Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1474
Revised: April 3, 2024
Accepted: April 25, 2024
Published online: May 27, 2024
Processing time: 135 Days and 1.6 Hours
Magnetic compression anastomosis is a promising treatment option for patients with complex esophageal atresia; but, at the present time, should not be the first therapeutic option in those cases where the surgeon can perform a primary anastomosis of the two ends of the esophagus with acceptable tension.
Core Tip: Esophageal magnetic compression anastomosis or magnamosis consists of a non-surgical esophageal anastomosis performed with the use of magnets positioned into upper and lower esophageal pouches. The magnetic force applies compression on interposed tissues, resulting in spontaneous progressive esophageal anastomosis. It is a promising treatment option for patients with complex esophageal atresia; but, at the present time, should not be the first therapeutic option in those cases where the surgeon can perform a primary anastomosis of the two ends of the esophagus with acceptable tension.
- Citation: Pérez-Bertólez S, Godoy-Lenz J. Primary repair of esophageal atresia Gross type C via thoracoscopic magnetic compression anastomosis: Is it the best option? World J Gastrointest Surg 2024; 16(5): 1474-1481
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1474.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1474
The use of magnets for the treatment of esophageal atresia (EA) was first described in 1975 by Hendren and Hale[1] and was initially proposed as lengthening procedure. In recent years, there is a renewed interest in magnetic compression for EA, focusing on magnetic compression force acting on interposed esophageal tissues, leading to simultaneous central necrosis and peripheral mucosal bridging, ultimately forming an esophageal anastomosis or “magnamosis”[2,3].
We have read with interest a case report by Zhang et al[4], who presented a case of thoracoscopic-assisted esophageal magnetic compression anastomosis. We would like to add some considerations on their paper.
The fundamental goal of EA repair remains the restoration of esophageal continuity, allowing for nutritional autonomy via oral intake. Thoracoscopic repair of EA represents one of the most challenging advanced pediatric endoscopic procedures. In general, minimally invasive surgery is preferred due to its association with reduced tissue trauma, decreased pain, shorter hospital stays, and comparable or even superior clinical outcomes compared to standard surgical approaches. Studies have shown that cerebral oxygenation remains stable within the normal range during and after thoracoscopic primary anastomosis for EA[5]. However, it is essential to recognize that the vast majority of senior pediatric surgeons are proficient in performing this surgery satisfactorily via thoracotomy.
In general terms, thoracotomy is more invasive than thoracoscopy, which, in turn, is more invasive than endoscopy. We disagree with the statement “open approaches are extremely invasive and there are many postoperative complications”. A significant proportion of children develop musculoskeletal deformities after thoracotomy, but most of them are subclinical. An axillary muscle-sparing technique has been shown to significantly decrease the incidence of these deformities[6-10]. Other complications (leakage, dehiscence, stenosis…) are not directly related to the surgical approach, but are influenced by factors such as the type of EA, patient characteristics and technical details.
Although the patient comes from a county hospital, the delay in diagnosis is conspicuous, as the patient exhibited classic clinical signs of EA, including drooling of saliva, dyspnea, and vomiting immediately after feeding. Additionally, prenatal detection of polyhydramnios further highlights the need for early recognition and intervention.
The authors mention “angiography” in their paper, but the imaging technique they describe is more accurately termed an “esophagogram.” Angiography is typically used to visualize vascular structures by detecting contrast injected into blood vessels. In this context, esophagograms are valuable for assessing the size and location of the proximal pouch. However, their systematic implementation is not recommended due to the risk of aspiration. An alternative approach involves insufflating air during X-rays, which is less hazardous.
The authors correctly placed a tube in the proximal esophageal pouch during physical examination. However, it is essential to clarify that this was not a “gastric tube” since it did not reach the stomach. Precise terminology ensures accurate communication.
The classification system used by the authors corresponds to that described by Robert E Gross, a revered pioneer in pediatric surgery. Given that it bears his surname, it should be written in capital letters: EA Gross type C.
The statement “To avoid the complications associated with open surgery” in the context of parental consent warrants clarification. Notably, a laparotomy (open surgery) was performed to access the stomach. Therefore, we recommend omitting this phrase to accurately reflect the surgical approach.
To validate the cosmetic outcome mentioned by the authors, we recommend including a photograph of the patient’s abdominal scar. Visual evidence would enhance transparency and allow readers to assess the aesthetic impact of the procedure.
The authors should provide the code of approval from their hospital’s ethics committee. Transparency in ethical considerations is essential for readers and ensures adherence to established guidelines.
While the authors describe their approach as less invasive, we respectfully disagree. The presented case of type C EA represents an ideal scenario for surgery, since it is a full-term newborn with a good weight (3500 g), without severe cardiopathy or other associated anomalies (if present, they should be described) and proximity of both pouches. In our opinion, the optimal procedure for this specific case would have been to close the tracheoesophageal fistula and perform an end-to-end esophageal anastomosis. This approach would have avoided an unnecessary laparotomy.
The authors meticulously describe their technique, including 3 ligatures (2 in the azygos vein and one in the fistula) and two purse strings (one in each esophageal pouch), the laparotomy, the measurement with the lower esophagus probes, the placement of the magnets, the progression of the transanastomotic probe through the magnets, the measurement of the ideal site for the placement of the silk stitch over the probe and its proper positioning, the closure of the stomach and the laparotomy… However, we question whether this multifaceted approach is indeed easier, faster, and superior to a straightforward termino-terminal esophageal anastomosis, which typically requires no more than ten stitches.
It would be valuable to include information on the anesthetic time and the overall duration of the surgical procedure. These details contribute to understanding the practical aspects of the technique.
Clarification is essential. Did the authors modify the patient’s position for the laparotomy? Were the esophageal pouches overlapping? Did the authors fix both pouches together with sutures? Describing and discussing these technical aspects would enhance the reader’s understanding and provide insights into surgical strategy.
A point of controversy lies in the preservation of the azygos vein. If not preserved, should it be ligated or coagulated? Addressing this topic would enrich the discussion.
Skilled surgeons proficient in thoracoscopic repair of EA can complete the procedure in less than an hour, as exemplified by Dr. Godoy’s impressive 37-min timeframe[11].
We seek clarification on several critical points: How long was the patient intubated and under relaxation? What was the duration of NICU admission? How many days did the patient spend in the hospital postoperatively?
The decision not to administer enteral nutrition through the transanastomotic tube warrants discussion. By utilizing the transanastomotic catheter for enteral nutrition, the patient could have potentially avoided 23 days of parenteral nutrition. Why didn't they administer enteral nutrition through the transanastomotic tube? Were they trying to prevent a stomach leak of the gastrotomy? After a non-complicated procedure of tracheoesophageal fistula closure and an end-to-end esophageal anastomosis, the esophagogram is routinely performed on the 5th-7th postoperative day and can start oral feeding. In addition, the patient has previous received enteral nutrition through the transanastomotic catheter. This approach aligns with the goal of early enteral feeding.
The authors report a minor leak on postoperative day 15. However, several questions arise: Were any other studies performed before this point? How many days did the patient have a chest tube, and what was the drainage pattern? The images of the case reported by Zhang et al[4], reveal atelectasis and pachypleuritis in the right hemithorax, suggesting that the leak may have been initially more significant than observed. The images also depicts effusion in the right costophrenic angle. We disagree with the authors’ statement that “the contrast agent did not enter the pleural cavity.” Given the transpleural approach used, the mediastinum and pleura were indeed connected.
While the abstract aligns with the manuscript content, the absence of explicit mention of the leakage is concerning. The statement “No leakage existed when the transoral feeding started” could mislead readers into assuming there was no leak throughout the entire course. Anastomotic leakage is a serious complication in EA, although rarely reported in magnamosis[12,13]. Transparency in reporting complications is essential for advancing our understanding and improving patient care.
Thinking about potential causes of leak… Could the leak be related to an area where the esophageal tissue did not connect due to the interposed purse string suture? To mitigate a possible suture interposition, alternative strategies can be considered:
(1) Transanastomotic tube avoidance: If the transanastomotic tube is not going to be used for feeding, it would not be necessary. Thus, the opening and subsequent purse string suture of the proximal esophageal pouch could have been circumvented.
(2) Optimal magnet sizing: The choice of magnets with appropriate diameters (proximal magnet larger than distal esophagus) further reduces the likelihood of displacement into the stomach. In this scenario, the silk “plug” attached to the transanastomotic probe could also have been avoided.
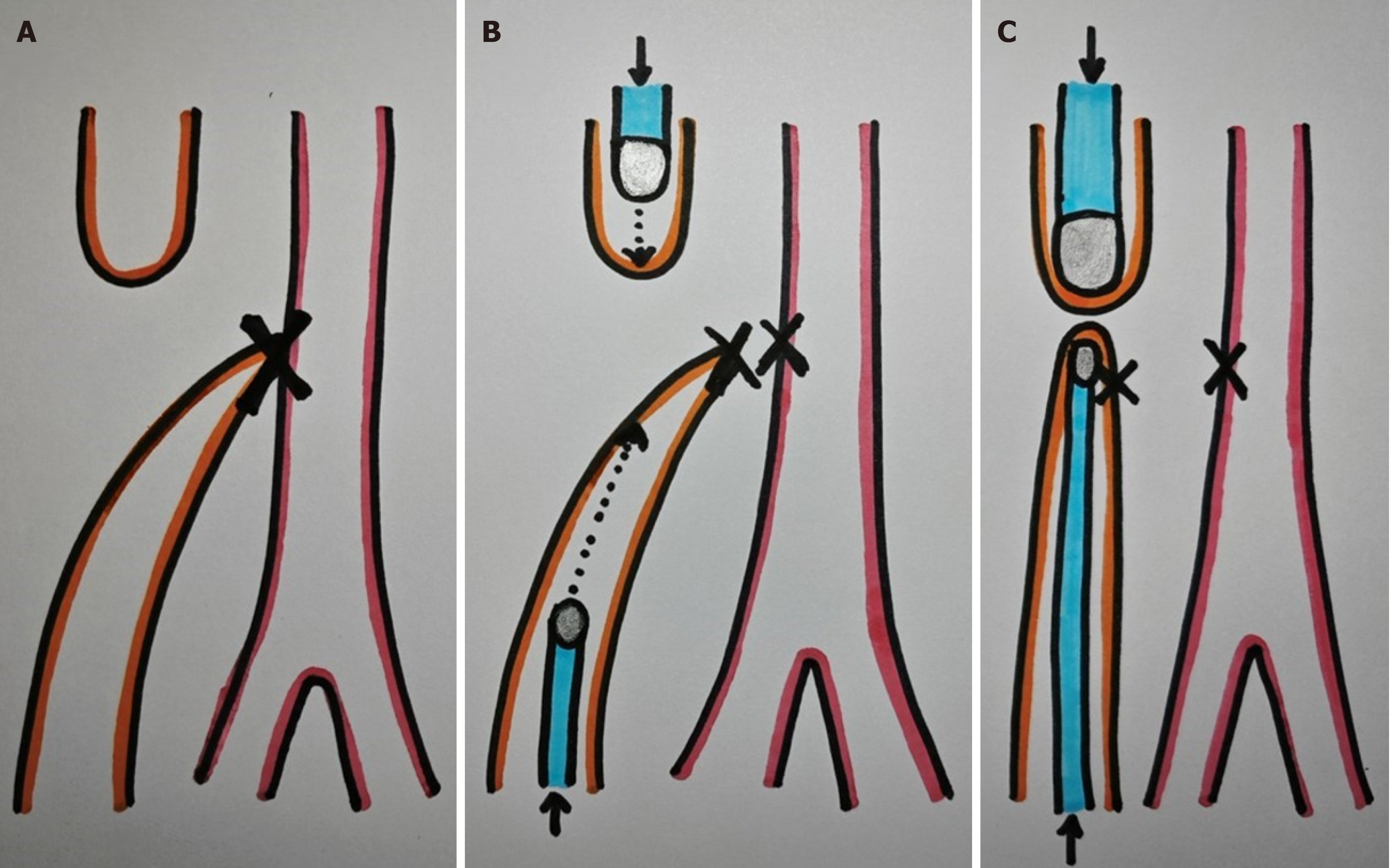
And (3) Alternative approach: End-to-side anastomosis: To prevent interposition of the distal purse string suture, consider sectioning the tracheoesophageal fistula between two ligatures and placing the magnet to create an end-to-side anastomosis (Figure 1).
The authors removed the magnets on postoperative day 23, but the method of removal remains unspecified. Clarification on this critical step would enhance our understanding.
The image of the control esophagogram is suboptimal due to faint staining of the esophagus. Clearer visualization would provide more robust evidence.
An essential aspect missing from the manuscript is the patient’s short, medium, and long-term evolution. Recalcitrant stenosis is a common complication after magnamosis[2,8,13-15], often manifesting at a later stage[16-19]. Reporting on the patient’s progress beyond the immediate postoperative period would be valuable.
There are some radiation exposure considerations. Magnamosis involves increased exposure to ionizing radiation, as both magnet positioning and removal typically occur under pulsed fluoroscopy. Although the authors do not mention this data, direct viewing by thoracoscopy may have reduced radiation dose.
Although thoracoscopic repair of EA is one of the most challenging advanced pediatric endoscopic procedures, nowadays the availability of step-by-step descriptions and documented procedures facilitates learning and training. Simulation-based training offers a promising avenue for acquiring technical skills[20]. Although it is expected that this technique will become the gold standard as robotic surgery evolves and miniaturizes, reducing the challenges of the thoracoscopic approach.
We acknowledge that the use of magnets has its specific indications. Dr. Godoy’s experience with 9 cases-comprising 2 type A long gap EA, 1 type B long gap EA, one congenital esophageal stenosis, and 5 severe esophageal strictures after prior EA surgery-provides valuable insights.
Based on this personal experience and an extensive review of the literature, we believe that magnamosis should be approached with caution and it is essential a proper patient selection. Magnamosis is not indicated nowadays when tension-free esophageal anastomosis surgery is feasible. However, we recognize that certain patients, particularly those with high-risk physiologic and anatomic comorbidities, may benefit from magnamosis.
We observed improved outcomes when both esophageal pouches are in contact with each other. However, for long gap EA cases, the choice of magnet type still needs to evolve, refining magnet design and optimizing the balance between attraction and tissue preservation. The ideal balance lies in creating an attraction between the magnets within the esophageal pouches, allowing for esophageal growth similar to tissue expanders. Excessive magnet force ultimately leads to severe stenosis, compromising patient outcomes. We hypothesize that the observed phenomenon may be due to two possible mechanisms. First, excessive traction may cause esophageal fissures, leading to the formation of fibrous and scar tissue, similar to cicatricial phimosis following forced preputial retraction. Second, the magnets may necrotize the ends of the distant esophageal pouches, and the path between the two pouches may resemble a fibrin sheath, without esophageal tissue, similar to the sheath that forms around a port-a-cath. In order to determine the true cause, anatomopathological studies of these tissues would be necessary.
Our best results emerged in the treatment of refractory strictures following EA repair[21]. By combining magnamosis with dynamic stents[22], we achieved definitive resolution of esophageal strictures in three patients. Long-term follow-up corroborates the efficacy of this strategy.
We hypothesize that dynamic stents are an effective treatment option for esophageal cicatricial strictures because they bring together several therapeutic options for the treatment of postoperative scars[23], such as silicone gel sheets (the silicone is in direct contact with the stenotic scar), pressure dressings and scar massage with a moisturizing substance (with the patient's movement and esophageal peristalsis, direct pressure and massage is exerted on the scar and moisturized with the patient's own saliva).
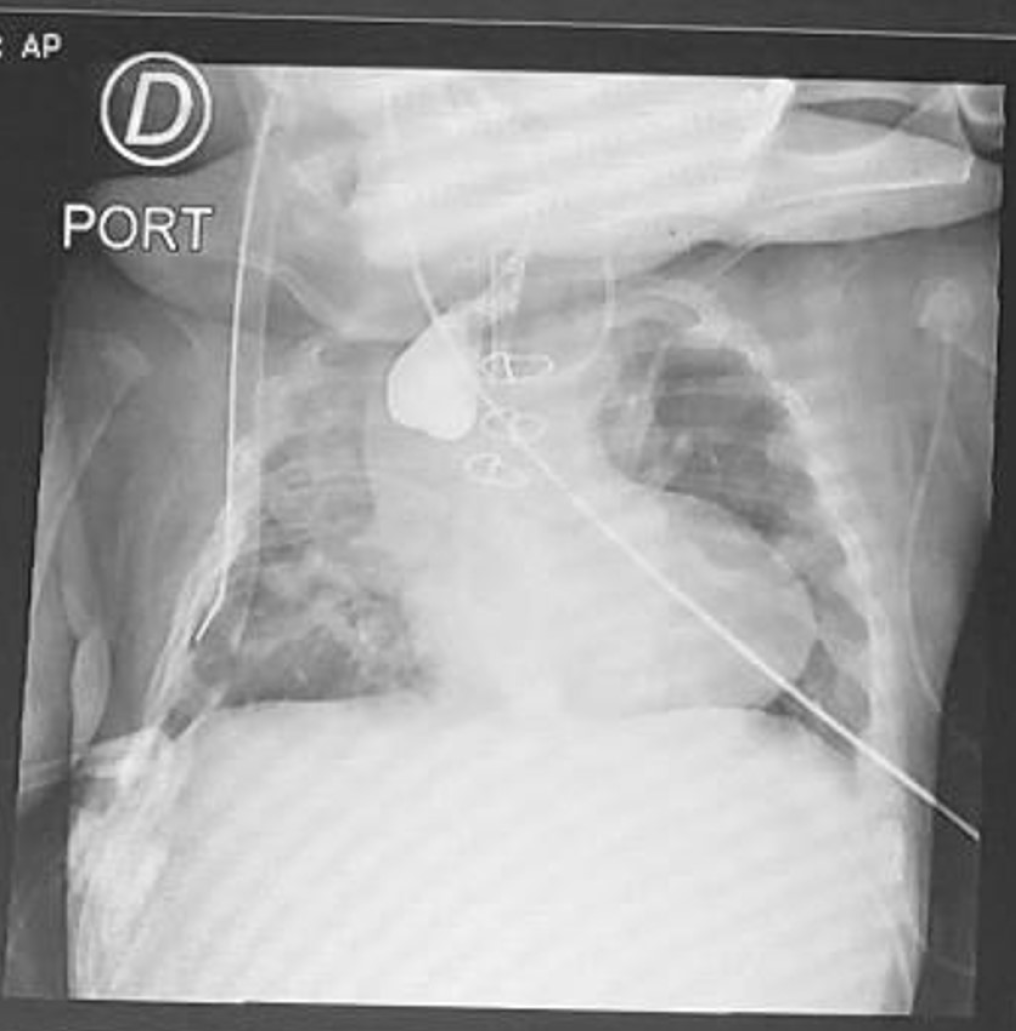
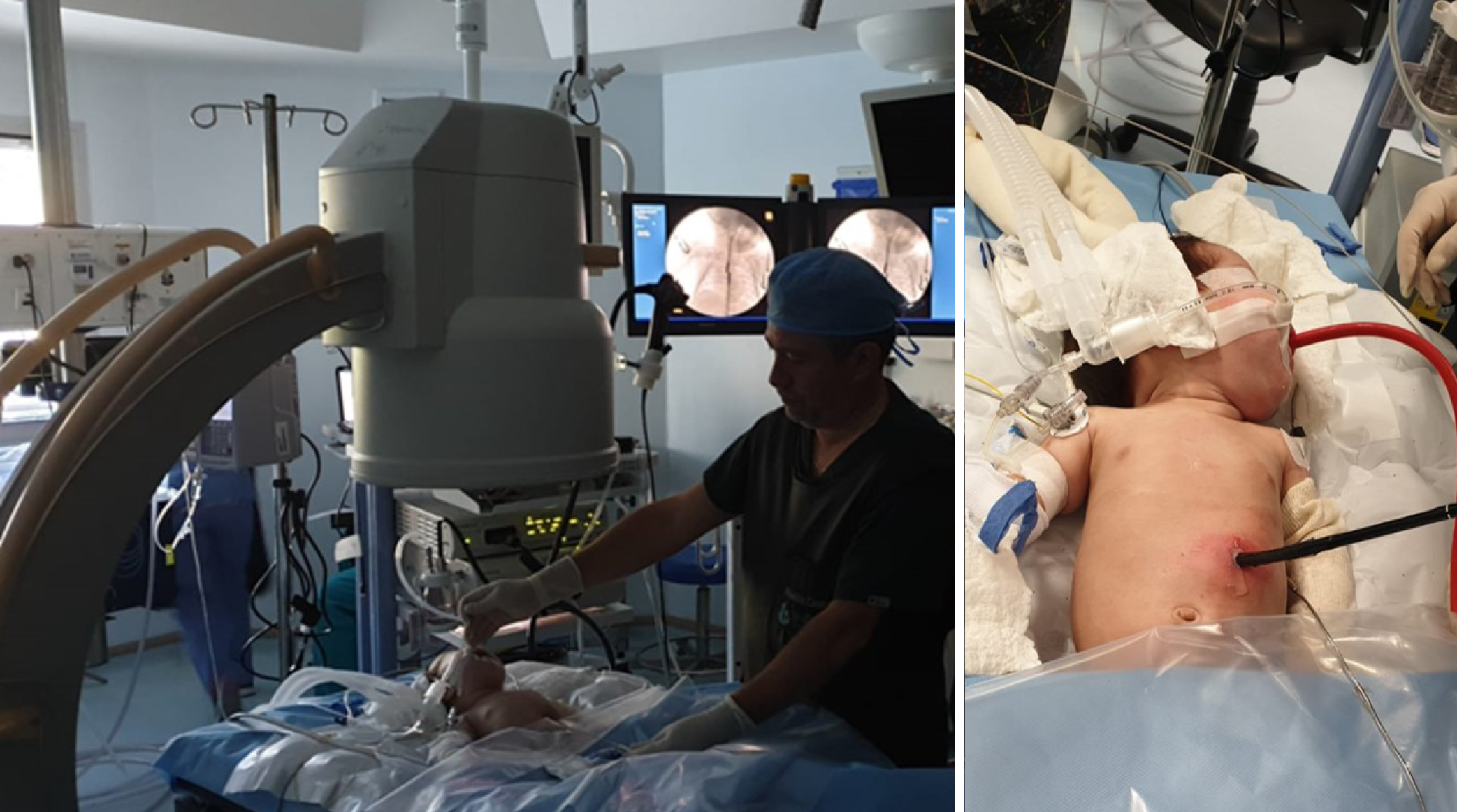
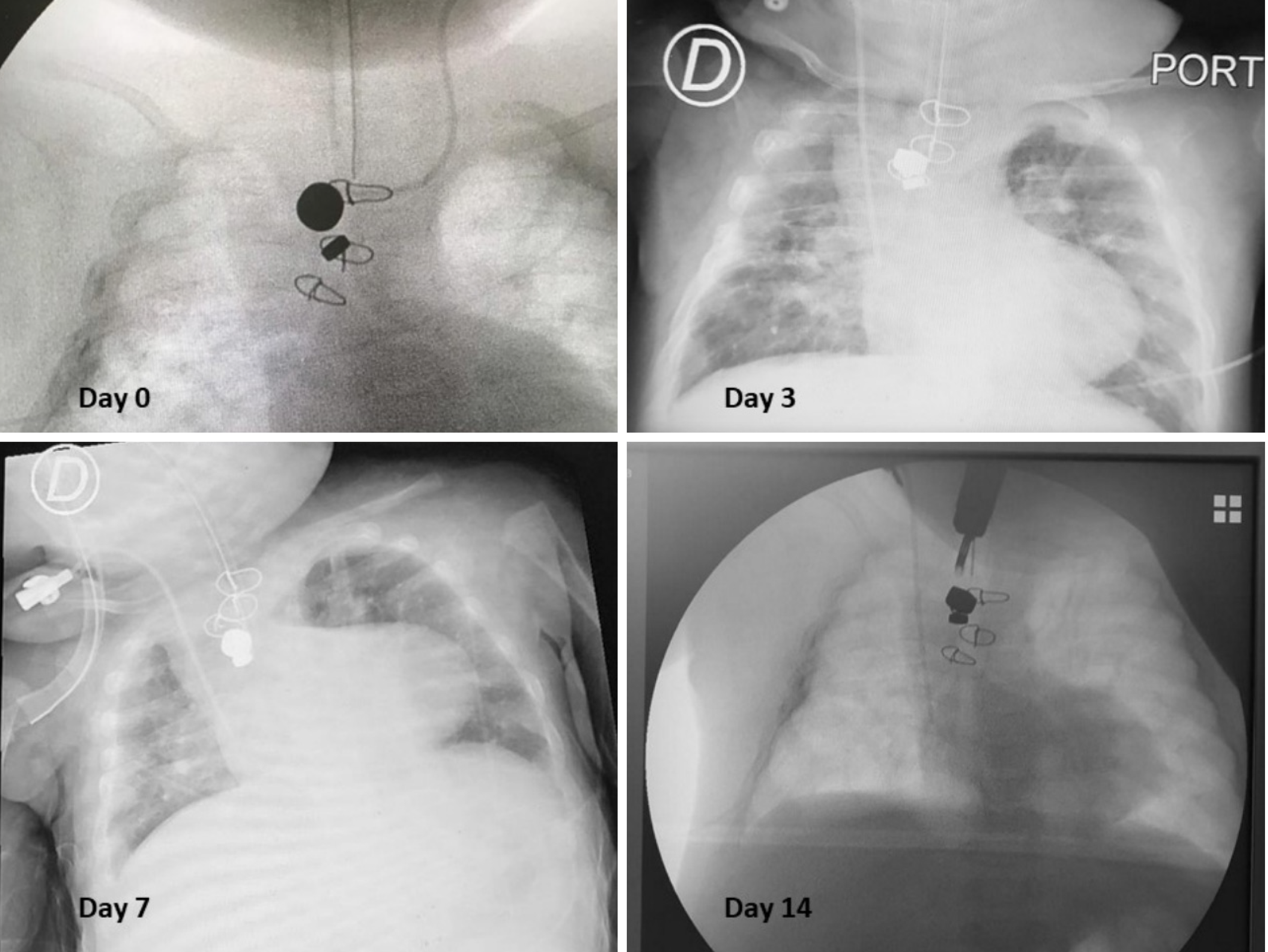
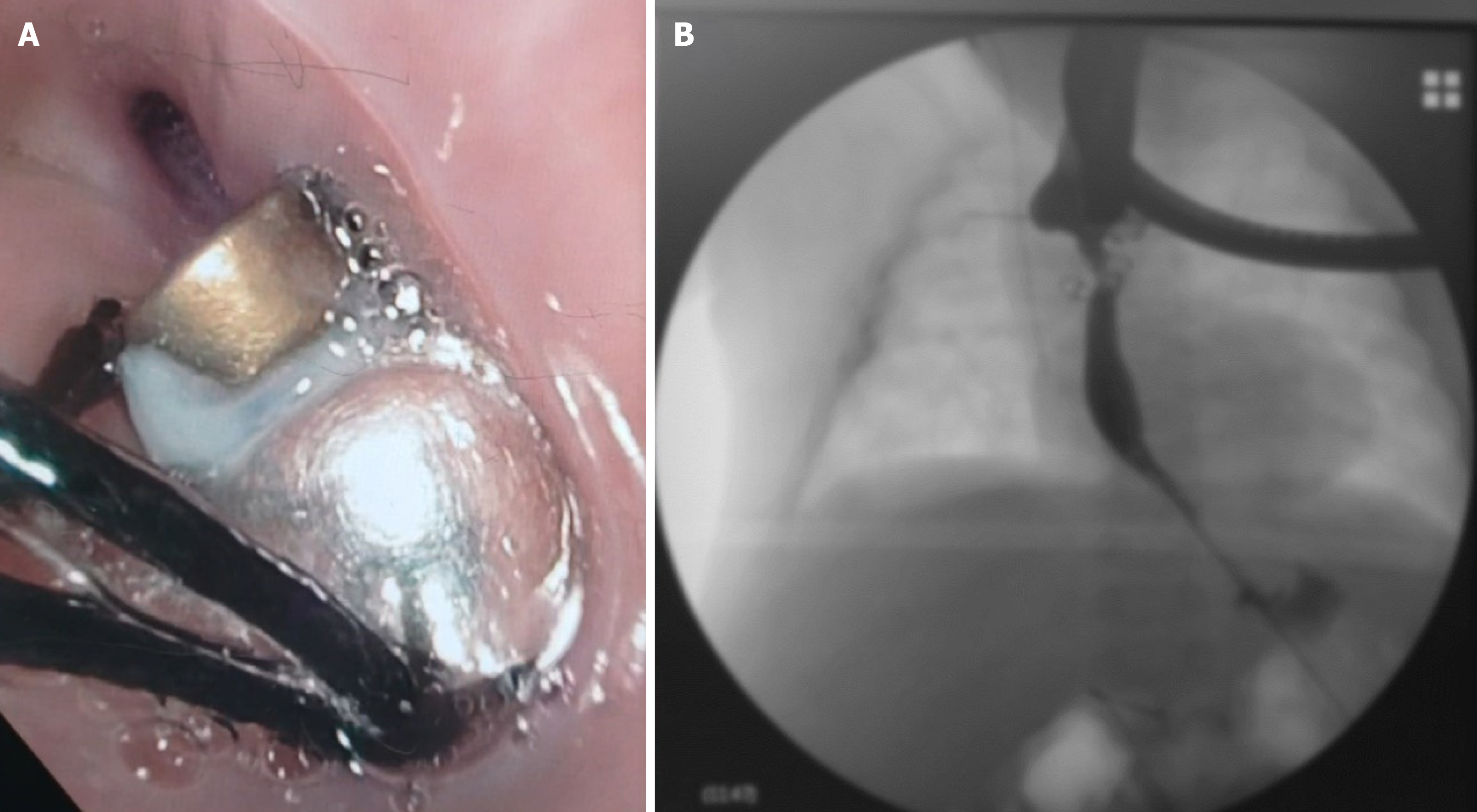
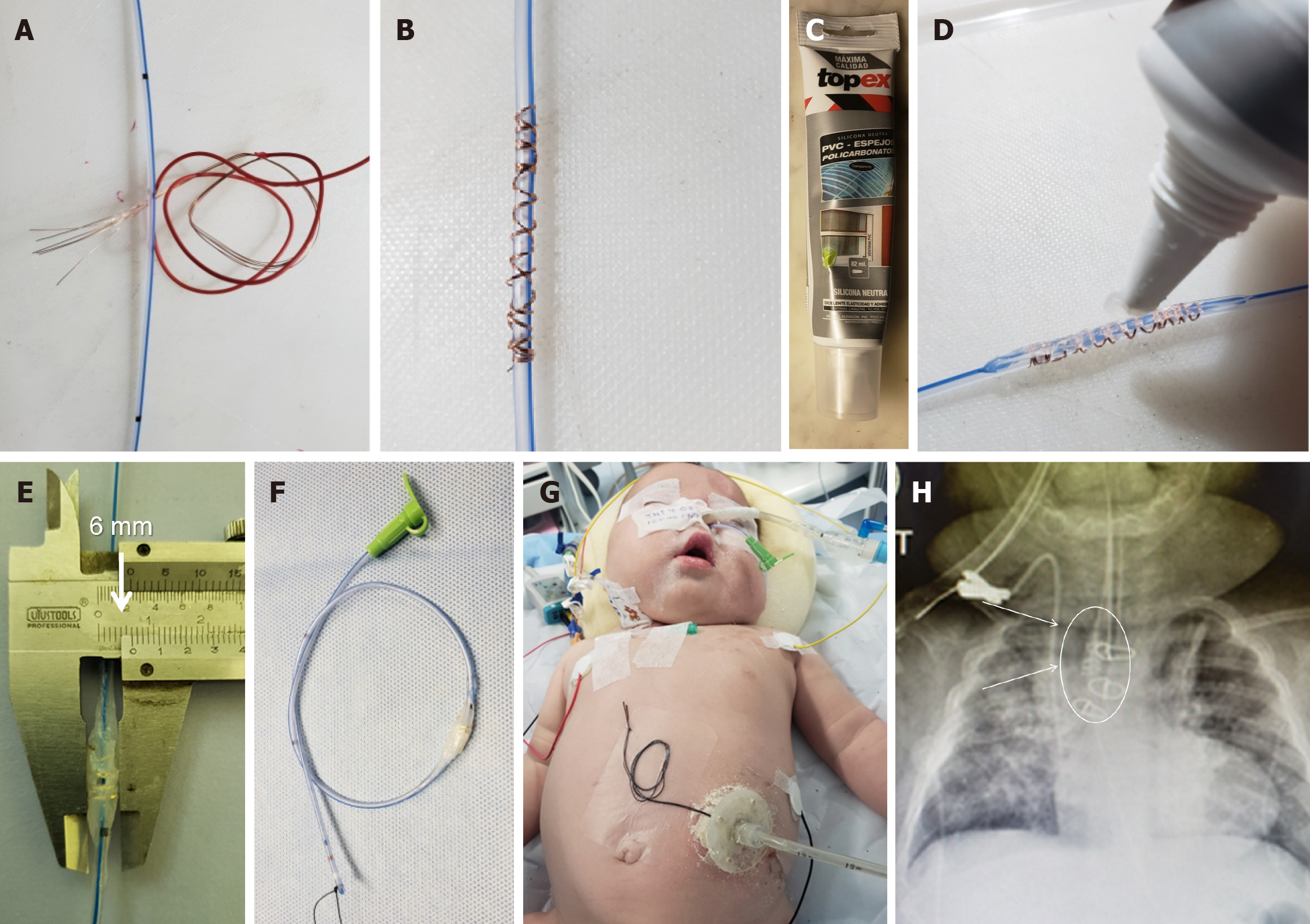
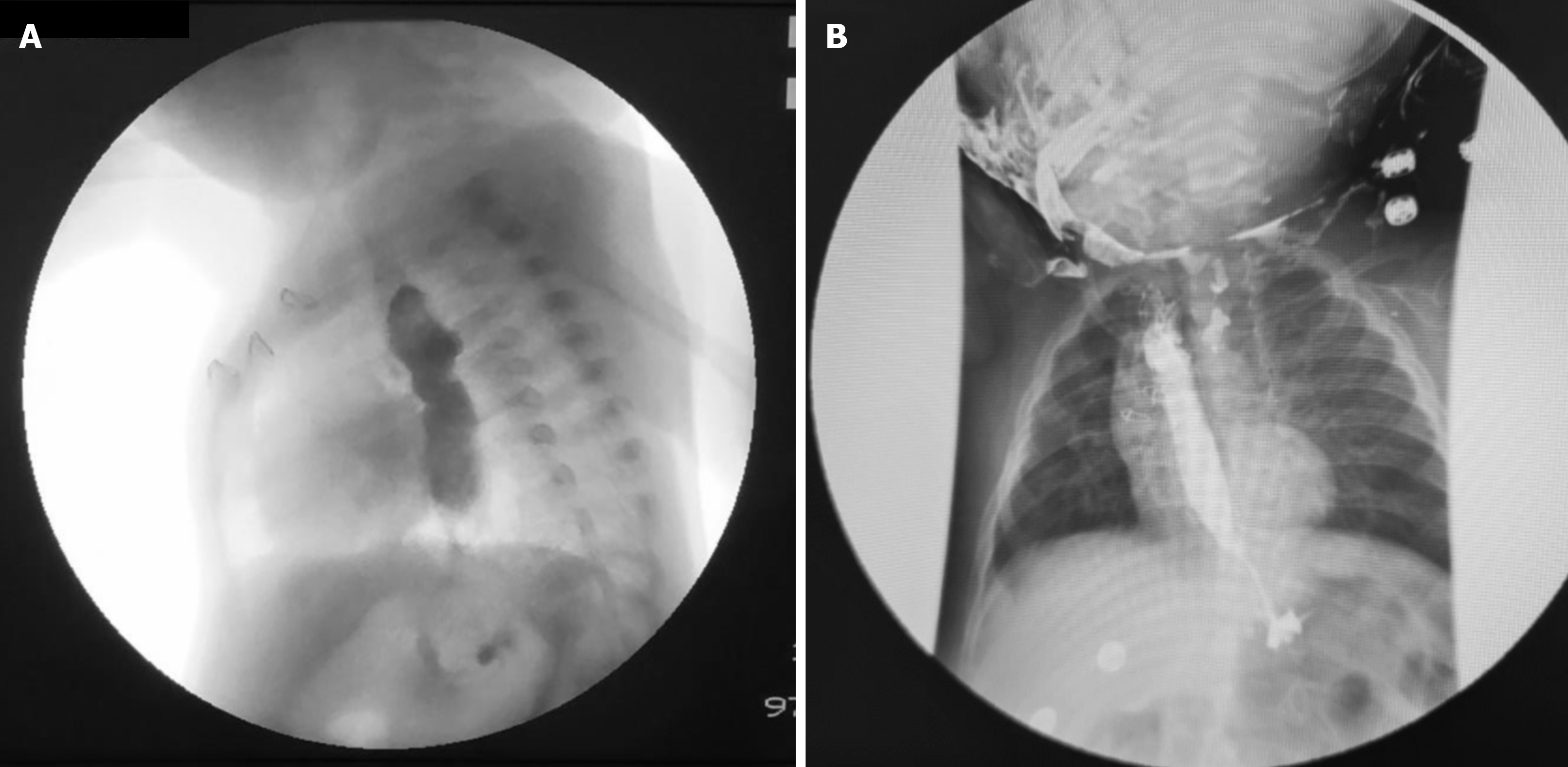
Herein, we present a case of a 5-month-old boy with a multioperated type C long gap EA who developed a severe esophageal stricture with complete obliteration (Figure 2). The esophagus was repermeabilized by magnamosis (Figures 3-5), and subsequently, a dynamic stent was implanted for 2 months (Figure 6). Video 1 shows the patient eating normally one year after the procedure (recorded by his mother; Supplementary material).
There has been no recurrence of the stricture or other complications after more than 3 years of follow-up (Figure 7).
We emphasize the importance of individualizing each case. Feasibility does not necessarily equate to the best or least invasive option for a patient. Considering patient-specific factors and tailoring the approach accordingly ensures optimal outcomes.
In conclusion, while magnamosis holds promise, judicious patient selection, ongoing evaluation, and a commitment to individualized care remain paramount.
| 1. | Hendren WH, Hale JR. Electromagnetic bougienage to lengthen esophageal segments in congenital esophageal atresia. N Engl J Med. 1975;293:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anastomosis in pediatric patients. J Pediatr Surg. 2014;49:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Lee WG, Evans LL, Chen CS, Fuchs JR, Zamora IJ, Bruzoni M, Harrison MR, Muensterer OJ. Lessons Learned From the First-In-Human Compassionate Use of Connect-EA™ in Ten Patients With Esophageal Atresia. J Pediatr Surg. 2024;59:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zhang HK, Li XQ, Song HX, Liu SQ, Wang FH, Wen J, Xiao M, Yang AP, Duan XF, Gao ZZ, Hu KL, Zhang W, Lv Y, Zhou XH, Cao ZJ. Primary repair of esophageal atresia gross type C via thoracoscopic magnetic compression anastomosis: A case report. World J Gastrointest Surg. 2023;15:2919-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Tytgat SH, van Herwaarden MY, Stolwijk LJ, Keunen K, Benders MJ, de Graaff JC, Milstein DM, van der Zee DC, Lemmers PM. Neonatal brain oxygenation during thoracoscopic correction of esophageal atresia. Surg Endosc. 2016;30:2811-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Allal H, Pérez-Bertólez S, Maillet O, Forgues D, Doan Q, Chiapinelli A, Kong V. [Comparative study of thoracoscopy versus thoracotomy in esophageal atresia]. Cir Pediatr. 2009;22:177-180. [PubMed] |
| 7. | Drevin G, Andersson B, Svensson JF. Thoracoscopy or Thoracotomy for Esophageal Atresia: A Systematic Review and Meta-analysis. Ann Surg. 2021;274:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Muensterer OJ, Evans LL, Sterlin A, Sahlabadi M, Aribindi V, Lindner A, König T, Harrison MR. Novel Device for Endoluminal Esophageal Atresia Repair: First-in-Human Experience. Pediatrics. 2021;148:e2020049627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hornok Z, Kubiak R, Csukas D, Ferencz A, Cserni T. Esophageal Magnetic Anastomosis Device (EMAD) to Simplify and Improve Outcome of Thoracoscopic Repair for Esophageal Atresia with Tracheoesophageal Fistula: A Proof of Concept Study. J Pediatr Surg. 2023;58:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Safa N, Wei S, Saran N, Guadagno E, Laberge JM, Emil S. Musculoskeletal deformities after thoracic surgery in children: An observational long-term follow-up study. J Pediatr Surg. 2021;56:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Atresia esofagica - thorascopic repair of esophageal atresia. Available from: https://youtu.be/PnUtzEkZYRs?si=5eCztjPYokHWHnx3. |
| 12. | Liu SQ, Lv Y, Fang Y, Luo RX, Zhao JR, Luo RG, Li YM, Zhang J, Zhang PF, Guo JZ, Li QH, Han MX. Magnetic compression for anastomosis in treating an infant born with long-gap oesophageal atresia: A case report. Medicine (Baltimore). 2020;99:e22472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Shieh HF, Jennings RW, Manfredi MA, Ngo PD, Zendejas B, Hamilton TE. Cautionary tales in the use of magnets for the treatment of long gap esophageal atresia. J Pediatr Surg. 2022;57:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Slater BJ, Borobia P, Lovvorn HN, Raees MA, Bass KD, Almond S, Hoover JD, Kumar T, Zaritzky M. Use of Magnets as a Minimally Invasive Approach for Anastomosis in Esophageal Atresia: Long-Term Outcomes. J Laparoendosc Adv Surg Tech A. 2019;29:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wolfe E, Zidane M, Hancock BJ, Lum Min SA, Zaritzky M, Keijzer R. Magnamosis for esophageal atresia is associated with anastomotic strictures requiring an increased number of dilatations. J Pediatr Surg. 2020;55:821-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Lovvorn HN, Baron CM, Danko ME, Novotny NM, Bucher BT, Johnston KK, Zaritzky MF. Staged repair of esophageal atresia: Pouch approximation and catheter-based magnetic anastomosis. J Pediatr Surg Case Rep. 2014;2:170-175. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Dorman RM, Vali K, Harmon CM, Zaritzky M, Bass KD. Repair of esophageal atresia with proximal fistula using endoscopic magnetic compression anastomosis (magnamosis) after staged lengthening. Pediatr Surg Int. 2016;32:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Ellebaek MBB, Qvist N, Rasmussen L. Magnetic Compression Anastomosis in Long-Gap Esophageal Atresia Gross Type A: A Case Report. European J Pediatr Surg Rep. 2018;6:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Conforti A, Pellegrino C, Valfré L, Iacusso C, Schingo PMS, Capolupo I, Sgro' S, Rasmussen L, Bagolan P. Magnamosis for long gap esophageal atresia: Minimally invasive "fatal attraction". J Pediatr Surg. 2023;58:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Maricic MA, Bailez MM, Rodriguez SP. Validation of an inanimate low cost model for training minimal invasive surgery (MIS) of esophageal atresia with tracheoesophageal fistula (AE/TEF) repair. J Pediatr Surg. 2016;51:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Woo R, Wong CM, Trimble Z, Puapong D, Koehler S, Miller S, Johnson S. Magnetic Compression Stricturoplasty For Treatment of Refractory Esophageal Strictures in Children: Technique and Lessons Learned. Surg Innov. 2017;24:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Caldaro T, Torroni F, De Angelis P, Federici di Abriola G, Foschia F, Rea F, Romeo E, Dall'Oglio L. Dynamic esophageal stents. Dis Esophagus. 2013;26:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Commander SJ, Chamata E, Cox J, Dickey RM, Lee EI. Update on Postsurgical Scar Management. Semin Plast Surg. 2016;30:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |