Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1430
Revised: March 26, 2024
Accepted: April 11, 2024
Published online: May 27, 2024
Processing time: 179 Days and 6.7 Hours
Spontaneous visceral artery dissection (SVAD) is a rare condition that affects the visceral arteries, such as the celiac, superior mesenteric, and inferior mesenteric arteries, without involving the aorta. Organ ischemia or hemorrhage from vessel rupture can occur in SVAD; therefore, prompt detection and management is es
A 53-year-old male presented with complaints of poor appetite and abnormal liver function for the past 6 months. He had previously undergone transabdo
Multi-modal imaging is effective in diagnosing SVAD, and conservative treatment is a choice for asymptomatic patients.
Core Tip: Spontaneous visceral artery dissection (SVAD) is a rare condition. Imaging examinations play an important role in the diagnosis of SVAD. Contrast-enhanced computed tomography has been used to diagnose most of the previous cases, but few studies have explored the potential of contrast-enhanced ultrasound (CEUS) for early detection of this disease. In our case, CEUS was used for early detection of the dissection, which was confirmed using other imaging modalities, and the condition was successfully managed with conservative therapy. This case demonstrates the diagnostic value of multi-modal imaging for this uncommon disease.
- Citation: Pu Y, Luo Y. Multi-modal imaging for the diagnosis of spontaneous visceral artery dissection: A case report. World J Gastrointest Surg 2024; 16(5): 1430-1435
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1430.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1430
Artery dissection is a life-threatening condition that occurs when a tear or rupture in the arterial lining allows blood to enter the arterial wall, separating its layers, and disrupting blood flow. Aortic dissection is a severe and fatal vascular disease with an annual incidence of 3.5-6/100000 per year[1]. Spontaneous visceral artery dissection (SVAD), which oc
Most of the previous cases have been diagnosed by contrast-enhanced computed tomography (CECT)[2,6], but the potential of contrast-enhanced ultrasound (CEUS) for early detection of this disease remains underexplored. We used CEUS to identify a case of artery dissection involving the celiac and common hepatic arteries at an early stage.
A 53-year-old male was admitted to our hospital with complaints of poor appetite and abnormal liver function during the previous 6 months.
Blood tests at the local hospital showed abnormalities in liver and renal functions, and ultrasound showed a hypoechoic nodule in the liver. The patient was transferred to our hospital for further diagnosis and treatment.
The patient had a history of viral hepatitis B for more than 20 years and had undergone transabdominal splenectomy, esophagogastric devascularization, and cholecystectomy 4 years earlier at our hospital for gallstones and severe portal hypertension. The patient had no history of hypertension, diabetes mellitus, or other abdominal surgery.
There was no other personal or family history of acute or chronic disease.
The patient showed no jaundice on visual examination and no tenderness, rebound tenderness, or muscle tension on abdominal palpation.
Liver function tests were performed at baseline, 3 months, and 6 months. Alanine aminotransferase was 45, 46, and 34 IU/L, respectively (reference range, < 50 IU/L). Aspartate aminotransferase was 38, 35, and 36 IU/L, respectively (reference range, < 40 IU/L). Total bilirubin was 31.9, 32.7, and 34.4 µmol/L, respectively (reference range, 5 µmol/L to 28 µmol/L).
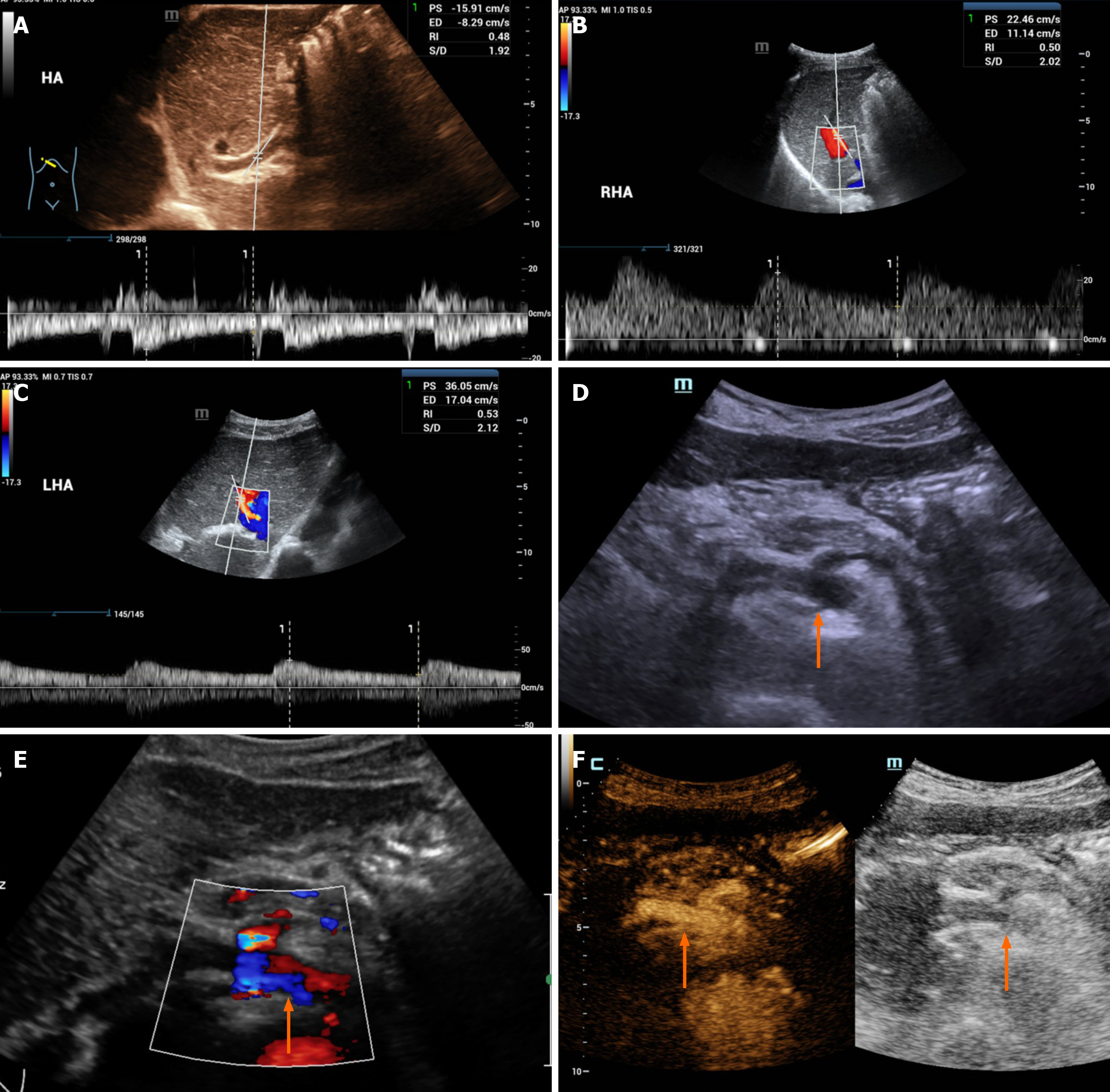
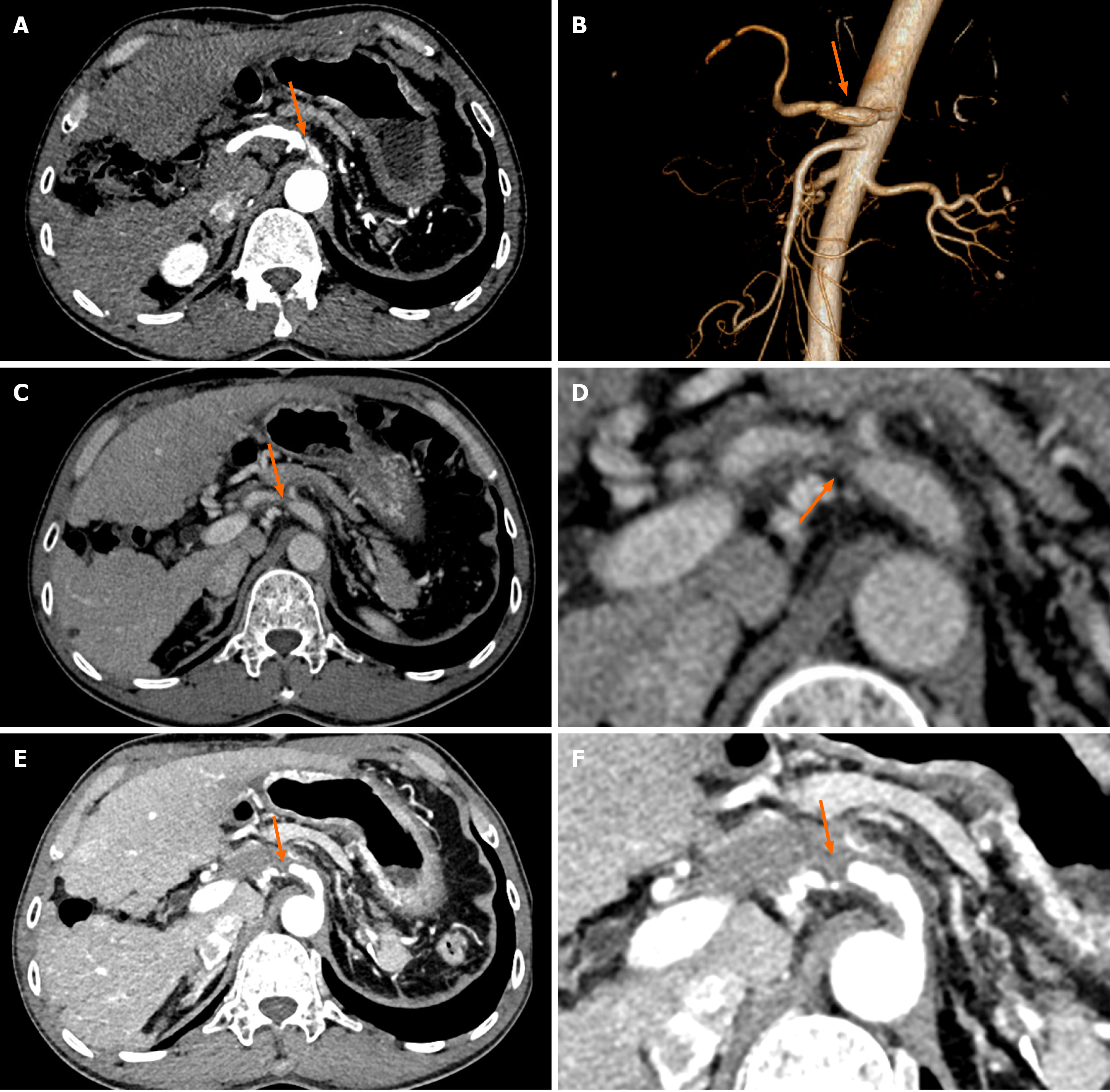
Color Doppler imaging showed that the flow of blood in the common hepatic artery was slowed, and the resistance index was reduced. Additionally, right hepatic artery blood flow accelerating time was significantly prolonged, and blood flow was slowed (Figure 1A and B). A dissection of the celiac artery was detected, with two opposite flow signals in the lumen (Figure 1C and D). In the CEUS imaging, the contrast agent extravasated the vessel wall, and the common hepatic artery was narrowed (Figure 1E and F). The CECT images revealed a linear low-density area in the celiac artery wall, heterogeneous thickening of the celiac artery, and thinning of the common hepatic artery, with low-density material attached to their walls (Figure 2A and B); at 3 months of follow-up, the low-density material increased, and the common hepatic artery was narrowed (Figure 2C and D); at 6 months of follow-up, the celiac artery wall had more low-density material, and the common hepatic artery was occluded with evidence of collateralization (Figure 2E and F).
Based on the analysis of multiple imaging information, the patient was finally diagnosed with SVAD.
The patient chose conservative treatment rather than surgical intervention. During follow-up, the patient was treated with a daily oral dose of 0.5 mg entecavir, 12.5 mg carvedilol, and 5 mg amlodipine besylate.
During the follow-up period of 6 months, the patient did not experience any symptoms (e.g., abdominal pain), and the liver function tests did not show any significant abnormal changes.
The three main branches of the visceral arteries are the celiac, superior mesenteric, and inferior mesenteric arteries. An SVAD is a condition in which these arteries experience a tear or rupture in their lining without affecting the aorta. Su
According to a recent systematic review, approximately 60% of patients with CAD exhibited nonspecific clinical sym
Imaging plays a crucial role in the diagnosis of CAD, which has increased in prevalence with the advancement of ima
The rates of conservative and surgical treatments for CAD vary widely, largely because of its unpredictable natural course[11]. For asymptomatic patients, doctors mostly recommend conservative treatment or regular follow-up. Conservative treatment consists mainly of antihypertensive, anticoagulant, and/or antiplatelet therapy[7]. Anticoagulation or antiplatelet therapy can help prevent thrombosis and maintain organ perfusion; however, the criteria for use of these agents are unclear. CAD can lead to thrombus formation at the entry site or in the false lumen, which can cause stenosis of the true lumen and obstruct blood flow, resulting in organ ischemia[12]. In our case, even though the hepatic artery was occluded, we did not provide surgical treatment because the liver is an organ with a dual blood supply. The CECT suggested that collateral circulation had formed around the occluded artery, and laboratory tests suggested that the patient did not have significant liver function abnormalities. A significant change in liver function would have been an indication that the CAD may have involved the hepatic artery, resulting in inadequate blood supply to the liver, which did not occur in our case. An additional concern was the possibility of splenic infarction if inhomogeneous splenic per
SVAD is a rare condition that requires prompt diagnosis and treatment to prevent organ ischemia or vascular rupture that results in massive bleeding. We identified this case early via CEUS, confirmed the diagnosis with other imaging modalities, and successfully managed it with conservative therapy. This case demonstrated the diagnostic value of multi-modal imaging for this uncommon disease. Conservative treatment is a reasonable choice for asymptomatic patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Ghimire R, Nepal S-Editor: Zhang L L-Editor: A P-Editor: Xu ZH
| 1. | Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Shi Y, Guo J, Dong J, Chen X, Luo L, Shen Y. Comparative analysis of prevalence, evaluation, management, and rehabilitation outcome of spontaneous isolated visceral artery dissection: a systematic review and meta-analysis of 80 reports. Int J Surg. 2023;109:469-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Li S, Luo J, Yin L, Yan C, Zhu Y, Wang J, Gao Z, Liu Z, Chen B. Aneurysmal celiac trunk dissection caused by median arcuate ligament syndrome successfully treated by endovascular technique: a case report. AME Case Rep. 2021;5:37. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Lin TS, Chiang YC, Chen CL, Concejero AM, Cheng YF, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC. Intimal dissection of the hepatic artery following transarterial embolization for hepatocellular carcinoma: an intraoperative problem in adult living donor liver transplantation. Liver Transpl. 2009;15:1553-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kang SH, Park HS, Yoon CJ, Shin CS, Yoo KC, Lee T. Mid- to Long-Term Outcomes in Management of Spontaneous Isolated Coeliac Artery Dissection (SICAD). Eur J Vasc Endovasc Surg. 2020;59:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Tanaka Y, Yoshimuta T, Kimura K, Iino K, Tamura Y, Sakata K, Hayashi K, Takemura H, Yamagishi M, Kawashiri MA. Clinical characteristics of spontaneous isolated visceral artery dissection. J Vasc Surg. 2018;67:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hosaka A, Nemoto M, Miyata T. Outcomes of conservative management of spontaneous celiac artery dissection. J Vasc Surg. 2017;65:760-765.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Poggiali E, Negri C, Mosso F, Petrini M, Michieletti E, Vercelli A. A rare and unusual cause of acute abdominal pain: A case of spontaneous isolated dissection of the celiac trunk. ECJ. 2022;18. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Shimata K, Sugawara Y, Irie T, Sambommatsu Y, Kadohisa M, Ibuki S, Kawabata S, Isono K, Honda M, Yamamoto H, Hibi T. Asymptomatic hepatic artery dissection early after living-donor liver transplantation with simultaneous splenectomy: two case reports. BMC Gastroenterol. 2020;20:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wu B, Wu X, Wang K, Tian F, Cheng L, Jia Z. Combination of Colour Duplex and Contrast Enhanced Ultrasound as an Alternative to Computed Tomography Angiography in Isolated Mesenteric Artery Dissection Surveillance. Eur J Vasc Endovasc Surg. 2019;58:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kim SR, Park TK, Choi SH, Kim SM, Choe YH, Heo SH, Park YJ, Kim DI, Kim YW, Kim DK. Natural history of spontaneous isolated celiac artery dissection after conservative treatment. J Vasc Surg. 2018;68:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Pateman Aciu S, Petrochko J, Bassik N, Fisher J. Spontaneous isolated celiac and splenic artery dissection with splenic infarction. Radiol Case Rep. 2022;17:2085-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Hosaka S, Fujita Y, Amagai T. A useful modality of CT angiography image to identify medical emergency in isolated celiac artery dissection type I: A case report with longest follow-up and literature review. Radiol Case Rep. 2023;18:4294-4298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Zettervall SL, Karthaus EG, Soden PA, Buck DB, Ultee KH, Schermerhorn ML, Wyers MC. Clinical presentation, management, follow-up, and outcomes of isolated celiac and superior mesenteric artery dissections. J Vasc Surg. 2017;65:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Galastri FL, Cavalcante RN, Motta-Leal-Filho JM, De Fina B, Affonso BB, de Amorim JE, Wolosker N, Nasser F. Evaluation and management of symptomatic isolated spontaneous celiac trunk dissection. Vasc Med. 2015;20:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Chen ZL, Zhang XC, Pan GR, Sun Y, Xu M, Li XQ. Clinical Features and Therapeutic Options for Isolated Visceral Artery Dissection. Ann Vasc Surg. 2016;30:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |