Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1548
Revised: May 13, 2024
Accepted: May 22, 2024
Published online: June 27, 2024
Processing time: 163 Days and 19.2 Hours
Laparoscopic low anterior resection (LLAR) has become a mainstream surgical method for the treatment of colorectal cancer, which has shown many advantages in the aspects of surgical trauma and postoperative rehabilitation. However, the effect of surgery on patients' left coronary artery and its vascular reconstruction have not been deeply discussed. With the development of medical imaging technology, 3D vascular reconstruction has become an effective means to evaluate the curative effect of surgery.
To investigate the clinical value of preoperative 3D vascular reconstruction in LLAR of rectal cancer with the left colic artery (LCA) preserved.
A retrospective cohort study was performed to analyze the clinical data of 146 patients who underwent LLAR for rectal cancer with LCA preservation from January to December 2023 in our hospital. All patients underwent LLAR of rectal cancer with the LCA preserved, and the intraoperative and postoperative data were complete. The patients were divided into a reconstruction group (72 patients) and a nonreconstruction group (74 patients) according to whether 3D vascular reconstruction was performed before surgery. The clinical features, operation conditions, complications, pathological results and postoperative recovery of the two groups were collected and compared.
A total of 146 patients with rectal cancer were included in the study, including 72 patients in the reconstruction group and 74 patients in the nonreconstruction group. There were 47 males and 25 females in the reconstruction group, aged (59.75 ± 6.2) years, with a body mass index (BMI) (24.1 ± 2.2) kg/m2, and 51 males and 23 females in the nonreconstruction group, aged (58.77 ± 6.1) years, with a BMI (23.6 ± 2.7) kg/m2. There was no significant difference in the baseline data between the two groups (P > 0.05). In the submesenteric artery reconstruction group, 35 patients were type I, 25 patients were type II, 11 patients were type III, and 1 patient was type IV. There were 37 type I patients, 24 type II patients, 12 type III patients, and 1 type IV patient in the nonreconstruction group. There was no significant difference in arterial typing between the two groups (P > 0.05). The operation time of the reconstruction group was 162.2 ± 10.8 min, and that of the nonreconstruction group was 197.9 ± 19.1 min. Compared with that of the reconstruction group, the operation time of the two groups was shorter, and the difference was statistically significant (t = 13.840, P < 0.05). The amount of intraoperative blood loss was 30.4 ± 20.0 mL in the reconstruction group and 61.2 ± 26.4 mL in the nonreconstruction group. The amount of blood loss in the reconstruction group was less than that in the control group, and the difference was statistically significant (t = -7.930, P < 0.05). The rates of anastomotic leakage (1.4% vs 1.4%, P = 0.984), anastomotic hemorrhage (2.8% vs 4.1%, P = 0.672), and postoperative hospital stay (6.8 ± 0.7 d vs 7.0 ± 0.7 d, P = 0.141) were not significantly different between the two groups.
Preoperative 3D vascular reconstruction technology can shorten the operation time and reduce the amount of intraoperative blood loss. Preoperative 3D vascular reconstruction is recommended to provide an intraoperative reference for laparoscopic low anterior resection with LCA preservation.
Core Tip: Through the observation of left coronary artery three-dimensional vascular reconstruction in patients with colorectal cancer after laparoscopic low anterior resection, the effect of surgery on the vascular system and its curative effect were discussed. The preservation of the left coronary artery and its possible effects were evaluated by comparing preoperative and postoperative vascular remodeling. The results of this study will help to deeply understand the impact of laparoscopic surgery on the cardiovascular system of patients with colorectal cancer, and provide an important reference for postoperative management and clinical treatment, and improve the safety and effect of surgery.
- Citation: Wang Y, Liu ZS, Wang ZB, Liu S, Sun FB. Efficacy of laparoscopic low anterior resection for colorectal cancer patients with 3D-vascular reconstruction for left coronary artery preservation. World J Gastrointest Surg 2024; 16(6): 1548-1557
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1548.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1548
Colorectal cancer is a malignant tumor of the digestive system with a high incidence worldwide, and surgical treatment has always been one of the main treatment methods[1-3]. In terms of surgical methods, low anterior resection is favored because of its lower trauma and faster recovery speed[4]. However, with improvements in surgical techniques, the need for preoperative evaluation is also increasing. As an accurate preoperative evaluation method, 3D vascular reconstruction imaging provides clearer anatomical information for the surgical process, especially for patients with left coronary artery preservation, which has unique value[5]. In recent years, with the continuous development of laparoscopic technology, colorectal cancer surgery has gradually evolved into a minimally invasive and individualized approach[6-8]. In this context, low anterior resection, a common surgical method, has attracted much attention. The purpose of this retro
LLAR to preserve the left colic artery (LCA) is a surgical procedure for rectal cancer. Its advantages include a good blood supply for anastomosis and better protection of autonomic nerves, especially for elderly patients with diabetes, hypertension, and extensive arterial disease[9]. Although there is still controversy and debate about the preservation of the LCA, many surgeons have chosen to perform this procedure to dissect and preserve the LCA while thoroughly clearing the regional lymph nodes[10]. The enlarged field of view and fine anatomy provided by laparoscopic surgery make it relatively easier and more feasible to preserve the LCA than open surgery. Preoperative computed tomography (CT) angiography and 3D reconstruction can provide 3D visual reconstruction of the inferior mesenteric artery (IMA) and its branches and provide the necessary reference for colorectal cancer surgery. However, the effect of preoperative 3D vascular reconstruction on LCA-preserving rectal surgery is inconclusive[11-13].
Therefore, we conducted this study to evaluate the clinical application of 3D vascular reconstruction technology in the surgical and postoperative outcomes of LLAR of rectal cancer patients with left colon preservation, to provide new ideas and evidence supporting the individualized treatment of colorectal cancer surgery and to promote the wider application of minimally invasive technology in clinical practice.
A total of 146 patients with left colon-preserved LLAR who underwent gastrointestinal surgery at the National University Hospital of Singapore from January to December 2023 were included in this retrospective cohort study. The inclusion criteria for patients were as follows: (1) Had a diagnosis of colonoscopy-confirmed and pathological biopsy-confirmed colorectal adenocarcinoma; (2) Had a tumor location 7 cm–15 cm from the anal margin; (3) Underwent LLAR of rectal cancer with the LCA preserved; and (4) Had a pathological stage of I–III. The exclusion criteria were as follows: (1) Had a diagnosis of stage IV rectal cancer; (2) Had a previous history of abdominal surgery; (3) Had intestinal obstruction, bleeding, perforation, etc., or needed emergency surgery; and (4) Had incomplete relevant case data and data. The main indications include large tumors, high surgical difficulty, and a need to improve surgical feasibility. In the pretreatment evaluation, we paid attention to the joint decision-making of the medical team and comprehensively considered preoperative chemotherapy, radiotherapy, and other treatment methods according to individual conditions.
A Philips or GE 64-slice CT scanner was used for enhanced CT. The tube voltage was 120 kV, the tube current was automatic MA, the layer thickness was 1.25 mm, the interval was 0.8 mm, and the detector collimation was 320 mm × 0.5 mm. The samples were fasted for 4 h before the examination. The patient was supine and scanned from the apex of the diaphragm to the level of the ischial tubercle. After a normal scan, 85 mL of the enhancer (iodohydramol, iodiproamine, etc., at a concentration of 350-370 mg/mL) was applied. Intravenously, 2 to 5 mL/s. The delay time of arterial phase scanning was 30 s, and 3D vascular reconstruction was performed using a Philips or GE workstation.
Patients in both groups were given general anesthesia with tracheal intubation, lithotomy at the head lower and foot higher, and 3D laparoscopic surgery. A 1 cm incision was made at the upper edge of the navel to establish a CO2 pneumoperitoneum, and the pressure was maintained at 12 mmHg-14 mmHg (1 mmHg = 0.133 kPa). The five-hole method was used. The whole abdominal cavity, location and size of the tumor and whether there was distant metastasis were investigated. Through the middle approach, the sigmoid colon is traversed with an ultrasound knife or electrotome.
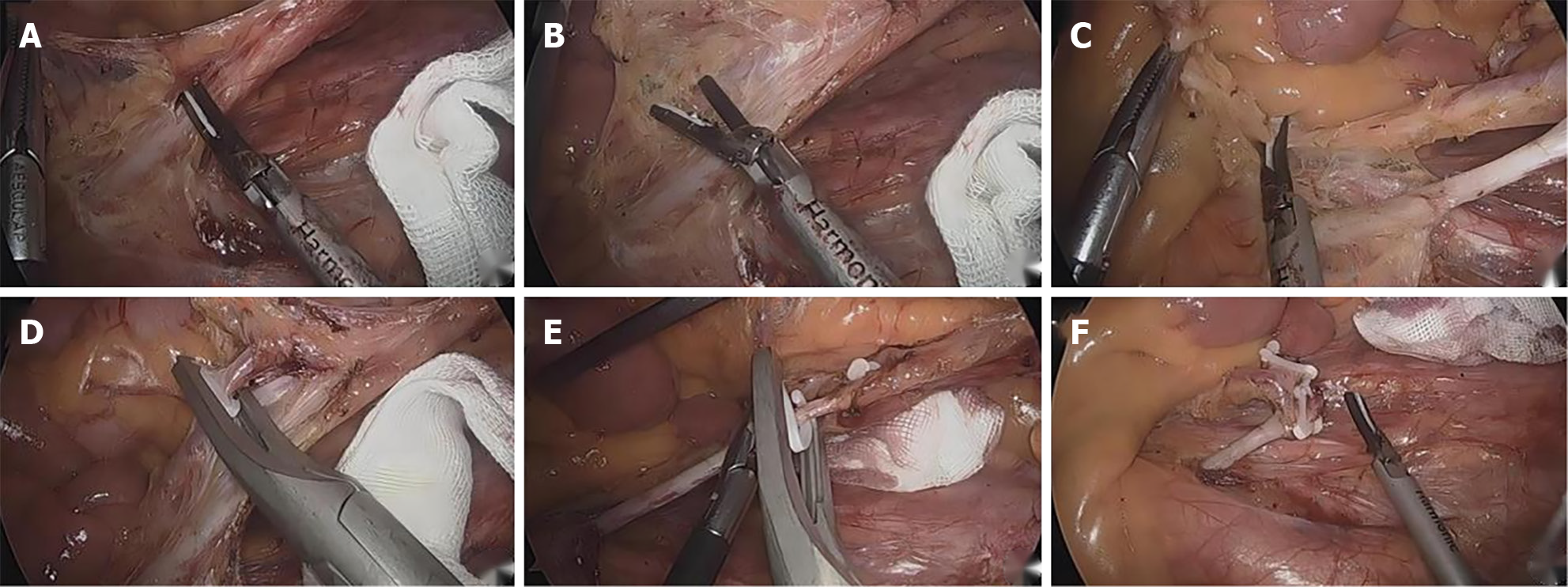
The serous membrane at the yellow-white junction of the mesangial root was opened to fully nip the IMA to avoid nerve injury. From the inside to the outside and from the head to the side, the IMA roots reach the Toldt space to skeletonize the IMA and protect the submesenteric plexus. The IMA branch was clearly dissected, and the lymph nodes and adipose tissue of Group 253 were completely cleared to the LCA branch. After completing the lymph node dissection in Group 253, the IMA branches were clearly dissected, ligation was performed below the bifurcation of the LCA, the LCA was retained, and the other branches were ligated and severed (Figure 1).
The Toldt space was fully extended, and the pelvic nerve plexus was fully protected. The submesenteric vein was separated to the left, and the surrounding fat and lymph nodes were removed. The mesosigmoid was trimmed along the LCA branch, preserving the marginal vascular arch. The intestinal tube was cut at a distance of more than 2 cm from the tumor, and all the specimens were removed from the lower abdomen in a sterile specimen bag. End-to-end anastomosis was performed to reconstruct the digestive tract. After the abdominal cavity was cleaned, the drainage tube was placed and fixed, and the operating hole was sutured.
SPSS software package 25.0 was used for the statistical analysis. Clinical and pathological data are presented as the mean ± SD, and statistical data are presented as the quantity and percentage. The t test was used for measurement data, and the chi-square test or Fisher’s exact test was used for counting data. A P value (bilateral) < 0.05 was considered to indicate statistical significance.
Between January 2023 and December 2023, we enrolled 146 patients who underwent LLAR-preserving LCA based on the inclusion criteria. In our study, preoperative treatment included chemotherapy, radiotherapy, or other related treatments to reduce tumor volume, improve surgical feasibility, and improve patients' postoperative quality of life. In particular, we observed that a subset of patients in the retrospective cohort received radiation therapy, and this decision was based on a comprehensive assessment of the individual's condition and the clinical team. Table 1 summarizes the basic information and clinical features of the patients in the reconstruction group (n = 72, 49.3%) and the nonreconstruction group (n = 74, 50.7%). There were 47 males and 25 females in the reconstruction group, aged (59.75 ± 6.2) years, with a body mass index (BMI) (24.1 ± 2.2) kg/m2, and 51 males and 23 females in the nonreconstruction group, aged (58.77 ± 6.1) years, with a BMI (23.6 ± 2.7) kg/m2. There was no significant difference in the baseline data between the two groups (P > 0.05). There were no statistically significant differences between the two groups in terms of sex, age, BMI, American Society of Anesthesiology (ASA) score, or neoadjuvant therapy.
| Clinical features | 3D vascular reconstruction group (n = 72) | Non-3D vascular reconstruction group (n = 74) | t value | P value |
| Gender | 0.219 | 0.64 | ||
| Male | 47 (65.3) | 51 (68.9) | ||
| Female | 25 (34.7) | 23 (31.1) | ||
| Age (year) | 59.75 ± 6.2 | 58.77 ± 6.1 | 0.961 | 0.338 |
| BMI (kg/m2) | 24.1 ± 2.2 | 23.6 ± 2.7 | 1.123 | 0.263 |
| Neoadjuvant therapy | 12 (16.7) | 13 (17.6) | 0.021 | 0.885 |
| ASA score | 0.174 | 0.917 | ||
| ASA I | 24 (33.3.0) | 23 (31.3) | ||
| ASA II | 37 (51.4) | 38 (51.4) | ||
| ASA III | 11 (15.3) | 13 (17.6) |
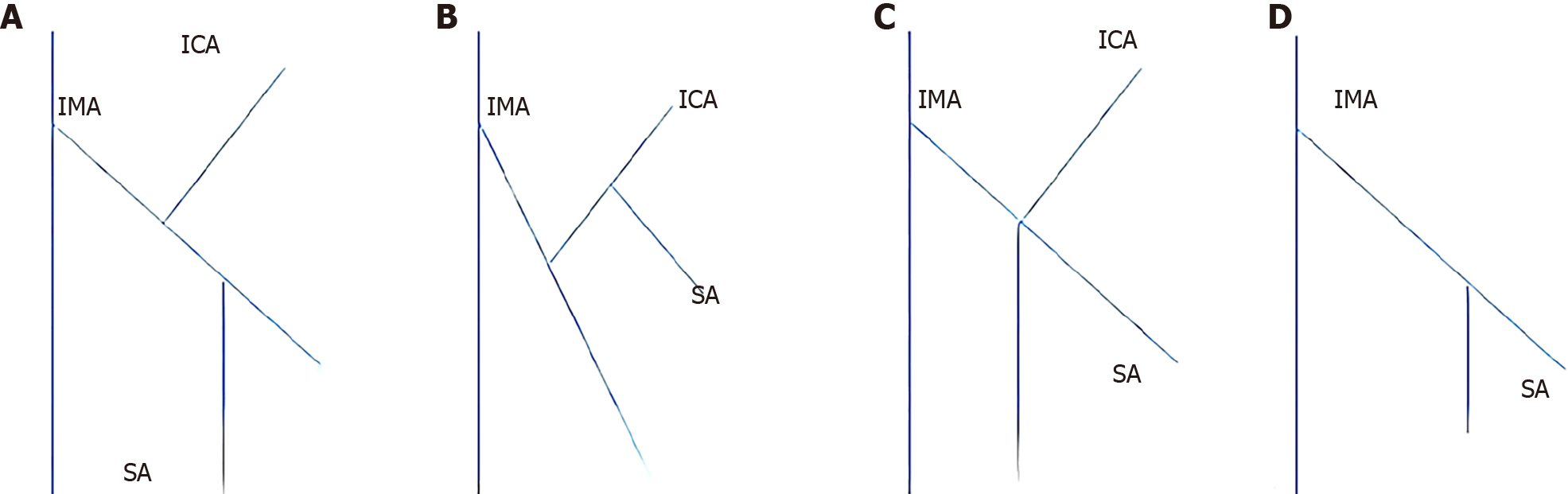
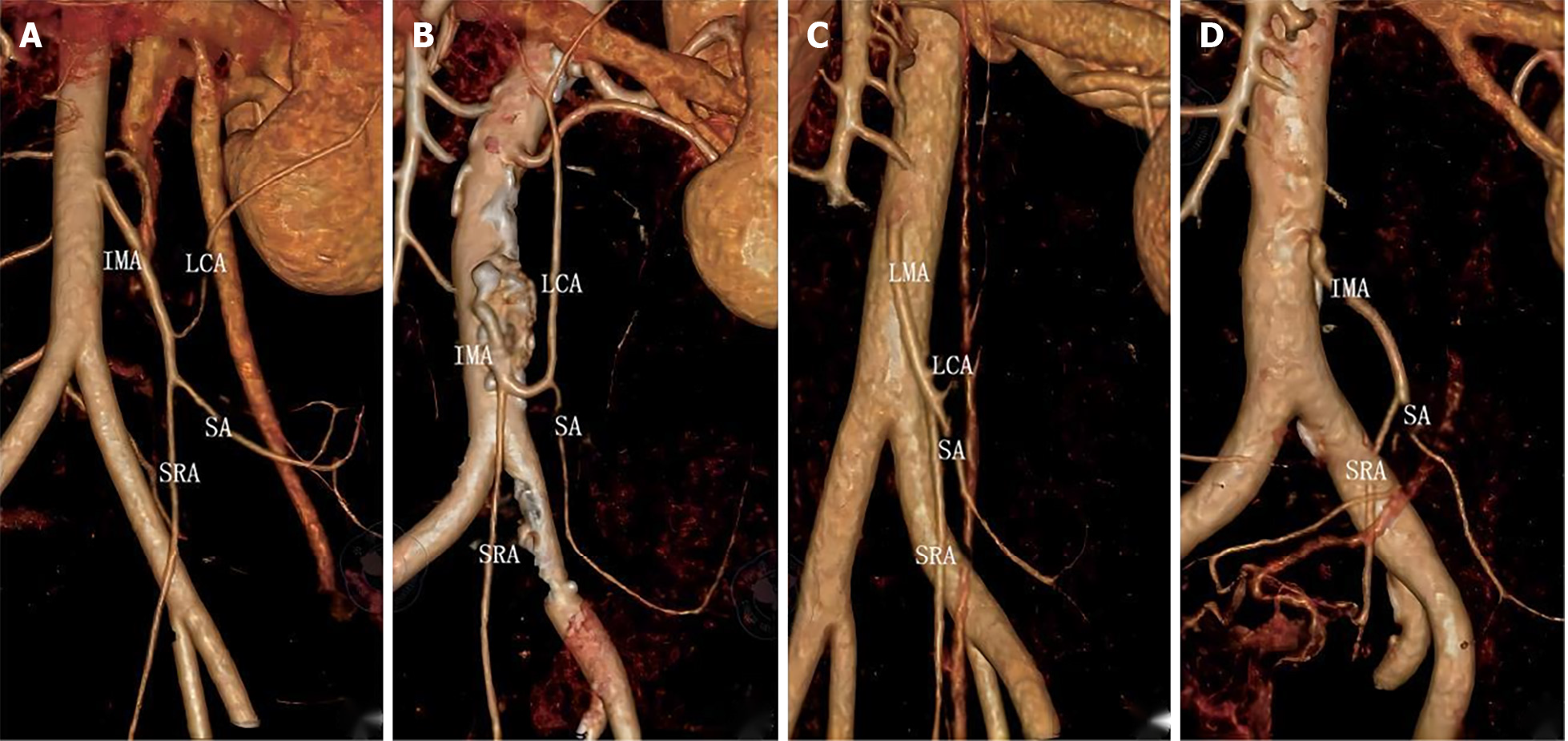
Preoperative 3D vascular reconstruction or surgery was used to study the IMA and its branches in a total of 146 patients. Four IMA blood vessel types were identified in our study. The four blood vessel types are as follows: Type I (Figures 2A and 3A), the LCA alone from the IMA, the sigmoid artery (SA) and the superior rectal artery (SRA) together; Type II (Figures 2B and 3B), the LCA and the SA originate together from a single backbone, and the SRA originates independently from the IMA. In type III (Figures 2C and 3C), all three branches of the IMA emanated at the same level; in type IV and type IV (Figures 2D and 3D), there was no LCA, and only the LCA and SA branched out from the IMA. There was no significant difference in blood vessel types between the two groups (χ2 = 0.092, P = 0.993) (Table 2).
| Arterial classification | 3D vascular reconstruction group (n = 72) | Non-3D vascular reconstruction group (n = 74) | χ2 | P value |
| Branch type | 0.092 | 0.993 | ||
| Type I | 35 (48.6) | 37 (50.0) | ||
| Type II | 25 (34.7) | 24 (32.4) | ||
| Type III | 11 (15.3) | 12 (16.2) | ||
| Type IV | 1 (1.4) | 1 (1.4) |
In the reconstruction group, there were 35 cases of IMA type I, 25 cases of type II, 11 cases of type III, and 1 case of type IV. There were 37 type I patients, 24 type II patients, 12 type III patients, and 1 type IV patient in the nonreconstruction group. There was no significant difference in arterial typing between the two groups (χ2 = 0.092, P = 0.993). In terms of branch type, both the reconstructed group and the nonreconstructed group were classified as type I (35 patients in the reconstructed group, 48.6% vs 37 patients (50.0%) in the nonreconstruction group and type II (25 patients (34.7%) in the reconstruction group vs 24 patients (32.4%) in the nonreconstruction group), followed by type III and type IV.
The surgical data and related surgical complications are shown in Tables 2 and 3. According to our results, the operation time for LLAR to retain the LCA was shorter in the reconstructive group (162.2 ± 10.8 min vs 197.9 ± 19.1 min, t = -13.840; P < 0.05). Intraoperative blood output was also significantly lower in the reconstruction group than in the nonreconstruction group (30.4 ± 20.0 vs 61.2 ± 26.4 mL, t = -7.930, P = 0.000). All procedures in both groups were performed by the same group of experienced surgeons specializing in colorectal cancer. There was no conversion to laparotomy in either group. There was no significant difference in postoperative complications, including anastomotic leakage (1 case, 1.4% vs 1 case, 1.4%, χ2 = 0.000, P = 0.984) or anastomotic hemorrhage (2 cases, 2.8% vs 3 cases, 4.1%, χ2 = 0.180, P = 0.672), between the two groups. All of these complications were successfully resolved. There was no significant difference in postoperative recovery between the reconstruction group (6.8 ± 0.7) and the nonreconstruction group (7.0 ± 0.7) (t = 1.480, P = 0.141).
| Intraoperative and postoperative indicators | 3D vascular reconstruction group (n = 72) | Non-3D vascular reconstruction group (n = 74) | χ2 | P value |
| Operation time (min) | 162.2 ± 10.8 | 197.9 ± 19.1 | -13.84 | 0.001 |
| Conversion to laparotomy | 0 | 0 | ||
| Intraoperative blood loss (mL) | 30.4 ± 20.0 | 61.2 ± 26.4 | -7.93 | 0.001 |
| Anastomotic leak | 1 (1.4%) | 1 (1.4%) | 0.001 | 0.984 |
| Anastomotic hemorrhage | 2 (2.8%) | 3 (4.1%) | 0.18 | 0.672 |
| Postoperative hospital stay | 6.8 ± 0.7 | 7.0 ± 0.7 | 1.48 | 0.141 |
The pathological results of the surgical patients are summarized in Table 4. The tumor size (3.1 ± 0.8 vs 2.9 ± 0.9 cm, t = 1.309, P = 0.193), differentiation degree (χ2 = 0.085, P = 0.958) and TNM stage (χ2 = 0.248, P = 0.958) of the two groups were evaluated. P = 0.883, and there was no significant difference (P > 0.05). In terms of lymph node dissection, the number of lymph nodes in the reconstruction group (18.3 ± 2.7) was slightly greater than that in the nonreconstruction group (17.6 ± 2.1), but the difference was not statistically significant (t = 1.720, P = 0.088).
| Pathological index | 3D vascular reconstruction group (n = 72) | Non-3D vascular reconstruction group (n = 74) | χ2 | P value |
| Tumor size (cm) | 3.1 ± 0.8 | 2.9 ± 0.9 | 1.309 | 0.193 |
| Number of lymph nodes | 18.3 ± 2.7 | 17.6 ± 2.1 | 1.72 | 0.088 |
| Degree of differentiation | 0.085 | 0.958 | ||
| Low | 20 (27.8) | 19 (25.7) | ||
| Middle | 39 (54.2) | 41 (55.4) | ||
| High | 13 (18.1) | 14 (18.9) | ||
| Pathological stage | 0.248 | 0.883 | ||
| Stage I | 14 (19.4) | 13 (17.6) | ||
| Stage II | 35 (48.6) | 39 (52.7) | ||
| Stage III | 23 (31.9) | 22 (29.7) |
Colorectal cancer is the third most commonly diagnosed cancer internationally, and laparoscopic colorectal cancer surgery has been widely accepted worldwide[14-16]. Preserving the LCA means that the IMA ligation is located far from the origin of the LCA, which is one of the surgical options for rectal cancer[17]. Our study showed that preoperative 3D vascular reconstruction can provide a preoperative reference for LCA-sparing rectal cancer surgery and can shorten the operation time and reduce the amount of intraoperative blood loss.
3D vascular reconstruction technology has been widely used in a variety of diseases, such as coronary artery disease, lower limb artery stenosis, aortic aneurysm, aortic dissection, and hepatic artery variation. Coronary CT and 3D reconstruction of blood vessels have become the gold standard noninvasive methods for detecting coronary artery disease in clinical practice[18-20]. Previous studies[21-24] have shown that 3D vascular reconstruction techniques are 100% accurate at identifying variations and stenoses in the arteries that supply the lower limbs and the liver, respectively.
With regard to submesenteric artery branch types, we identified four submesenteric artery types using 3D vascular reconstruction techniques and surgical dissection[25]. In our study, both the reconstructed and nonreconstructed groups included four types, of which types I and II were the most common, type III was the most common, and type IV was the least common. In type IV patients, the LCA could not be maintained, but it was impossible to determine whether the patients in the nonreconstruction group would not have the LCA ahead of time[26-28]. This could only be proven by separating the organs along the main IMA during surgery[29]. Therefore, for strict control between the two groups, type IV patients were included in the study. This type of study also highlights the important reference significance of preoperative 3D vascular reconstruction technology for surgical planning and design.
Our study showed that surgical time was shorter in the reconstruction group than in the nonreconstruction group. Although there are no studies that have compared the use of 3D vascular reconstruction in LCA-sparing rectal surgery, a study involving 112 patients who underwent right hemicolectomy, left hemicolectomy, or prerectal resection reported similar results and shortened the time required for surgery for colon and rectal cancer. One study showed that in colorectal cancer surgery, the surgery time in the reconstruction group was significantly shorter than that in the control group[30-32]. According to their experience, preoperative 3D vascular reconstruction may show significant advantages in identifying blood vessels, even in special cases of vascular anatomical variation or obesity[33]. Because less time is spent dissecting blood vessels and looking for aberrant or missing blood vessels, this approach can help surgeons shorten the operation time. Another study showed that in laparoscopic right hemicolectomy, the surgical time in the reconstruction group was shorter than that in the control group (154.7 ± 25.9 min and 177.6 ± 24.4 min, respectively).
In terms of operation time, according to our experience, the application of preoperative vascular reconstruction has shortened the operation time for the following reasons. First, 3D vascular reconstruction helps to accurately plan surgical strategies and programs before surgery. Similarly, many scholars have proposed similar effects of 3D reconstruction technology on surgical planning and design in stomach and liver operations[34]. Second, 3D reconstruction of blood vessels can help clinicians predict the type of blood vessels before surgery, find blood vessels during surgery, and correctly anatomize blood vessels. This is especially helpful for people who are very overweight, have experienced major changes in their anatomy, or have severe abdominal adhesions[35]. Third, 3D vascular reconstruction technology may help avoid some intraoperative complications, such as visceral damage, vascular damage, and bleeding, which are some of the main reasons for prolonged surgical time. Preoperative 3D vascular reconstruction can provide surgeons with basic information on arterial classification and variation before surgery, which is of great help and important reference significance for safe and effective colorectal cancer surgery[36-38]. Compared with digital subtraction angiography, 3D vascular reconstruction is a better way to quickly reconstruct IMA branching patterns with less damage[39]. Moreover, with the rapid development of CT technology, 3D reconstruction of blood vessels can now be performed more quickly and at a lower cost. In terms of postoperative pathology, there was no significant difference in tumor size, number of lymph nodes, degree of differentiation, or pathological stage between the reconstructed group and the nonreconstructed group. Some studies[40-42] have shown that vascular reconstruction technology may have a positive impact on the number of lymph nodes because vascular reconstruction can help surgeons better cut the blood vessels at the root and clean the lymph nodes at the root of the blood vessels to clear the local lymph nodes more thoroughly. Although the number of lymph nodes in the reconstructed group was slightly greater than that in the nonreconstructed group (18.3 ± 2.7 vs 17.6 ± 2.1), the difference was not statistically significant in our study. This may be related to the fact that we were the same group of surgeons who performed the operation, and the location of the vessel ligation and the scope of regional lymph node dissection were the same. Whether vascular reconstruction techniques have a positive effect on the number of lymph nodes obtained in colorectal cancer surgery is also a hot issue, and multicenter, prospective clinical studies may provide a greater amount of evidence[43-45].
Our study also has several limitations. First, the study design was retrospective in nature. Prospective studies may better confirm future results. Second, the study represents the experience of a single center, which may limit its external validation validity. Therefore, conducting large-scale, multicenter studies is the direction of further research to better verify the conclusions of this study.
Our study suggested that preoperative 3D vascular reconstruction can shorten the operative time and reduce intraoperative blood loss during LLAR of rectal cancer while preserving the LCA. Therefore, for LCA-sparing rectal cancer surgery, preoperative 3D vascular reconstruction is recommended.
| 1. | Garcia-Granero A, Jerí Mc-Farlane S, Gamundí Cuesta M, González-Argente FX. Application of 3D-reconstruction and artificial intelligence for complete mesocolic excision and D3 lymphadenectomy in colon cancer. Cir Esp (Engl Ed). 2023;101:359-368. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Yotsov T, Karamanliev M, Maslyankov S, Iliev S, Ramadanov N, Dimitrov D. Mesenteric Vascular Evaluation with Pre-operative Multidetector Computed Tomographic Angiography and Intraoperative Indocyanine Green Angiography to Reduce Anastomotic Leaks after Minimally Invasive Surgery for Colorectal Cancer. JSLS. 2022;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Andersen BT, Stimec BV, Kazaryan AM, Rancinger P, Edwin B, Ignjatovic D. Re-interpreting mesenteric vascular anatomy on 3D virtual and/or physical models, part II: anatomy of relevance to surgeons operating splenic flexure cancer. Surg Endosc. 2022;36:9136-9145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Fletcher J, Ilangovan R, Hanna G, Miskovic D, Lung P. The impact of three-dimensional reconstruction and standardised CT interpretation (AMIGO) on the anatomical understanding of mesenteric vascular anatomy for planning complete mesocolic excision surgery: A randomised crossover study. Colorectal Dis. 2022;24:388-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Cotsoglou C, Granieri S, Bassetto S, Bagnardi V, Pugliese R, Grazi GL, Guglielmi A, Ruzzenente A, Aldrighetti L, Ratti F, De Carlis L, De Carlis R, Centonze L, De Angelis N, Memeo R, Delvecchio A, Felli E, Izzo F, Belli A, Patrone R, Ettorre GM, Berardi G, Di Benedetto F, Di Sandro S, Romano F, Garancini M, Scotti MA, Bianchi G, Germini A, Gjoni E, Bonomi A, Bruno F, Paleino S, Pugliese G. Dynamic surgical anatomy using 3D reconstruction technology in complex hepato-biliary surgery with vascular involvement. Results from an international multicentric survey. HPB (Oxford). 2024;26:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 6. | Brown PJ, Toh EW, Smith KJ, Jones P, Treanor D, Magee D, Burke D, Quirke P. New insights into the lymphovascular microanatomy of the colon and the risk of metastases in pT1 colorectal cancer obtained with quantitative methods and three-dimensional digital reconstruction. Histopathology. 2015;67:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Miyamoto R, Nagai K, Kemmochi A, Inagawa S, Yamamoto M. Three-dimensional reconstruction of the vascular arrangement including the inferior mesenteric artery and left colic artery in laparoscope-assisted colorectal surgery. Surg Endosc. 2016;30:4400-4404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Andersen BT, Stimec BV, Edwin B, Kazaryan AM, Maziarz PJ, Ignjatovic D. Re-interpreting mesenteric vascular anatomy on 3D virtual and/or physical models: positioning the middle colic artery bifurcation and its relevance to surgeons operating colon cancer. Surg Endosc. 2022;36:100-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (3)] |
| 9. | Giusti I, Carnevali P, Bertoglio CL, Giani A, Achilli P, Grimaldi S, Origi M, Mazzola M, Magistro C, Ferrari G. Laparoscopic right hemicolectomy for hepatic flexure adenocarcinoma with complete mesocolic excision and 3D-CT vascular reconstruction. Tech Coloproctol. 2022;26:1003-1004. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Miyamoto R, Tadano S, Sano N, Inagawa S, Yamamoto M. The Impact of Laparoscopic-assisted Colorectal Surgery Using 3-dimensional Reconstruction for Highly Obese Patients With Colorectal Cancer. Surg Laparosc Endosc Percutan Tech. 2017;27:175-178. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Kobayashi M, Morishita S, Okabayashi T, Miyatake K, Okamoto K, Namikawa T, Ogawa Y, Araki K. Preoperative assessment of vascular anatomy of inferior mesenteric artery by volume-rendered 3D-CT for laparoscopic lymph node dissection with left colic artery preservation in lower sigmoid and rectal cancer. World J Gastroenterol. 2006;12:553-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Forgione A, Barberio M, Agnus V, Swanström L, Marescaux J, Diana M, Gallix B. Precision image-guided colonic surgery: proof of concept for enhanced preoperative and intraoperative vascular imaging. Surg Endosc. 2021;35:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Pérez-Corbal L, Trujillo-Diaz JC, Alarcón I, Licardie E, Senent A, Morales-Conde S. Interactive 3D vascular reconstruction: A navigation tool to improve safety in laparoscopic D3 right colectomy - a video vignette. Colorectal Dis. 2021;23:3030-3032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Bonomi AM, Kersik A, Bracchetti G, Cotsoglou C. 3D reconstruction in complex parenchymal sparing liver surgery. Heliyon. 2023;9:e13857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 17. | Miyamoto R, Tadano S, Sano N, Inagawa S, Adachi S, Yamamoto M. The impact of three-dimensional reconstruction on laparoscopic-assisted surgery for right-sided colon cancer. Wideochir Inne Tech Maloinwazyjne. 2017;12:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Bodensteiner C, Darolti C, Schweikard A. Vascular tree reconstruction with discrete tomography: intensity based camera correction for 3D reconstruction. Int J Comput Assist Radiol Surg. 2009;4:189-202. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Groves LA, VanBerlo B, Veinberg N, Alboog A, Peters TM, Chen ECS. Automatic segmentation of the carotid artery and internal jugular vein from 2D ultrasound images for 3D vascular reconstruction. Int J Comput Assist Radiol Surg. 2020;15:1835-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Luzon JA, Kumar RP, Stimec BV, Elle OJ, Bakka AO, Edwin B, Ignjatovic D. Semi-automated vs. manual 3D reconstruction of central mesenteric vascular models: the surgeon's verdict. Surg Endosc. 2020;34:4890-4900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Toutouzas K, Chatzizisis YS, Riga M, Giannopoulos A, Antoniadis AP, Tu S, Fujino Y, Mitsouras D, Doulaverakis C, Tsampoulatidis I, Koutkias VG, Bouki K, Li Y, Chouvarda I, Cheimariotis G, Maglaveras N, Kompatsiaris I, Nakamura S, Reiber JH, Rybicki F, Karvounis H, Stefanadis C, Tousoulis D, Giannoglou GD. Accurate and reproducible reconstruction of coronary arteries and endothelial shear stress calculation using 3D OCT: comparative study to 3D IVUS and 3D QCA. Atherosclerosis. 2015;240:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 23. | Melville JC, Manis CS, Shum JW, Alsuwied D. Single-Unit 3D-Printed Titanium Reconstruction Plate for Maxillary Reconstruction: The Evolution of Surgical Reconstruction for Maxillary Defects-A Case Report and Review of Current Techniques. J Oral Maxillofac Surg. 2019;77:874.e1-874.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Gao B, Jing H, Gao M, Wang S, Fu W, Zhang X, He X, Zheng J. Long-segmental tracheal reconstruction in rabbits with pedicled Tissue-engineered trachea based on a 3D-printed scaffold. Acta Biomater. 2019;97:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Li F, Chenoune Y, Ouenniche M, Blanc R, Petit E. Segmentation and reconstruction of cerebral vessels from 3D rotational angiography for AVM embolization planning. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5522-5525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Arkill KP, Neal CR, Mantell JM, Michel CC, Qvortrup K, Rostgaard J, Bates DO, Knupp C, Squire JM. 3D reconstruction of the glycocalyx structure in mammalian capillaries using electron tomography. Microcirculation. 2012;19:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprint. 2024;10:1256. [DOI] [Full Text] |
| 28. | Waechter I, Bredno J, Weese J, Barratt DC, Hawkes DJ. Using flow information to support 3D vessel reconstruction from rotational angiography. Med Phys. 2008;35:3302-3316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 30. | Lu TW, Chen WT, Ji T. Accuracy of Mandibular Reconstruction with a Vascularised Iliac Flap Using 3D Templates: a Systematic Review. Chin J Dent Res. 2022;25:37-43. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Kresakova L, Danko J, Andrejcakova Z, Petrovova E, Vdoviakova K, Cizkova D, Maloveska M, Toth T, Tomco M, Vrzgula A, Teleky J, Supuka P. 3D Reconstruction and Evaluation of Accessory Hepatic Veins in Right Hemilivers in Laboratory Animals by Metrotomography: Implications for Surgery. Med Sci Monit. 2019;25:920-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 33. | Coste E, Vasseur C, Rousseau J. 3D reconstruction of the cerebral arterial network from stereotactic DSA. Med Phys. 1999;26:1783-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Qiu MG, Zhang SX, Liu ZJ, Tan LW, Wang YS, Deng JH, Tang ZS. Plastination and computerized 3D reconstruction of the temporal bone. Clin Anat. 2003;16:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Miyashita Y, Ikeda T, Shinto E, Okano S, Korehisa S, Shimazaki H, Oki E, Ueno H, Oda Y, Mori M. Three-dimensional imaging of intramural perineural invasion in colorectal cancer: Three-dimensional reconstruction approach with multiple immunohistochemically stained sections. Pathol Int. 2022;72:293-299. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Misri H, Pöckler-Schöniger C, Gaa J, Georgi M, Sturm J, Ihle V. [Ultrafast contrast-enhanced 3D MR angiography in the preoperative diagnosis of liver tumors]. Rofo. 1998;169:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Adam DR, Burstein P. Vascular imaging by ultrasound: 3D reconstruction of flow velocity fields for endothelial shear stress calculation. Adv Exp Med Biol. 1997;430:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 39. | DeFazio MV, Arribas EM, Ahmad FI, Le-Petross HT, Liu J, Chu CK, Santiago L, Clemens MW. Application of Three-Dimensional Printed Vascular Modeling as a Perioperative Guide to Perforator Mapping and Pedicle Dissection during Abdominal Flap Harvest for Breast Reconstruction. J Reconstr Microsurg. 2020;36:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Romano G, Di Buono G, Galia M, Agnello F, Anania G, Guerrieri M, Milone M, Silecchia G, Buscemi S, Agrusa A. Role of preoperative CT angiography with multimodality imaging reconstruction to perform laparoscopic Complete Mesocolic Excision (CME) and Central Vascular Ligation (CVL) in right-sided colon cancer: Is it really useful? A prospective clinical study. Eur J Surg Oncol. 2023;49:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Yano M, Okazaki S, Kawamura I, Ito S, Nozu S, Ashitomi Y, Suzuki T, Kamio Y, Hachiya O. A three-dimensional computed tomography angiography study of the anatomy of the accessory middle colic artery and implications for colorectal cancer surgery. Surg Radiol Anat. 2020;42:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Ueda T, Morita K, Koyama F, Teramura Y, Nakagawa T, Nakamura S, Matsumoto Y, Inoue T, Nakamoto T, Sasaki Y, Kuge H, Takeda M, Ohbayashi C, Fujii H, Sho M. A detailed comparison between the endoscopic images using blue laser imaging and three-dimensional reconstructed pathological images of colonic lesions. PLoS One. 2020;15:e0235279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Ke J, Cai J, Wen X, Wu X, He Z, Zou Y, Qiu J, He X, Lian L, Zhou Z, Lan P. Anatomic variations of inferior mesenteric artery and left colic artery evaluated by 3-dimensional CT angiography: Insights into rectal cancer surgery - A retrospective observational study. Int J Surg. 2017;41:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Griffini P, Smorenburg SM, Verbeek FJ, van Noorden CJ. Three-dimensional reconstruction of colon carcinoma metastases in liver. J Microsc. 1997;187:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |