0.001). Table 3 shows the changes in liver function indices before and after treatment.
| Before (n = 51) | After (n = 51) | t | P value | |
| ALT (U/L) | 75.11 ± 4.62 | 60.62 ± 4.09 | 16.771 | 0 |
| AST (U/L) | 119.47 ± 11.69 | 41.57 ± 6.88 | 41.013 | 0 |
| LDH (U/L) | 439.15 ± 20.62 | 236.73 ± 18.15 | 52.623 | 0 |
| AFP-13 (%) | 18.52 ± 5.37 | 11.31 ± 2.65 | 8.598 | 0 |
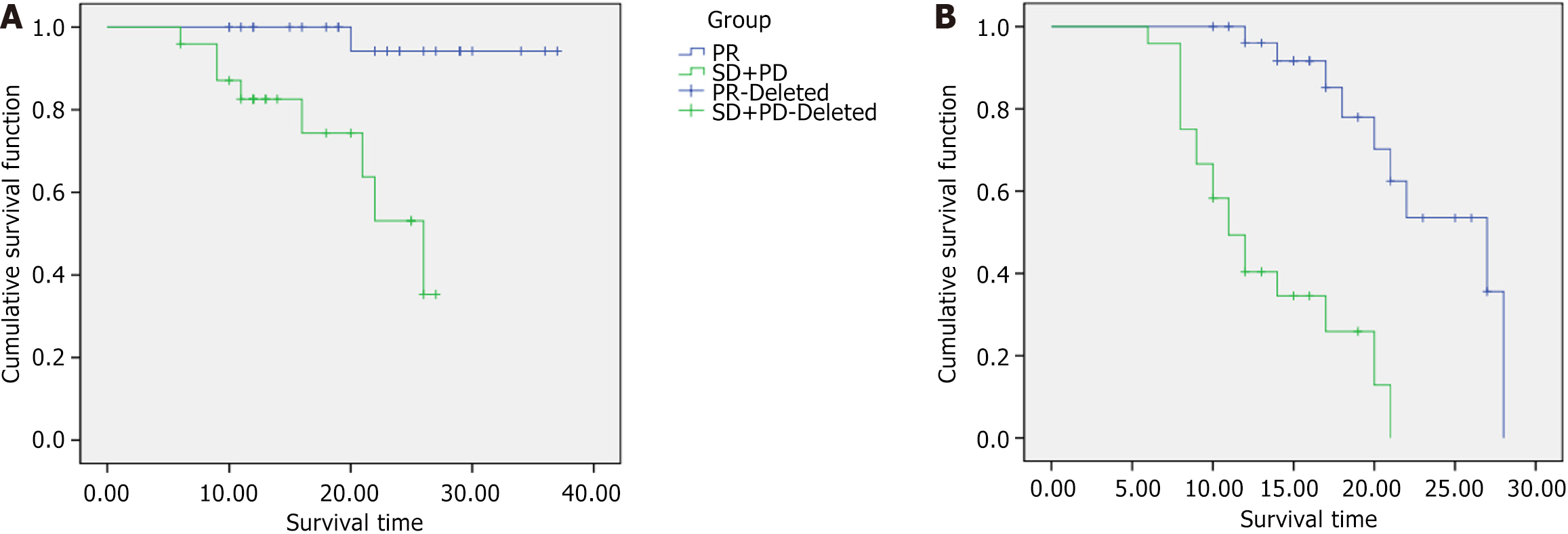
The median OS times for patients in the PR group and the SD + PD group were 26 months [95% confidence interval (95%CI): 19.946-32.054] and 19 months (95%CI: 14.156-23.125), respectively, which were significantly different (P = 0.015). Figure 1A shows a comparison of OS curves between the PR group and the SD + PD group. The median PFS times for patients in the PR group and the SD + PD group were 20 months (95%CI: 18.441-34.559) and 12 months (95%CI: 8.745-13.425), respectively, which were significantly different (P = 0.002). Figure 1B shows a comparison of PFS curves between the PR group and the SD + PD group.
During the treatment of the 51 patients with primary liver cancer, there were no treatment-related deaths. The common types of adverse reactions included nausea and vomiting (39.22%), platelet reduction (27.45%), and leukocyte reduction (25.49%). The most common adverse reactions were hypertension (7.84%), leukocyte reduction (5.88%), and platelet reduction (3.92%). All adverse reactions in the patients improved with corresponding symptomatic treatment and adjustment of the targeted drug dosage. Table 4 shows the details of the occurrence of adverse events.
| Incidence | Grade 3-5 | |
| Hypertension | 10 (19.61) | 4 (7.84) |
| Platelet reduction | 14 (27.45) | 2 (3.92) |
| ALT elevation | 7 (13.73) | 1 (1.96) |
| Leukocyte reduction | 13 (25.49) | 3 (5.88) |
| Nausea and vomiting | 20 (39.22) | 1 (1.96) |
| Fever | 3 (5.88) | 0 (0.00) |
| Hypothyroidism | 2 (3.92) | 0 (0.00) |
| Abdominal pain | 4 (7.84) | 0 (0.00) |
| Hyperbilirubinemia | 6 (11.76) | 0 (0.00) |
| Ascites | 3 (5.88) | 0 (0.00) |
| Hypokalemia | 2 (3.92) | 0 (0.00) |
The pathogenesis of primary liver cancer is complex, and early-stage cases often lack obvious symptoms. By the time symptoms such as liver pain, fatigue, weight loss, and decreased appetite manifest, the disease is usually in the advanced stage, and the optimal treatment window is often missed[8]. TACE is the main treatment method for advanced primary liver cancer. Although TACE has shown substantial efficacy in recent years, long-term outcomes are inevitably affected by the development of collateral vessels or revascularization, increasing the risk of tumor recurrence and distant metastasis and thus limiting the effectiveness of TACE as a standalone treatment for advanced primary liver cancer[9,10]. Therefore, it is highly important to explore other safer and more effective methods to improve the long-term prognosis of patients with advanced primary liver cancer, especially in combination with TACE.
Gamma Knife® technology is a stereotactic radiotherapy technique that uses high-dose focused radiation to treat tumors and protect normal tissue by irradiating the tumor tissue locally. In recent years, a large number of studies have indicated[11,12] that the combination of Gamma Knife® and TACE can further improve treatment outcomes for patients with primary liver cancer and promote disease improvement. Reports in the literature[13] indicate that compared to TACE alone, the combination of Gamma Knife® therapy and TACE for primary liver cancer treatment can enhance short-term treatment efficacy without increasing adverse reactions in patients and prolong survival time. The mechanism of TACE in treating primary liver cancer involves the direct killing of tumor cells and interruption of the tumor blood supply by delivering antitumor drugs and embolic agents into the hepatic artery. Additionally, TACE treatment can increase the expression of hypoxia-inducible factors, thereby upregulating the expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor (PDGFR), promoting tumor angiogenesis, and hindering antitumor effects[14,15]. In recent years, studies have indicated[16] that tyrosine kinase receptor inhibitors such as lenvatinib and sorafenib can inhibit the expression of VEGFR and PDGFR, further enhancing the therapeutic effect of TACE. Therefore, the combination of TACE and targeted drugs can to some extent compensate for the limitations of TACE.
Currently, various immune checkpoint inhibitors have been successfully used in clinical treatment. Among them, PD-1 monoclonal antibodies, which are widely used, can block the binding of PD-1 to its ligands PD-L1 and PD-L2, relieving immune suppression, activating T-cell function, eliciting tumor immune responses, and exerting antitumor effects[17]. Immune drug combinations with targeted therapy have become the standard first-line treatment for advanced primary liver cancer. In recent years, phase I/II studies of immune checkpoint inhibitors combined with targeted drugs such as lenvatinib combined with regorafenib and anlotinib combined with apatinib have shown promising results[18]. The results of the IMbrave150 trial showed that compared to sorafenib alone, the combination of atezolizumab and bevacizumab significantly prolonged the median PFS and OS of liver cancer patients[19]. Due to the unique and complex immune microenvironment of hepatocellular carcinoma, which is characterized mainly by immune suppression, monotherapy with immune checkpoint inhibitors has limited efficacy. Therefore, we predict that combining immune therapy with Gamma Knife® therapy, TACE, and targeted drugs may be effective. Studies[20] have shown that compared with TACE alone in the treatment of patients with liver cancer, targeted therapy and immunotherapy used in conjunction with TACE can further prolong the survival time of patients and is safe and effective.
The results of this study showed that among 51 patients with primary liver cancer who received Gamma Knife® therapy combined with TACE and immune-targeted therapy, liver function indicators significantly improved. The clinical evaluation revealed a PR rate of 52.94%, an SD1 rate of 31.37%, a PD rate of 15.69%, an objective response rate of 52.94%, and a disease control rate of 84.31%, similar to previous reports[21], indicating good efficacy of Gamma Knife® combined with TACE and immune-targeted therapy for primary liver cancer. From the comparison of OS and PFS between the PR group and the SD + PD group, the PR group was shown to exhibit significantly greater OS and PFS than the SD + PD group; this result suggests that Gamma Knife® combined with TACE and immune targeted therapy may increase the possibility of achieving curative resection in the conversion treatment of initially unresectable advanced primary liver cancer, potentially improving OS and recurrence-free survival. It is speculated that the reason may be that Gamma Knife® combined with TACE and immune-targeted therapy for primary liver cancer. TACE plays an antitumor role by directly killing and cutting off vascular nutrients. Immunotherapy can play a good antitumor role by generating a tumor immune response, while Gamma Knife® enables the delivery of higher doses of radiotherapy for smaller target lesions. The combination of the three in the treatment of patients with liver cancer can further improve the clinical benefit rate and prolong the survival time of patients[11]. At the same time, in the present study, the common types of adverse reactions during the treatment of patients were nausea and vomiting (39.22%), thrombocytopenia (27.45%), leukopenia (25.49%), etc., and there were no treatment-related death events, confirming the safety of Gamma Knife® combined with TACE and immune-targeted therapy.
Gamma Knife® combined with TACE and immune-targeted therapy is safe and effective for treating primary liver cancer, improving clinical benefits and enhancing liver function in patients. This study has certain limitations, such as its small sample size and single-center retrospective nature. In the future, expanding the sample size and conducting multicenter clinical trials will be important for further validation of the research findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade C
P-Reviewer: Roth G, France; Zamboni F, Italy S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
Zhang XD
| 1. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1771] [Article Influence: 442.8] [Reference Citation Analysis (1)] |
| 2. | Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 888] [Article Influence: 177.6] [Reference Citation Analysis (2)] |
| 3. | Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, Zhou JY, Li YN, Qiu FN, Li B, Yan ML. Lenvatinib Combined with Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2021;8:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Zhang C, Dai YH, Lian SF, Liu L, Zhao T, Wen JY. Efficacy of transcatheter arterial chemoembolization using pirarubicin-loaded microspheres combined with lobaplatin for primary liver cancer. World J Clin Cases. 2022;10:9650-9656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 6. | Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Cai Y, Chang Q, Xiao E, Shang QL, Chen Z. Transcatheter arterial chemoembolization (TACE) combined with γ-knife compared to TACE or γ-knife alone for hepatocellular carcinoma. Medicine (Baltimore). 2018;97:e10890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 9. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Wang J, Li W, Liu S, Li Z, Hu X. Microwave ablation combined with I125 seed implantation for treatment of residual lesions of liver cancer after TACE. J Cancer Res Ther. 2022;18:1392-1396. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 585] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 12. | Wang J, Luo J, Yin X, Huang W, Cao H, Wang G, Wang J, Zhou J. Jiedu Granule Combined with Transcatheter Arterial Chemoembolization and Gamma Knife Radiosurgery in Treating Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Biomed Res Int. 2019;2019:4696843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, Nakahara T, Naeshiro N, Ono A, Miyaki D, Nagaoki Y, Kawaoka T, Takaki S, Hiramatsu A, Ishikawa M, Kakizawa H, Kenjo M, Takahashi S, Awai K, Nagata Y, Chayama K. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 246] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 15. | Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, Huang M. TACE Combined with Lenvatinib and Camrelizumab for Unresectable Multiple Nodular and Large Hepatocellular Carcinoma (>5 cm). Technol Cancer Res Treat. 2023;22:15330338231200320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Shimose S, Iwamoto H, Niizeki T, Tanaka M, Shirono T, Moriyama E, Noda Y, Nakano M, Suga H, Kuromatsu R, Torimura T, Koga H, Kawaguchi T. Efficacy of Lenvatinib Combined with Transcatheter Intra-Arterial Therapies for Patients with Advanced-Stage of Hepatocellular Carcinoma: A Propensity Score Matching. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 18. | Wu JY, Wu JY, Li YN, Qiu FN, Zhou SQ, Yin ZY, Chen YF, Li B, Zhou JY, Yan ML. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: A multicenter retrospective study. Front Oncol. 2022;12:985380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4633] [Article Influence: 926.6] [Reference Citation Analysis (2)] |
| 20. | Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 21. | Kondo Y, Morosawa T, Minami S, Tanaka Y. DEB-TACE combined with hepatic artery infusion chemotherapy might be an affordable treatment option for advanced stage of HCC. Sci Rep. 2022;12:16868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |