Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1485
Revised: April 29, 2024
Accepted: May 15, 2024
Published online: June 27, 2024
Processing time: 194 Days and 22.7 Hours
Colorectal cancer is the third most common cancer in the world. Surgery is man
Core Tip: In terms of oncological outcomes and quality of resection, laparoscopic approach allows to do just as well as open surgery, in particular the number of the lymph nodes removed is identical, regardless of the access. However, the laparoscopic approach is not recommended when the neoplasm presents with urgency, in the occlusive or perforated phase, as well as it is not recommended for locally advanced tumors. When the tumor involved the serosal layer or invades an adjacent organ, open “en-bloc” excision is recommended.
- Citation: Cariati M, Brisinda G, Chiarello MM. Has the open surgical approach in colorectal cancer really become uncommon? World J Gastrointest Surg 2024; 16(6): 1485-1492
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1485.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1485
Colorectal cancer is the third most common cancer in the world. In 2019, new cases of colorectal cancer in the world were 1931590, corresponding to 10% of new cancer diagnoses, and is responsible for approximately 750000 cancer-related dea
Curative resection aims to radically remove the segment of intestine in which the tumor is located. The resection must include at least 5 cm of healthy colon upstream and downstream of the lesion[6,7]. Furthermore, lymphadenectomy is fundamental for a systematic lymph node dissection and for removing all potentially metastatic lymph nodes[8-11]. In
In recent decades, the traditional surgical approach via laparotomy and direct access to the patient’s abdominal cavity has been joined by new minimally invasive surgical techniques. Laparoscopic surgery has now been validated through large randomized controlled studies conducted throughout the world. In this editorial, we aim to summarize the clinical and technical aspects which, even today, make the use of laparotomy relevant and necessary in the treatment of patients with colorectal cancer.
Laparoscopic surgery in colorectal cancer represents a correct alternative to open surgery, if performed by surgeons with adequate training in this specific procedure[13]. Even in laparoscopic surgery the proximal and distal resection margins are appropriate and proximal vessel ligation is performed safely. Curative resections are therefore obtained, with en-bloc removals and tumor-free radial margins (R0)[14].
The United Kingdom Medical Research Counsel (MRC) trial of conventional vs laparoscopic assisted surgery in colorectal cancer, published in 2005, which included patients with both colon and rectal cancer was the first randomized controlled trial to investigate the role of laparoscopy in colorectal cancer[15]. After this trial there were four subsequent randomized controlled trials comparing laparoscopy to open surgery in colorectal cancer. All these studies - the Com
Recently, the short-term results of a multicenter prospective randomized trial conducted in China comparing laparoscopic and open resection have been published[20]. The Laparoscopy-Assisted Surgery for Carcinoma of the Low Rectum study was conducted on a population of over 1000 patients. Specifically, 685 patients in the laparoscopic surgery group and 350 patients in the open surgery group were included. No significant differences in morbidity rate were observed between the two groups. Higher rates of sphincter preservation and shorter length of stay were observed in patients un
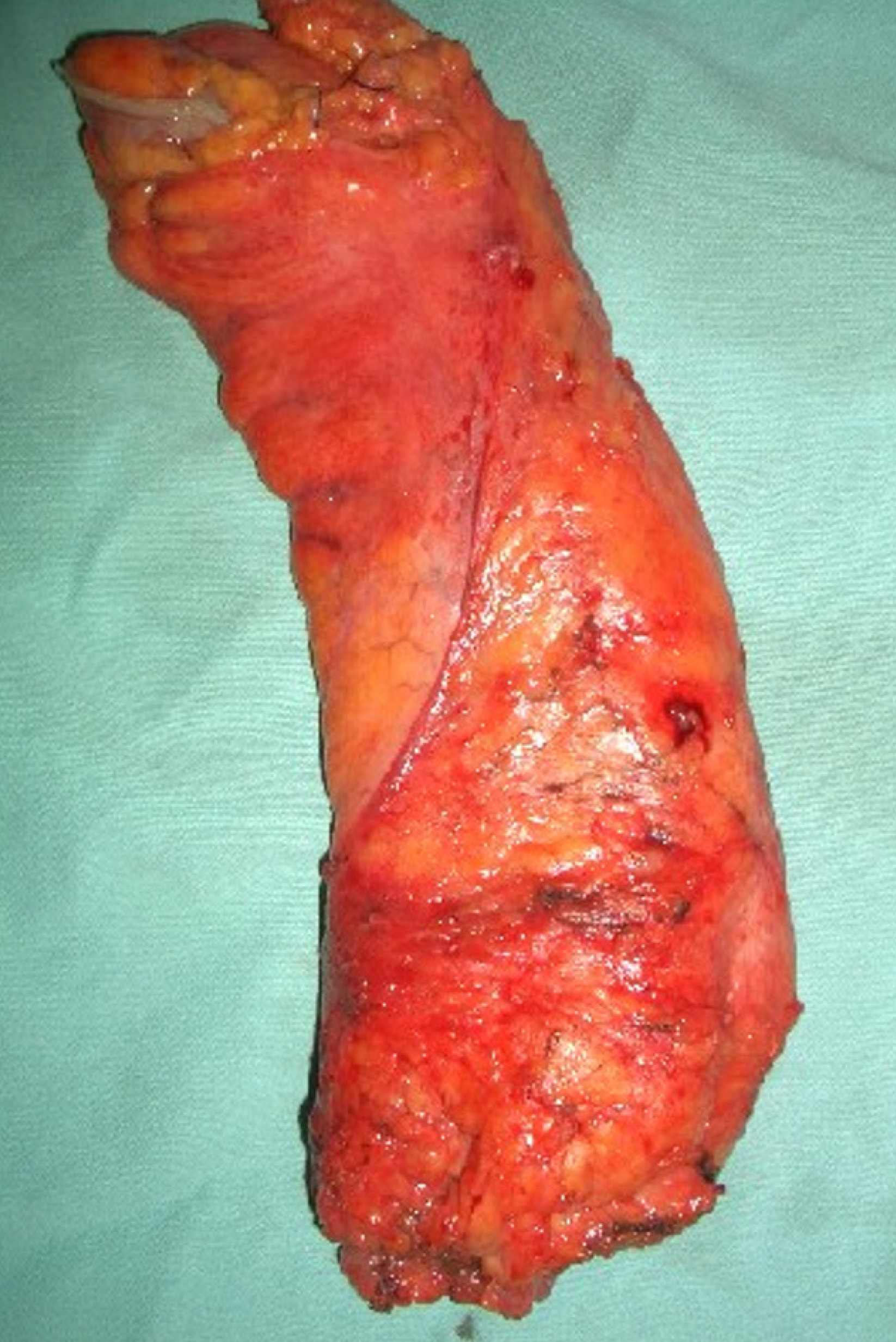
Extraperitoneal rectal carcinoma presents peculiar aspects. While the approach to carcinoma of high rectum does not differ from that of the recto-sigmoid junction and the sigmoid carcinoma, surgery of the mid-lower rectum presents technical difficulties that are best managed in high-volume specialist centers. The cornerstones of this surgery concern the total excision of the mesorectum (Figure 1), the preservation of the sympathetic and parasympathetic innervation (nerve-sparing technique), the distal and circumferential section margin free from neoplasia and, in locally advanced forms (T3-T4 and/or regional lymph node metastases) the use of neoadjuvant therapies. In these patients, laparoscopic total me
In colorectal cancer patients, laparoscopic surgery has some controversial aspects. A learning curve appears fundamental in the laparoscopic field. Both the surgeon and the operating room auxiliary staff are required to acquire advanced la
Furthermore, aspects of laparoscopic surgery have raised some initial concerns in the scientific community. The risk of a potential violation of oncological principles, the possible spread of neoplastic cells linked to carbon dioxide insufflation and the possibility of tumor recurrence in the access sites of the trocars have represented some of the controversial aspects[38,39]. However, it seems right to emphasize that these fears and these controversial aspects were found to be completely unjustified, both by evaluating some aspects of basic scientific research and by analyzing the results of large randomized and controlled studies.
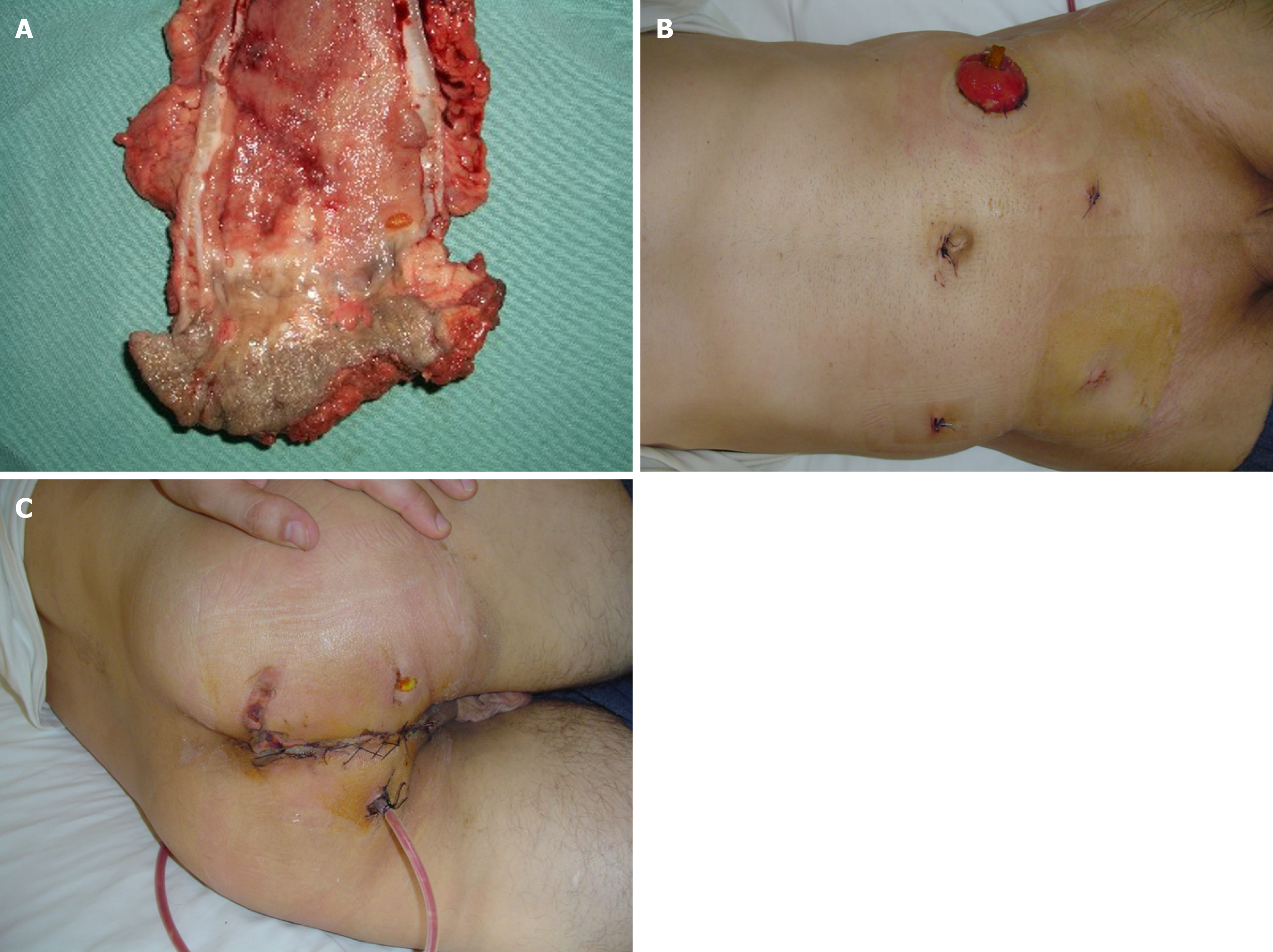
T4 tumors show an incidence of up to 15% in patients with colon cancer. Among patients with rectal cancer, 5% to 12% of patients have tumors adherent to adjacent organs[40-42]. In these patients, is recommended en-bloc resection to manage locally advanced colorectal cancer[43,44]. Thus is T4 colorectal cancer still an absolute contraindication to laparoscopic surgery? The answer is that T4 colon cancer is not an absolute contraindication. Obviously, the possibility of treating T4 colorectal cancer laparoscopically depends on local circumstances (e.g., organs involved in the enlarged demolition and factors related to the surgeon (e.g., skill and experience of the individual surgeon in performing a laparoscopic en-bloc resection). Intraoperative observation of a T4 lesion often requires conversion to open surgery, especially if the goal of the therapeutic approach is curative resection. This eventuality is necessary because en-bloc demolition in the presence of a T4 lesion is not always effective in laparoscopic surgery. However, en-bloc resection may not be possible using either te
In the UK MRC-CLASSIC trial, 34% of the patients randomized to the laparoscopic group underwent conversion to an open procedure. In this group of patients, a higher post-operative morbidity rate (P = 0.002) and a worsened overall sur
In rectal cancer, when using the minimally invasive approach, particularly for tumors in low rectum, a further cha
Complications of large bowel diseases account for 47% of gastrointestinal emergencies. Colorectal cancer presents as emergency in a wide range of patients (from 7% to 40% of the total). Large bowel obstruction represents almost 80% of the emergencies related to colorectal cancer, while perforation accounts for the remaining 20%. The most common location of bowel obstruction is the sigmoid colon, with 75% of the tumors located distal to the splenic flexure. Per
In case of colonic obstruction due to tumor of the right colon or proximal transverse colon, right hemicolectomy, classic or extended, with subsequent primary ileocolic anastomosis represents the most appropriate treatment. The general condition of the patient strongly affects the choice to perform an anastomosis. The patient’s condition, including hemo
In case of obstructing cancer of the left colon, a variety of options have been advocated[53]. Resection and primary anastomosis, with or without protective stoma, resection according to Hartmann, intraabdominal subtotal colectomy with ileostomy or ileorectal anastomosis are the most frequently used procedures. More recently, endoscopically placed co
The use of laparoscopy in the emergency treatment of colorectal cancer cannot be recommended and should be re
Risk factors for conversion for different populations have been widely reported in the literature. A recent meta-analysis documented an average conversion rate of 17.9%. An evaluation of the factors that negatively influence the completion of the laparoscopic surgical procedure has shown that the factors that are most responsible for the conversion to open surgery are male sex, a tumor localized in the extraperitoneal rectum, the T3/T4 stage and the presence of metastases to locoregional lymph nodes[56]. With increasing laparoscopic hospital volume, conversion decreases below 10% with only minimal impact of conversion on short-term postoperative outcome. To perform an early conversion can be an appro
Laparoscopy is a safe and effective surgical technique for the treatment of colorectal cancer. Laparoscopy remains an acceptable minimally invasive option in well trained hands. Surgeon represents a significant prognostic factor: His ope
Laparoscopic surgery for low rectal cancer, when performed by experienced surgeons, could produce pathological outcomes comparable to those of open surgery. In large surgical series and multicenter studies, no differences are ob
| 1. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 909] [Article Influence: 454.5] [Reference Citation Analysis (1)] |
| 2. | AIOM. I numeri del cancro in Italia 2023. Roma: Intermedia Editore, 2023. |
| 3. | Angenete E. The importance of surgery in colorectal cancer treatment. Lancet Oncol. 2019;20:6-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Sartori CA, D'Annibale A, Cutini G, Senargiotto C, D'Antonio D, Dal Pozzo A, Fiorino M, Gagliardi G, Franzato B, Romano G. Laparoscopic surgery for colorectal cancer: clinical practice guidelines of the Italian Society of Colo-Rectal Surgery. Tech Coloproctol. 2007;11:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Green CJ, Maxwell R, Verne J, Martin RM, Blazeby JM. The influence of NICE guidance on the uptake of laparoscopic surgery for colorectal cancer. J Public Health (Oxf). 2009;31:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Levine RA, Chawla B, Bergeron S, Wasvary H. Multidisciplinary management of colorectal cancer enhances access to multimodal therapy and compliance with National Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal Dis. 2012;27:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL; On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 8. | Wood TF, Saha S, Morton DL, Tsioulias GJ, Rangel D, Hutchinson W Jr, Foshag LJ, Bilchik AJ. Validation of lymphatic mapping in colorectal cancer: in vivo, ex vivo, and laparoscopic techniques. Ann Surg Oncol. 2001;8:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Bilchik AJ, Trocha SD. Lymphatic mapping and sentinel node analysis to optimize laparoscopic resection and staging of colorectal cancer: an update. Cancer Control. 2003;10:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Wu Z, Zhang S, Aung LH, Ouyang J, Wei L. Lymph node harvested in laparoscopic versus open colorectal cancer approaches: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Yeung TM, Wang LM, Colling R, Kraus R, Cahill R, Hompes R, Mortensen NJ. Intraoperative identification and analysis of lymph nodes at laparoscopic colorectal cancer surgery using fluorescence imaging combined with rapid OSNA pathological assessment. Surg Endosc. 2018;32:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Lykke J, Jess P, Roikjær O; Danish Colorectal Cancer Group. A high lymph node yield in colon cancer is associated with age, tumour stage, tumour sub-site and priority of surgery. Results from a prospective national cohort study. Int J Colorectal Dis. 2016;31:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Liang Y, Li G, Chen P, Yu J. Laparoscopic versus open colorectal resection for cancer: a meta-analysis of results of randomized controlled trials on recurrence. Eur J Surg Oncol. 2008;34:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S, Kuhrij E, Haglind E, Påhlman L; Transatlantic Laparoscopically Assisted vs Open Colectomy Trials Study Group. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2296] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 16. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 753] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 17. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1213] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 18. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 830] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 19. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 768] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 20. | Jiang WZ, Xu JM, Xing JD, Qiu HZ, Wang ZQ, Kang L, Deng HJ, Chen WP, Zhang QT, Du XH, Yang CK, Guo YC, Zhong M, Ye K, You J, Xu DB, Li XX, Xiong ZG, Tao KX, Ding KF, Zang WD, Feng Y, Pan ZZ, Wu AW, Huang F, Huang Y, Wei Y, Su XQ, Chi P; LASRE trial investigators. Short-term Outcomes of Laparoscopy-Assisted vs Open Surgery for Patients With Low Rectal Cancer: The LASRE Randomized Clinical Trial. JAMA Oncol. 2022;8:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 930] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 22. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1816] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 23. | Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 24. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1679] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 25. | Tjandra JJ, Chan MK, Yeh CH. Laparoscopic- vs. hand-assisted ultralow anterior resection: a prospective study. Dis Colon Rectum. 2008;51:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Morino M, Parini U, Giraudo G, Salval M, Brachet Contul R, Garrone C. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg. 2003;237:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Cheung HY, Chung CC, Wong JC, Yau KK, Li MK. Laparoscopic rectal cancer surgery with and without neoadjuvant chemo-irradiation: a comparative study. Surg Endosc. 2009;23:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Hasegawa H, Ishii Y, Nishibori H, Endo T, Watanabe M, Kitajima M. Short- and midterm outcomes of laparoscopic surgery compared for 131 patients with rectal and rectosigmoid cancer. Surg Endosc. 2007;21:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Miyajima N, Fukunaga M, Hasegawa H, Tanaka J, Okuda J, Watanabe M; Japan Society of Laparoscopic Colorectal Surgery. Results of a multicenter study of 1,057 cases of rectal cancer treated by laparoscopic surgery. Surg Endosc. 2009;23:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Franks PJ, Bosanquet N, Thorpe H, Brown JM, Copeland J, Smith AM, Quirke P, Guillou PJ; CLASICC trial participants. Short-term costs of conventional vs laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial). Br J Cancer. 2006;95:6-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Marusch F, Gastinger I, Schneider C, Scheidbach H, Konradt J, Bruch HP, Köhler L, Bärlehner E, Köckerling F; Laparoscopic Colorectal Surgery Study Group (LCSSG). Experience as a factor influencing the indications for laparoscopic colorectal surgery and the results. Surg Endosc. 2001;15:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Schlachta CM, Mamazza J, Grégoire R, Burpee SE, Pace KT, Poulin EC. Predicting conversion in laparoscopic colorectal surgery. Fellowship training may be an advantage. Surg Endosc. 2003;17:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Schlachta CM, Mamazza J, Seshadri PA, Cadeddu M, Gregoire R, Poulin EC. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 34. | Künzli BM, Friess H, Shrikhande SV. Is laparoscopic colorectal cancer surgery equal to open surgery? An evidence based perspective. World J Gastrointest Surg. 2010;2:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Arteaga González I, Díaz Luis H, Martín Malagón A, López-Tomassetti Fernández EM, Arranz Duran J, Carrillo Pallares A. A comparative clinical study of short-term results of laparoscopic surgery for rectal cancer during the learning curve. Int J Colorectal Dis. 2006;21:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Akiyoshi T, Kuroyanagi H, Ueno M, Oya M, Fujimoto Y, Konishi T, Yamaguchi T. Learning curve for standardized laparoscopic surgery for colorectal cancer under supervision: a single-center experience. Surg Endosc. 2011;25:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Chen G, Liu Z, Han P, Li JW, Cui BB. The learning curve for the laparoscopic approach for colorectal cancer: a single institution's experience. J Laparoendosc Adv Surg Tech A. 2013;23:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 227] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Martel G, Crawford A, Barkun JS, Boushey RP, Ramsay CR, Fergusson DA. Expert opinion on laparoscopic surgery for colorectal cancer parallels evidence from a cumulative meta-analysis of randomized controlled trials. PLoS One. 2012;7:e35292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Ng DC, Co CS, Cheung HY, Chung CC, Li MK. The outcome of laparoscopic colorectal resection in T4 cancer. Colorectal Dis. 2011;13:e349-e352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Bretagnol F, Dedieu A, Zappa M, Guedj N, Ferron M, Panis Y. T4 colorectal cancer: is laparoscopic resection contraindicated? Colorectal Dis. 2011;13:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Kim IY, Kim BR, Kim HS, Kim YW. Differences in clinical features between laparoscopy and open resection for primary tumor in patients with stage IV colorectal cancer. Onco Targets Ther. 2015;8:3441-3448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Sugarbaker PH. Revised guidelines for second-look surgery in patients with colon and rectal cancer. Clin Transl Oncol. 2010;12:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Turaga K, Levine E, Barone R, Sticca R, Petrelli N, Lambert L, Nash G, Morse M, Adbel-Misih R, Alexander HR, Attiyeh F, Bartlett D, Bastidas A, Blazer T, Chu Q, Chung K, Dominguez-Parra L, Espat NJ, Foster J, Fournier K, Garcia R, Goodman M, Hanna N, Harrison L, Hoefer R, Holtzman M, Kane J, Labow D, Li B, Lowy A, Mansfield P, Ong E, Pameijer C, Pingpank J, Quinones M, Royal R, Salti G, Sardi A, Shen P, Skitzki J, Spellman J, Stewart J, Esquivel J. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1112] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 46. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 47. | Sawyer MA, Sawyer EM. Controversies in laparoscopic surgery for colorectal cancer. Curr Surg. 2004;61:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 48. | Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 49. | Fleshman J, Marcello P, Stamos MJ, Wexner SD; American Society of Colon and Rectal Surgeons (ASCRS); Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Focus Group on Laparoscopic Colectomy Education as endorsed by The American Society of Colon and Rectal Surgeons (ASCRS) and The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum. 2006;49:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Kleespies A, Füessl KE, Seeliger H, Eichhorn ME, Müller MH, Rentsch M, Thasler WE, Angele MK, Kreis ME, Jauch KW. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis. 2009;24:1097-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Tebala GD. Colorectal surgery in a rural setting. Updates Surg. 2015;67:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Smyth R, Darbyshire A, Mercer S, Khan J, Richardson J. Trends in emergency colorectal surgery: a 7-year retrospective single-centre cohort study. Surg Endosc. 2023;37:3911-3920. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Vallance AE, Keller DS, Hill J, Braun M, Kuryba A, van der Meulen J, Walker K, Chand M. Role of Emergency Laparoscopic Colectomy for Colorectal Cancer: A Population-based Study in England. Ann Surg. 2019;270:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Marcello PW, Milsom JW, Wong SK, Brady K, Goormastic M, Fazio VW. Laparoscopic total colectomy for acute colitis: a case-control study. Dis Colon Rectum. 2001;44:1441-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Gash K, Chambers W, Ghosh A, Dixon AR. The role of laparoscopic surgery for the management of acute large bowel obstruction. Colorectal Dis. 2011;13:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Clancy C, O'Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, Myers E. A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Dis. 2015;17:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Casillas S, Delaney CP, Senagore AJ, Brady K, Fazio VW. Does conversion of a laparoscopic colectomy adversely affect patient outcome? Dis Colon Rectum. 2004;47:1680-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | White I, Greenberg R, Itah R, Inbar R, Schneebaum S, Avital S. Impact of conversion on short and long-term outcome in laparoscopic resection of curable colorectal cancer. JSLS. 2011;15:182-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | de Neree Tot Babberich MPM, van Groningen JT, Dekker E, Wiggers T, Wouters MWJM, Bemelman WA, Tanis PJ; Dutch Surgical Colorectal Audit. Laparoscopic conversion in colorectal cancer surgery; is there any improvement over time at a population level? Surg Endosc. 2018;32:3234-3246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |