Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1592
Revised: February 21, 2024
Accepted: April 30, 2024
Published online: June 27, 2024
Processing time: 157 Days and 2.9 Hours
Malignant obstructive jaundice (MOJ) is a condition characterized by varying degrees of bile duct stenosis and obstruction, accompanied by the progressive development of malignant tumors, leading to high morbidity and mortality rates. Currently, the two most commonly employed methods for its management are percutaneous transhepatic bile duct drainage (PTBD) and endoscopic ultrasound-guided biliary drainage (EUS-BD). While both methods have demonstrated favorable outcomes, additional research needs to be performed to determine their relative efficacy.
To compare the therapeutic effectiveness of EUS-BD and PTBD in treating MOJ.
This retrospective analysis, conducted between September 2015 and April 2023 at The Third Affiliated Hospital of Soochow University (The First People’s Hospital of Changzhou), involved 68 patients with MOJ. The patients were divided into two groups on the basis of surgical procedure received: EUS-BD subgroup (n = 33) and PTBD subgroup (n = 35). Variables such as general data, preoperative and postoperative indices, blood routine, liver function indices, myocardial function indices, operative success rate, clinical effectiveness, and complication rate were analyzed and compared between the subgroups.
In the EUS-BD subgroup, hospital stay duration, bile drainage volume, effective catheter time, and clinical effectiveness rate were superior to those in the PTBD subgroup, although the differences were not statistically significant (P > 0.05). The puncture time for the EUS-BD subgroup was shorter than that for the PTBD subgroup (P < 0.05). Postoperative blood routine, liver function index, and myocardial function index in the EUS-BD subgroup were significantly lower than those in the PTBD subgroup (P < 0.05). Additionally, the complication rate in the EUS-BD subgroup was lower than in the PTBD subgroup (P < 0.05).
EUS-BD may reduce the number of punctures, improve liver and myocardial functions, alleviate traumatic stress, and decrease complication rates in MOJ treatment.
Core Tip: Malignant obstructive jaundice (MOJ) primarily manifests in advanced tumors, posing significant risks to patient survival and necessitating prompt treatment. The principal treatment methods for MOJ are endoscopic ultrasound-guided biliary drainage and percutaneous transhepatic bile duct drainage. However, the efficacy of these two procedures varies, and their clinical value and postoperative effects require further analysis. This study, involving 68 patients, compares the therapeutic outcomes of these two surgical treatments for MOJ, aiming to identify the optimal treatment strategy.
- Citation: Zhu QQ, Chen BF, Yang Y, Zuo XY, Liu WH, Wang TT, Zhang Y. Endoscopic ultrasound-guided biliary drainage vs percutaneous transhepatic bile duct drainage in the management of malignant obstructive jaundice. World J Gastrointest Surg 2024; 16(6): 1592-1600
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1592.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1592
Malignant obstructive jaundice (MOJ) is a type of jaundice disease arising from hepatic duct occlusion, which leads to impaired bile excretion and abnormal elevation of serum bilirubin[1]. MOJ commonly results in biliary tract infection or suppurative cholangitis. Without timely treatment, it can progress to abnormal coagulation function, impaired immune function, liver failure, and even death[2]. Currently, endoscopic retrograde cholangiopancreatography (ERCP) combined with biliary stent implantation is the primary standard treatment in clinical practice. However, intubation may pose a challenge in certain patients, with a failure rate reaching as high as 7%. Consequently, traditional percutaneous trans
This retrospective analysis included 68 patients with MOJ who were admitted to The Third Affiliated Hospital of Soochow University (The First People’s Hospital of Changzhou) between September 2015 and April 2023. On the basis of the treatment methods employed, these patients were divided into two groups: the EUS-BD subgroup (n = 33) and the PTBD subgroup (n = 35).
Inclusion criteria: MOJ diagnosis was confirmed through B-ultrasound, computed tomography (CT), or Magnetic resonance cholangiopancreatography prior to surgery; patients for whom conventional ERCP procedures had failed or were deemed unsuitable by endoscopists; availability of complete clinical data; absence of distant metastasis; normal coagulation function.
Exclusion criteria: Patients who had undergone radiotherapy or chemotherapy; those with hemophilia or other significant coagulation disorders; and patients suffering from severe infections.
Prior to surgery, patients underwent routine blood tests, coagulation function assessment, liver function tests, and general blood biochemistry analysis. They were required to fast for 4 to 6 hours before the procedure. Additionally, medications that affect platelet aggregation and anticoagulation, such as clopidogrel, warfarin, aspirin, and low-molecular-weight heparin, were discontinued. Imaging data from the planned puncture site (abdominal CT or magnetic resonance imaging) were carefully reviewed to ascertain the presence of any large vessels crossing or adjacent to the site. The procedure was performed under general anesthesia or intravenous sedation, with assistance from an anesthesiologist.
EUS-BD Subgroup: Using linear array endoscopic ultrasonography (UCT-260, Olympus Medical Systems, Tokyo, Japan), the appropriate puncture site was selected under guidance. Color Doppler was used to avoid blood flow signals in the puncture path. A 19G needle (ECHO-19, Cook Ireland Ltd, Limerick, Ireland) was inserted through the digestive tract into the intrahepatic bile duct. After confirming the aspiration fluid as bile, a contrast agent was injected to delineate the biliary system under X-ray guidance. A 0.035-inch guidewire was then introduced into the biliary system, exiting from the puncture needle. A cystotome along the guidewire was used to dilate the sinus tract, followed by the insertion of a nasobiliary tube or stent into the tract.
PTBD Subgroup: Under B-mode ultrasound guidance, bile duct dilatation was identified. The local skin was dis
General clinical data: This includes age, sex, body mass index (BMI), smoking history, cancer type, duration of the disease, duration of surgery, and length of hospital stay.
Surgery-related indicators: These encompass operation time, hospital stay duration, number of punctures, bile drainage flow rate, and effective duration of tube maintenance.
Blood routine before and 7 d after surgery: Measurements of red blood cells (RBC), white blood cells (WBC), and platelets (PLT).
Liver function indices before and 7 d after surgery: Levels of total protein (TP), albumin (ALB), serum alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), and total bile acid are determined, along with hypersensitive C-reactive protein (hs-CRP), using an automatic biochemical analyzer (DNM-9602, Beijing Pulang New Technology Co., Ltd).
Myocardial function before and 7 d after surgery: Assessments of lactate dehydrogenase (LDH), creatine kinase (CK), and creatine kinase isoenzyme (CK-MB).
Surgical success rate and clinical efficiency: Successful surgery is defined as the correct placement of the stent or drain in the desired position. The clinical effectiveness rate is determined on the basis of the reduction in TBIL levels before and 7 d after surgery, as well as the absence of stent blockage or displacement one month post-surgery. A significant effective rate is characterized by a decrease in TBIL of > 30% post-surgery, with no stent blockage or displacement within one month. Effectiveness is defined as a 10%-30% decrease in TBIL post-surgery, without stent blockage or displacement within one month. Ineffectiveness is indicated by a less than 10% decrease in postoperative TBIL and stent blockage or displacement within one month.
Postoperative complications: These include biliary fistula, cholangitis, bile duct bleeding, pneumoperitoneum, stent blockage, stent displacement, and mucosal laceration.
The measurement data, such as age, BMI, disease course, operation-related indicators, liver function indicators, and myocardial function indicators, are expressed as mean ± SD, and the t-test was used to analyze these data. Count data, such as sex, smoking history, cancer type, surgical success rate, clinical effective rate, and postoperative complications, are expressed as rates and were analyzed using the chi-square test. SPSS 27.0 software (IBM Corp.) was used to process the data. P < 0.05 was considered statistically significant.
The subgroups EUS-BD and PTBD were comparable in terms of age, sex, BMI, smoking history, cancer type, and duration of illness. No significant differences were observed between these groups (P > 0.05), as detailed in Table 1.
| EUS-BD (n = 33) | PTBD (n = 35) | t/χ2 | P value | |
| Age (mean SD, yr) | 66.13 ± 10.04 | 64.36 ± 9.28 | 0.755 | 0.453 |
| Sex, n (%) | 0.391 | 0.532 | ||
| Male | 24 (72.73) | 23 (65.71) | ||
| Female | 9 (27.27) | 12 (34.29) | ||
| BMI (mean SD, kg/m2) | 23.10 ± 3.18 | 23.25 ± 3.23 | 0.195 | 0.846 |
| History of smoking, n (%) | 0.000 | 0.994 | ||
| Yes | 17 (51.52) | 18 (51.43) | ||
| No | 16 (48.48) | 17 (48.57) | ||
| Cancer type, n (%) | 0.066 | 0.967 | ||
| Carcinoma of head of pancreas | 15 (45.46) | 17 (48.57) | ||
| Cholangiocarcinoma | 10 (30.30) | 10 (28.57) | ||
| Other | 8 (24.24) | 8 (22.86) | ||
| Disease course (mean SD, months) | 6.30 ± 3.04 | 6.31 ± 3.17 | 0.015 | 0.988 |
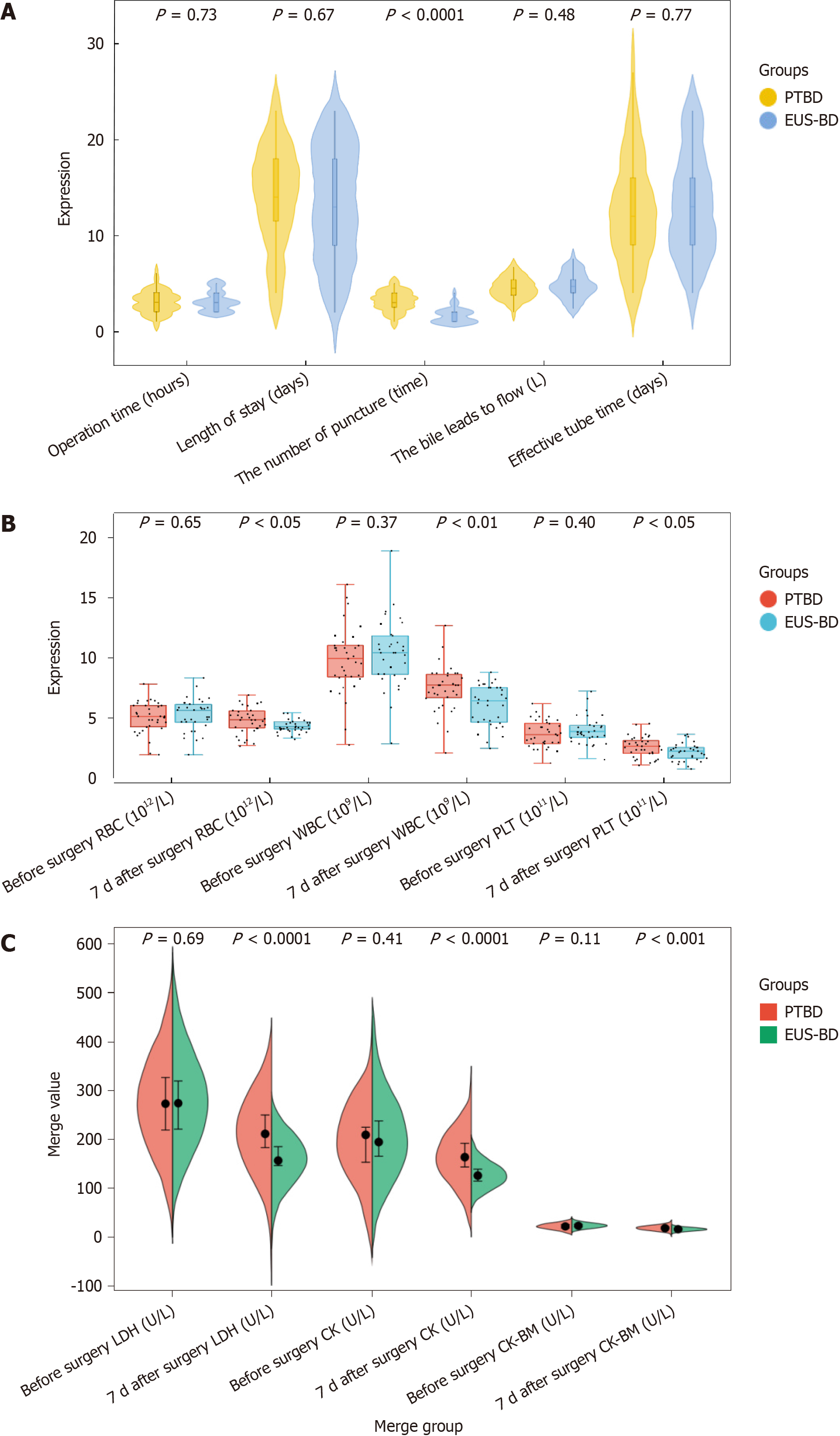
The EUS-BD subgroup exhibited shorter operative times and reduced lengths of hospital stay compared with the PTBD subgroup. Additionally, the EUS-BD subgroup demonstrated higher bile drainage volumes and longer effective tube times than the PTBD subgroup, though these differences were not statistically significant (P > 0.05). Notably, the number of punctures in the EUS-BD subgroup was fewer than in the PTBD subgroup (P < 0.05), as illustrated in Figure 1A.
Prior to surgery, the blood routine parameters of the two subgroups EUS-BD and PTBD showed no significant differences (P > 0.05). However, 7 d post-surgery, the levels of RBC, WBC, and PLT in the EUS-BD subgroup were significantly lower than those in the PTBD subgroup (P < 0.05), as illustrated in Figure 1B.
Prior to surgery, the serum indices of both groups EUS-BD and PTBD were similar (P > 0.05). Postoperatively, the levels of TP, ALP, adenosine triphosphate, ALT, AST, GGT, TBIL, DBIL, and hs-CRP in the EUS-BD subgroup were lower than those in the PTBD subgroup (P < 0.05), as shown in Table 2.
| Index | Time | EUS-BD (n = 33) | PTBD (n = 35) | t | P value |
| TP (g/L) | Before surgery | 61.85 ± 7.14 | 59.25 ± 7.12 | -1.498 | 0.139 |
| 7 d after surgery | 41.75 ± 5.80a,b | 55.13±6.82a | 8.687 | < 0.001 | |
| ALB (g/L) | Before surgery | 44.04 ± 6.98 | 40.97 ± 6.70 | -1.846 | 0.069 |
| 7 d after surgery | 31.20 ± 4.45a,b | 39.55 ± 6.07a | 6.439 | < 0.001 | |
| ALP (U/L) | Before surgery | 529.68 ± 182.52 | 505.45 ± 176.87 | -0.559 | 0.578 |
| 7 d after surgery | 285.39 ± 97.22a,b | 360.65 ± 150.02a | 2.439 | 0.017 | |
| ALT (U/L) | Before surgery | 148.76 ± 52.55 | 146.58 ± 53.35 | -0.169 | 0.866 |
| 7 d after surgery | 51.33 ± 6.61a,b | 73.58 ± 7.03a | 13.424 | < 0.001 | |
| AST (U/L) | Before surgery | 121.29 ± 37.58 | 107.62 ± 28.98 | -1.685 | 0.097 |
| 7 d after surgery | 42.02 ± 13.95a,b | 75.03 ± 16.25a | 8.969 | < 0.001 | |
| GGT (U/L) | Before surgery | 612.05 ± 179.24 | 575.13 ± 174.30 | -0.861 | 0.391 |
| 7 d after surgery | 213.67 ± 61.37a,b | 299.65 ± 65.12a | 5.595 | < 0.001 | |
| TBIL (mmol/L) | Before surgery | 228.02 ± 66.39 | 205.23 ± 76.28 | -1.311 | 0.194 |
| 7 d after surgery | 107.93 ± 52.43a,b | 144.79 ± 59.10a | 2.714 | 0.008 | |
| DBIL (mmol/L) | Before surgery | 204.18 ± 52.86 | 192.13 ± 49.66 | -0.969 | 0.336 |
| 7 d after surgery | 70.19 ± 17.16a,b | 84.48 ± 19.41a | 3.208 | 0.002 | |
| TBA (mmol/L) | Before surgery | 228.62 ± 55.45 | 208.90 ± 63.35 | -1.362 | 0.178 |
| 7 d after surgery | 66.31 ± 18.61a,b | 90.55 ± 20.11a | 5.152 | < 0.001 | |
| hs-CRP (mg/L) | Before surgery | 13.22 ± 3.29 | 11.60 ± 3.90 | -1.843 | 0.070 |
| 7 d after surgery | 4.58 ± 1.33a,b | 7.66 ± 2.36a | 6.576 | < 0.001 |
Postoperative myocardial function indices in the EUS-BD subgroup were significantly lower than those in the PTBD subgroup (P < 0.05), as illustrated in Figure 1C.
In the EUS-BD subgroup, 31 patients experienced successful biliary drainage, resulting in a success rate of 93.94%, which surpassed the 85.71% success rate observed in the PTBD subgroup. Among the EUS-BD subgroup, 16 patients (48.49%) demonstrated a postoperative decrease in TBIL of > 30%, a value of > 40.00% was observed in the PTBD subgroup. The proportion of patients whose TBIL decreased by 10%-30% post-surgery was 42.42% in the EUS-BD subgroup, comparable to that in the PTBD subgroup. Only 9.09% of the EUS-BD subgroup showed a TBIL decrease of < 10%, which was lower than the 17.14% in the PTBD subgroup. However, the differences in efficacy and clinical response between the two subgroups were not statistically significant (P > 0.05), as shown in Table 3.
| EUS-BD (n = 33) | PTBD (n = 35) | χ2 | P value | |
| Successful operation | 31 (93.94) | 30 (85.71) | 1.244 | 0.265 |
| Excellent | 16 (48.49) | 14 (40.00) | ||
| Effective | 14 (42.42) | 15 (42.86) | ||
| Ineffective | 3 (9.09) | 6 (17.14) | ||
| Total effective | 30 (90.91) | 29 (82.86) | 0.959 | 0.327 |
In the EUS-BD subgroup, complications were observed in 3 patients (9.09%), comprising 1 case of biliary fistula, 1 of stent blockage, and 1 of mucosal laceration. All these patients showed improvement following treatment. In the PTBD subgroup, 10 patients (28.57%) experienced complications, including 2 biliary fistulas, 2 instances of cholangitis, 1 bile duct hemorrhage, 1 pneumoperitoneum, 3 stent blockages, and 1 stent displacement, with all patients improving post-treatment. The EUS-BD subgroup had a significantly lower complication rate than the PTBD subgroup (P < 0.05), as shown in Table 4.
| Complication | EUS-BD (n = 33) | PTBD (n = 35) | χ2 | P value |
| Biliary fistula | 1 (3.03) | 2 (5.71) | ||
| Cholangitis | 0 (0.00) | 2 (5.71) | ||
| Bile tube bleeding | 0 (0.00) | 1 (2.86) | ||
| Pneumoperitoneum | 0 (0.00) | 1 (2.86) | ||
| Stent blockage | 1 (3.03) | 3 (8.57) | ||
| Stent displacement | 0 (0.00) | 1 (2.86) | ||
| Mucosal laceration | 1 (3.03) | 0 (0.00) | ||
| Total | 3 (9.09) | 10 (28.57) | 4.169 | 0.041 |
MOJ is a common surgical condition characterized by symptoms of bile duct obstruction, including yellowing of the skin, sclera, and other tissues. The pathogenesis of the disease mainly consists of compromised bile excretion and subsequent sepsis, causing infiltration of cytokines, oxidative stress, and disruption of physiological functions, resulting in hepatic cell injury and apoptosis[7-9]. The predominant clinical approach to MOJ is surgical intervention, yet the efficacy of various surgical techniques varies. ERCP is frequently used but can be technically challenging and carries a risk of postoperative biliary tract infection, thus affecting the surgical success rate. Recently, PTBD and EUS-BD have gained prominence in MOJ treatment owing to their minimal invasiveness. While both methods have shown effective outcomes and serve as alternatives for ERCP failures, determining which procedure holds greater clinical value remains a subject of ongoing exploration[10,11]. This study aims to compare and analyze the clinical effects of PTBD and EUS-BD in treating MOJ by examining surgery-related metrics, blood routine, liver and myocardial function indices, surgical success rate, clinical effectiveness, and complication rate, to identify a superior treatment approach.
PTBD and EUS-BD are comparable in terms of operation duration, hospital stay, bile flow, and effective drainage time. However, EUS-BD is characterized by a lower puncture frequency compared with PTBD. This may be attributed to the shorter puncture path in EUS-BD, its traversal through fewer organs, and its relatively higher accuracy. Additionally, EUS-BD offers a broader range of puncture sites. The stent can be positioned in the common bile duct under X-ray guidance, and its design often includes multiple side holes or a nasal cyst, enhancing the drainage effectiveness. In contrast, PTBD is more susceptible to complications like secondary biliary peritonitis and is limited by the extent of intrahepatic bile duct dilation, which can result in a lower puncture success rate[12,13].
In the context of blood routine, liver function, and myocardial function indices, the postoperative metrics for these parameters in the EUS-BD subgroup were lower than those in the PTBD subgroup. This suggests that EUS-BD causes less trauma to the body, can effectively improve serum levels, liver function, and myocardial function in patients, and inflicts minimal damage to the liver. Firstly, routine blood tests are fundamental in clinical diagnostics, encompassing a range of commonly used sensitive indicators that are responsive to various bodily disease changes and assist in disease asse
Secondly, MOJ frequently leads to abnormal coagulation function, as well as impairment of liver and kidney functions, often resulting in secondary infections. TBIL serves as a crucial liver function marker and a primary indicator for diag
This and other studies indicate that the success rate and clinical effectiveness of the EUS-BD subgroup were lower than those of the PTBD subgroup, although the difference was not statistically significant[23,24]. Regarding complication rates, the PTBD subgroup exhibited a higher incidence compared with the EUS-BD subgroup. While the PTBD procedure is straightforward with a high success rate, it often leads to complications, resulting in a poorer prognosis for some patients. In this study, the complication rate for patients with PTBD was as high as 28.57%, with common issues including cholangitis, stent blockage, and biliary fistula. In contrast, the complication rate in the EUS-BD subgroup was 9.09%, which could be managed with conservative treatment, effectively reducing patient discomfort[8,25]. Complications typically arise due to the following: (1) Mucosal tear and injury to peripheral blood vessels during endoscopic procedures; (2) Bile retrograding into blood, leading to infection; (3) Cholestasis causing biliary sludge formation, either from longitudinal tissue development or tumor growth causing stent obstruction; and (4) Injury to the pancreas, gallbladder, and bile duct during surgery. EUS-BD, conducted under endoscopic ultrasound and X-ray guidance, can enhance operational accuracy and reduce postoperative complications like infection and bleeding.
This study has the following limitations: (1) This study employed a retrospective research design, which retro
When comparing the EUS-BD subgroup with the PTBD subgroup, the differences in surgical success rates and clinical efficiency were not significant. However, the EUS-BD subgroup showed superiority in several aspects, including the number of punctures, blood routine, liver function, myocardial function, and complications, when compared with the PTBD subgroup. Consequently, it can be concluded that EUS-BD offers an effective therapeutic approach for patients with MOJ. Compared with PTBD, EUS-BD presents advantages such as reduced puncture frequency, improved liver and myocardial functions, alleviated traumatic stress, and fewer complications.
| 1. | Zerem E, Imširović B, Kunosić S, Zerem D, Zerem O. Percutaneous biliary drainage for obstructive jaundice in patients with inoperable, malignant biliary obstruction. Clin Exp Hepatol. 2022;8:70-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Wang T, Liu S, Zheng YB, Song XP, Sun BL, Jiang WJ, Wang LG. Clinical Study on Using (125)I Seeds Articles Combined with Biliary Stent Implantation in the Treatment of Malignant Obstructive Jaundice. Anticancer Res. 2017;37:4649-4653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Shao JH, Fang HX, Li GW, He JS, Wang BQ, Sun JH. Percutaneous transhepatic biliary drainage and stenting for malignant obstructive jaundice: A report of two cases. Exp Ther Med. 2015;10:1503-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Wang HB, Peng F, Wang M, Qin RY, Zhu F. Impact of Percutaneous Transhepatic Biliary Drainage on Clinical Outcomes of Patients with Malignant Obstructive Jaundice Undergoing Laparoscopic Pancreaticoduodenectomy. Curr Med Sci. 2021;41:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Hayat U, Bakker C, Dirweesh A, Khan MY, Adler DG, Okut H, Leul N, Bilal M, Siddiqui AA. EUS-guided versus percutaneous transhepatic cholangiography biliary drainage for obstructed distal malignant biliary strictures in patients who have failed endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:4-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Makar M, Zhao E, Tyberg A. Personalized Approach to the Role of Endoscopic Ultrasound in the Diagnosis and Management of Pancreaticobiliary Malignancies. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 7. | Zhao B, Cheng Q, Cao H, Zhou X, Li T, Dong L, Wang W. Dynamic change of serum CA19-9 levels in benign and malignant patients with obstructive jaundice after biliary drainage and new correction formulas. BMC Cancer. 2021;21:517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Duan F, Cui L, Bai Y, Li X, Yan J, Liu X. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: a systematic review and meta-analysis. Cancer Imaging. 2017;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Li J, Zhuo S, Chen B, Liu Y, Wu H. Clinical efficacy of laparoscopic modified loop cholecystojejunostomy for the treatment of malignant obstructive jaundice. J Int Med Res. 2020;48:300060519866285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Rizzo A, Ricci AD, Frega G, Palloni A, DE Lorenzo S, Abbati F, Mollica V, Tavolari S, DI Marco M, Brandi G. How to Choose Between Percutaneous Transhepatic and Endoscopic Biliary Drainage in Malignant Obstructive Jaundice: An Updated Systematic Review and Meta-analysis. In Vivo. 2020;34:1701-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Morita S, Sugawara S, Suda T, Hoshi T, Abe S, Yagi K, Terai S. Conversion of percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided biliary drainage. DEN Open. 2021;1:e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 12. | Liu JG, Wu J, Wang J, Shu GM, Wang YJ, Lou C, Zhang J, Du Z. Endoscopic Biliary Drainage Versus Percutaneous Transhepatic Biliary Drainage in Patients with Resectable Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Pedersoli F, Schröder A, Zimmermann M, Schulze-Hagen M, Keil S, Ulmer TF, Neumann UP, Kuhl CK, Bruners P, Isfort P. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts: technical considerations and complications. Eur Radiol. 2021;31:3035-3041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Jin H, Pang Q, Liu H, Li Z, Wang Y, Lu Y, Zhou L, Pan H, Huang W. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: A retrospective observational study. Medicine (Baltimore). 2017;96:e5895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Vinnik YS, Pakhomova RA, Kochetova LV, Voronova EA, Kozlov VV, Kirichenko AK. [Predictors of hepatic insufficiency in obstructive jaundice]. Khirurgiia (Mosk). 2018;37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sahinli H, Özet A. Prognostic and predictive factors in cancer patients with obstructive jaundice treated by percutaneous transhepatic biliary drainage: A single-center experience. J Cancer Res Ther. 2020;16:S99-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Liu W, Liu Q, Wang W, Wang P, Chen J, Hong T, Zhang N, Li B, Qu Q, He X. Differential diagnostic roles of the serum CA19-9, total bilirubin (TBIL) and the ratio of CA19-9 to TBIL for benign and malignant. J Cancer. 2018;9:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Chen WY, Kong CL, Meng MM, Chen WQ, Zheng LY, Mao JT, Fang SJ, Chen L, Shu GF, Yang Y, Weng QY, Chen MJ, Xu M, Ji JS. Percutaneous biliary stent combined with brachytherapy using 125I seeds for treatment of unresectable malignant obstructive jaundice: A meta-analysis. World J Clin Cases. 2021;9:10979-10993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 19. | Stupin V, Abramov I, Gahramanov T, Kovalenko A, Manturova N, Litvitskiy P, Balkizov Z, Silina E. Comparative Study of the Results of Operations in Patients with Tumor and Non-Tumor Obstructive Jaundice Who Received and Did Not Receive Antioxidant Therapy for the Correction of Endotoxemia, Glycolysis, and Oxidative Stress. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 20. | Ng TG, Damiris K, Trivedi U, George JC. Obstructive jaundice, a rare presentation of lung cancer: A case report. Respir Med Case Rep. 2021;33:101425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Segaran N, Sandrasegaran K, Devine C, Wang MX, Shah C, Ganeshan D. Features of primary pancreatic lymphoma: A bi-institutional review with an emphasis on typical and atypical imaging features. World J Clin Oncol. 2021;12:823-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Nakamura Y, Ito K, Takemura N, Inagaki F, Mihara F, Kokudo N. Elevation in creatine kinase isoenzyme-MM associated with hepatocellular carcinoma: a case report and review of literature. Clin J Gastroenterol. 2022;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Pang L, Wu S, Kong J. Comparison of Efficacy and Safety between Endoscopic Retrograde Cholangiopancreatography and Percutaneous Transhepatic Cholangial Drainage for the Treatment of Malignant Obstructive Jaundice: A Systematic Review and Meta-Analysis. Digestion. 2023;104:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 24. | Hasimu A, Gu JP, Ji WZ, Zhang HX, Zhu DW, Ren WX. Comparative Study of Percutaneous Transhepatic Biliary Stent Placement with or without Iodine-125 Seeds for Treating Patients with Malignant Biliary Obstruction. J Vasc Interv Radiol. 2017;28:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Turan AS, Jenniskens S, Martens JM, Rutten MJCM, Yo LSF, van Strijen MJL, Drenth JPH, Siersema PD, van Geenen EJM. Complications of percutaneous transhepatic cholangiography and biliary drainage, a multicenter observational study. Abdom Radiol (NY). 2022;47:3338-3344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |