Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1385
Revised: February 27, 2024
Accepted: April 16, 2024
Published online: May 27, 2024
Processing time: 187 Days and 18 Hours
Previous studies have validated the efficacy of both magnetic compression and surgical techniques in creating rabbit tracheoesophageal fistula (TEF) models. Magnetic compression achieves a 100% success rate but requires more time, while surgery, though less frequently successful, offers rapid model establishment and technical maturity in larger animal models.
To determine the optimal approach for rabbit disease modeling and refine the process.
TEF models were created in 12 rabbits using both the modified magnetic com
The modified magnetic compression technique achieved a 66.7% success rate, whereas the success rate of the surgery technique was 33.3%. Surviving surgical rabbits might not meet subsequent experimental requirements due to TEF-related inflammation. In the modified magnetic compression group, one rabbit died, possibly due to magnet corrosion, and another died from tracheal magnet obstruction. Similar events occurred during the second round of modified magnetic compression modeling, with one rabbit possibly succumbing to aggravated lung infection. The operation time of the first round of modified magnetic compression was 3.2 ± 0.6 min, which was significantly reduced to 2.1 ± 0.4 min in the second round, compared to both the first round and that of the original technique.
The modified magnetic compression technique exhibits lower stress responses, a simple procedure, a high success rate, and lower modeling costs, making it a more appropriate choice for constructing TEF models in rabbits.
Core Tip: Tracheoesophageal fistula (TEF) is a complex condition with both congenital and acquired forms and presents a significant clinical challenge. Despite its importance, the methods for creating TEF models have limitations, particularly in terms of success rates and practicality. We compared the modified magnetic compression technique with the conventional surgical method for creating TEF models in rabbits.
- Citation: Meng H, Nan FY, Kou N, Hong QY, Lv MS, Li JB, Zhang BJ, Zou H, Li L, Wang HW. Establishment of acquired tracheoesophageal fistula using a modified magnetic compression technique in rabbits and its postmodeling evaluation. World J Gastrointest Surg 2024; 16(5): 1385-1394
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1385.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1385
Tracheoesophageal fistula (TEF) is a pathological communication between the esophagus and trachea and is the most common digestive-respiratory tract fistula. Congenital TEF often accompanies esophageal atresia[1,2], while acquired TEF can result from factors such as tumors, surgeries, infections, trauma, or foreign objects[3-11]. Untreated, TEF leads to recurrent pneumonia or malnutrition in a short span[12].
Surgery is the preferred method for treating benign fistulas[13], although it can be traumatic, necessitating multiple surgeries, with feasibility and safety hinging on the patient's condition. Malignant TEF patients may opt for interventional treatment due to overall health constraints[12]. Effective postoperative airway management and complication prevention are essential for therapeutic success[14]. Alternative methods include endoscopic closure, vacuum therapy, fibrin glue, and cardiac occluders, but conclusive research on their clinical efficacy is lacking[12].
Establishing suitable animal models is crucial for exploring minimally invasive treatments. In 2003, Kiyan et al[15] surgically created a rabbit TEF model[15]. However, there have been no subsequent reports of successful surgical TEF model establishment in rabbits. In 2019, Gao et al[16] used a nonsurgical magnetic compression technique in rabbits, achieving a 100% success rate[16]. To further explore the best method for modeling TEF in rabbits and validate its repeatability and stability, our team conducted this study. This involved simplifying some of the original procedures, con
This study was approved by the Ethical Committee of Animal Experiments at Nongnong (Beijing) Biotechnology Co., Ltd. (No. 202307001). Twenty-seven adult New Zealand rabbits (3-4 kg, conventionally graded) from the Beijing Fangyuanyuan Breeding Farm were subjected to one week of adaptive feeding before the experiments (26 °C, 12 h/12 h light/dark, 50% humidity, with ad libitum access to food and water). Twelve rabbits were designated for comparison of the modified magnetic compression (MMC) technique and SM (6 per group). Fifteen rabbits were employed to validate the repeatability and stability of the MMC technique for modeling.
The experimental equipment used in this study included a tracheal intubation device (Guangzhou AMK Equipment Co., Ltd., Guangdong Medical Device Registration No. 20172660258), an X-ray machine (GE OEC Medical Systems, Inc., OEC 9900 Elite), a flexible bronchoscope (Zhejiang UE Medical Corp., EBC-380C), a laryngoscope (Shenzhen Teslong Technology Co., Ltd., TSL2080845), a puncher (Beijing Lab Anim Tech Development Co., Ltd., LAT-DK), nonabsorbable surgical sutures (Johnson & Johnson MedTech, National Medical Device Registration No. 20192022154), an anesthesia machine (Beijing Aerospace Changfeng Co., Ltd., 17-608-10-027), a monitor (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., CM-74153659), and an upside-down integrated fluorescence microscope (Revolve FL microscope, Echo-labs, San Diego, CA, United States).
The experimental reagents included pentobarbital sodium (Shanghai Pharma New Asia Pharma Co., Ltd.), methylene blue (Jumpcan Pharmaceutical Group Co., Ltd.), sodium penicillin for injection (Huabei Pharmaceutical Co., Ltd.), Tolfedine CS (Vétoquinol SA, France), potassium chloride (China Otsuka Pharmaceutical Co., Ltd.), 4% paraformaldehyde fixative solution (Shanghai Beyotime Biotech. Co., Ltd.), and hematoxylin-eosin staining (Shanghai Klamar Reagent Co., Ltd.).
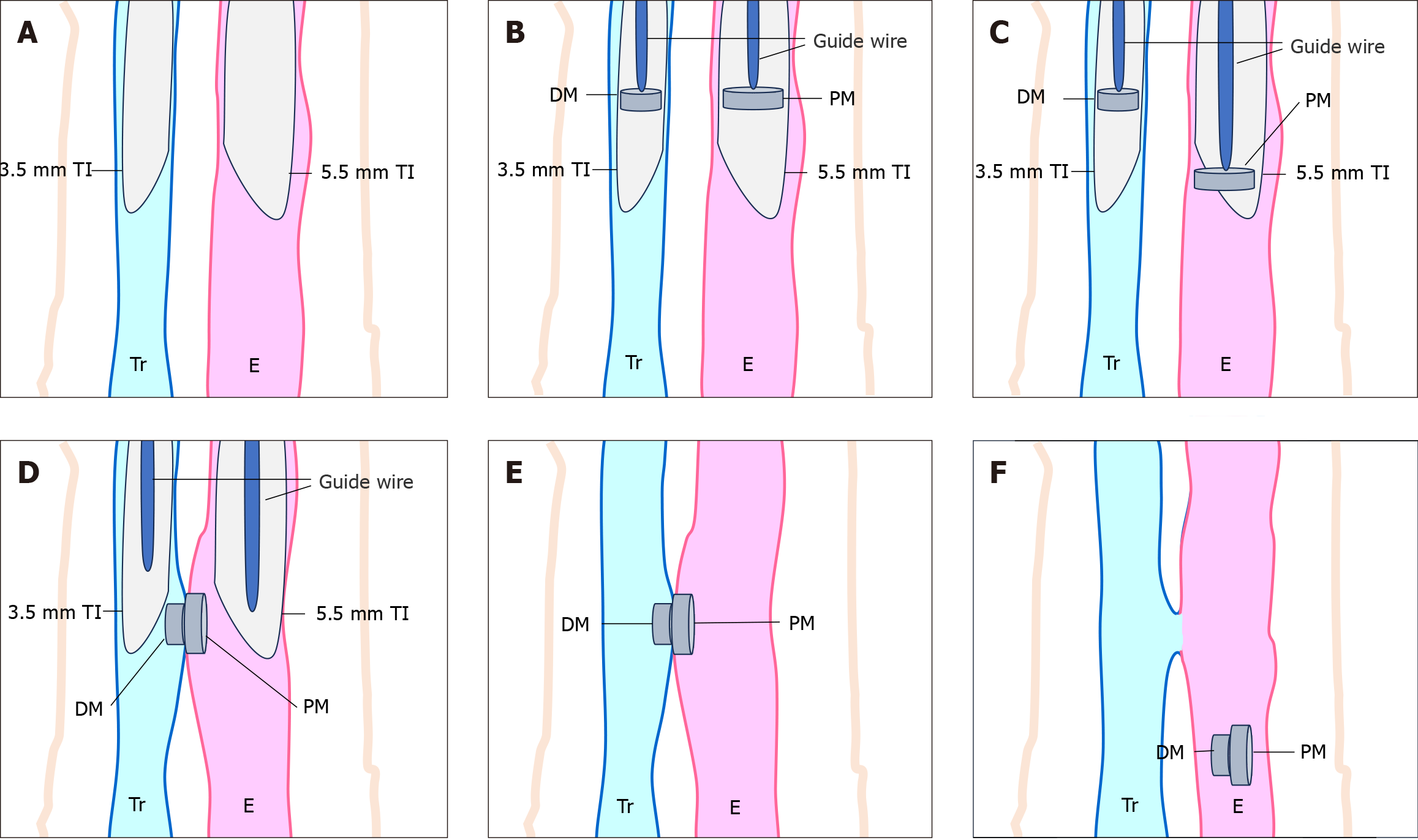
The magnets utilized included a parent magnet (PM, cylinder, diameter 5 mm, thickness 3 mm), a daughter magnet (DM, cylinder, diameter 3 mm, thickness 3 mm), and an anchor magnet (cylinder, diameter 8 mm, thickness 10 mm). All magnets were constructed from N52 NdFeB permanent magnetic material with zinc electroplating for corrosion resistance.
After 12 h of fasting and water restriction, the rabbits were weighed, and blood was collected. Anesthesia was induced via injection of 1.5% pentobarbital sodium (2 mL/kg) through the marginal ear vein. Once the rabbits were anesthetized, they were placed in a supine position and securely fastened to the operating table.
MMC technique (Figure 1): The rabbits' heads were fully extended, and the glottis was visualized using a laryn
SM technique: Rabbits underwent tracheal intubation to provide artificial respiration, and were connected to an ane
After the procedures, each rabbit received prophylactic antibiotics (25000 U/kg) to prevent infection and xylazine (4 mg/kg) for 3 d for pain relief. The model success rate, general observations (food and water intake, respiration, activity level), and survival duration were compared between the groups. To address modeling failures and adverse reactions, the sample size was increased to further clarify the feasibility and success rate of the MMC technique.
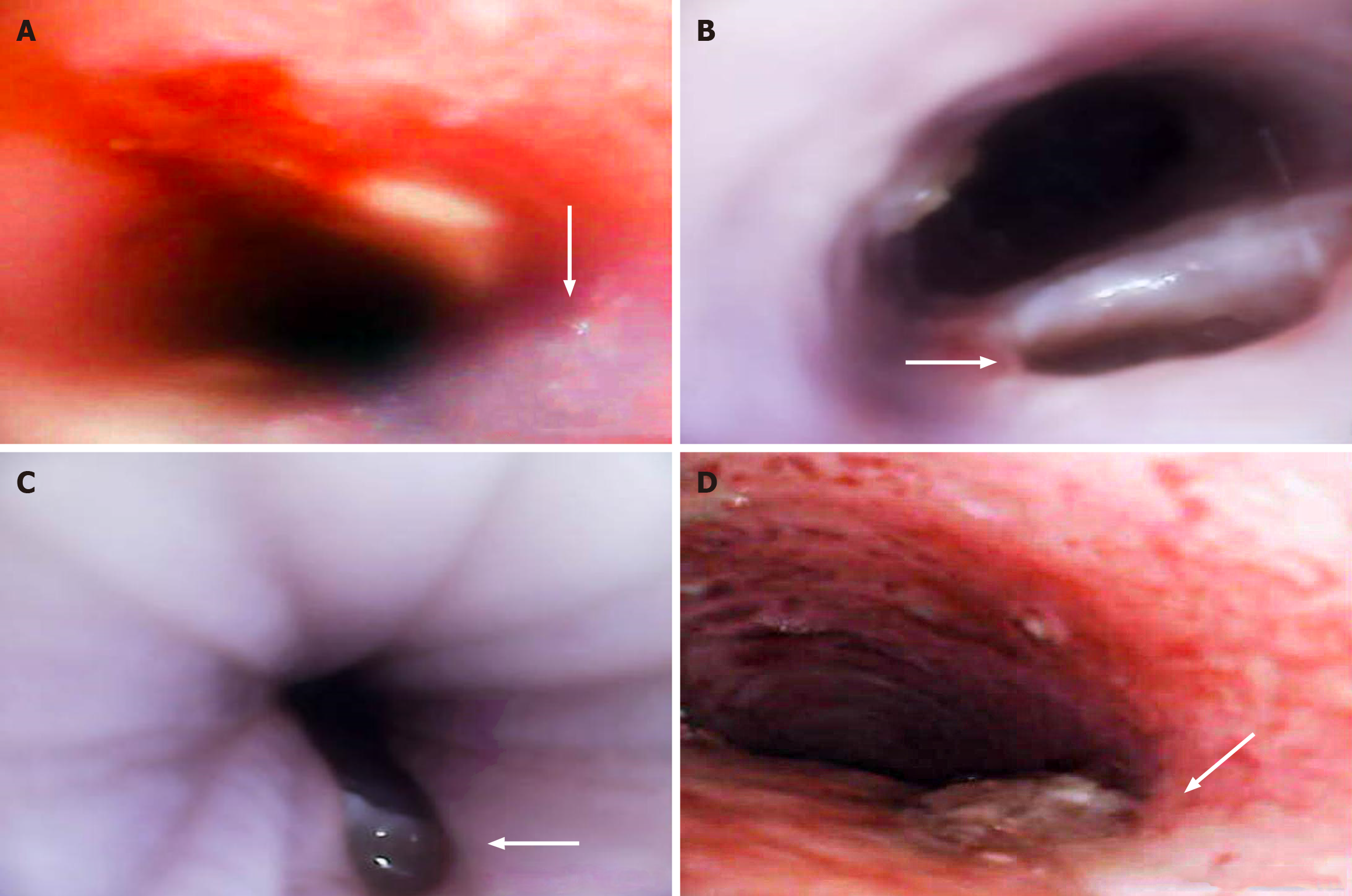
On the 3rd day after successful model establishment, blood samples were collected. After 5 d, the rabbits were weighed, anesthetized with pentobarbital sodium, placed in a supine position, and the morphology of the fistula was observed under a bronchoscope. Subsequently, euthanasia was performed using a large dose of 10% potassium chloride solution, and the fistula tissues were excised. After gross examination, the specimens were fixed in 4% paraformaldehyde, and the sections were stained with hematoxylin and eosin for light microscopy to assess histological changes. In the event of mortality during the experiment, fistula tissues were collected promptly for gross observation and histological analysis. The Smith score method was used for semiquantitative analysis of pulmonary inflammation: No injury (0), lesion range < 25% (1), lesion area 25% Mel 50% (2), lesion range 50% Mel 75% (3), and lesion range > 75% (4). Five nonrepetitive regional visual fields were observed for each animal, and the average value was taken.
Data analysis was conducted using SPSS 26.0 statistical software. Quantitative data are presented as the mean ± SD. Group comparisons were performed using one-way ANOVA for quantitative data. For quantitative data that met the normal distribution, Pearson correlation analysis was employed. Spearman correlation analysis was used to analyze the relationship between the quantitative and qualitative data. A P value less than 0.05 was considered to indicate statistical significance. Graphs and plots were generated using GraphPad Prism 8.0.
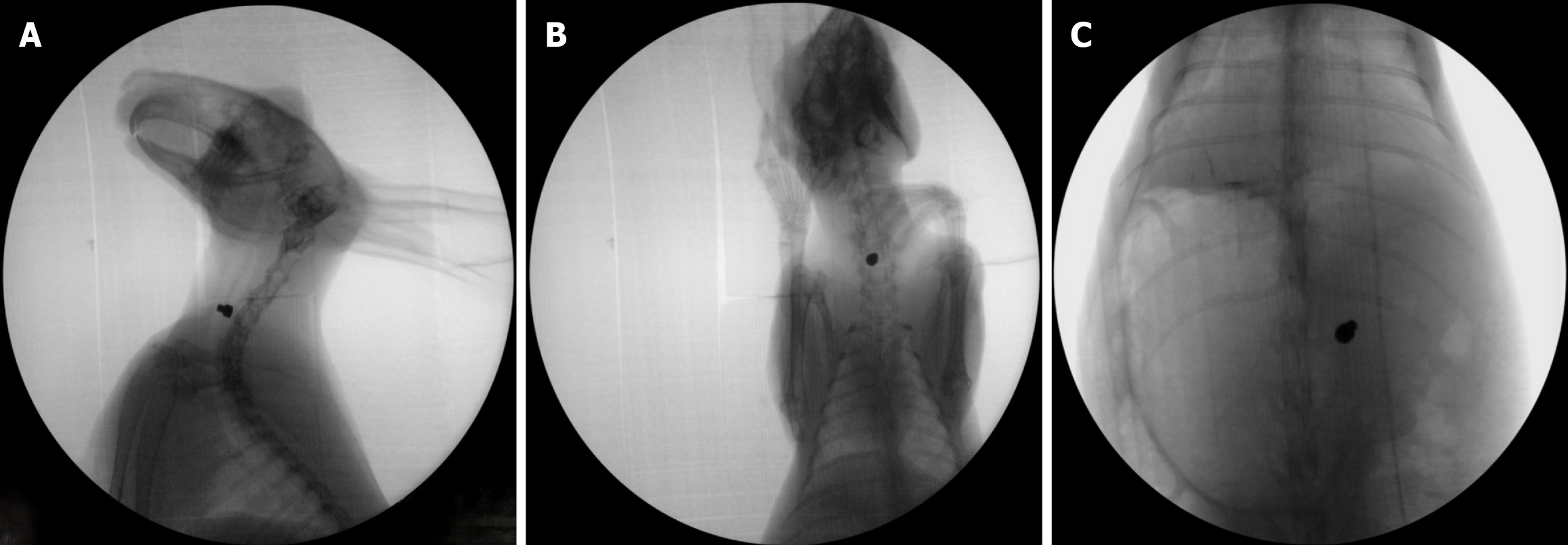
Model conditions: In the MMC group, the average operation time was 3.2 ± 0.6 min. Some rabbits showed reduced food and water intake. One rabbit died on the 6th day. In this rabbit, the magnet was still in the target position, and the tissue between the DM and PM was necrotic, the lungs were congested and the spleen vessels were bruised; changes in the color of the tracheal and esophageal mucosa were also observed (Supplementary Figure 2). Another rabbit experienced breathing difficulties, and bronchoscopy revealed that the magnets had entered the trachea on the 9th day. Attempts to remove the magnets were unsuccessful (Supplementary Figure 3). For the remaining 4 rabbits, the average time for the magnets to move away from the target position was 8.0 ± 1.4 d. These rabbits were able to eat and drink normally and showed good general activity, resulting in a model success rate of 66.7%.
In the SM group, the average operation time was 17.8 ± 3.2 min. The process of establishing the model was uneventful, but the condition of the rabbits deteriorated after model establishment. The rabbits did not eat or drink, showed rapid breathing, and some exhibited labored breathing. Within 3 d of model establishment, 4 rabbits died. One rabbit remained lethargic and did not eat or drink water. The final rabbit gradually improved and exhibited good activity. This group had a model success rate of 33.3%.
After model establishment, the MMC group showed a slight decrease in weight, while the SM group, with only 2 successful model cases, exhibited significant weight loss (Supplementary Table 1). Analysis highlighted a significant correlation (coefficient of 0.828, aP < 0.05) between the premodel weight in the SM group and model success, whereas no such correlation was observed in the MMC group.
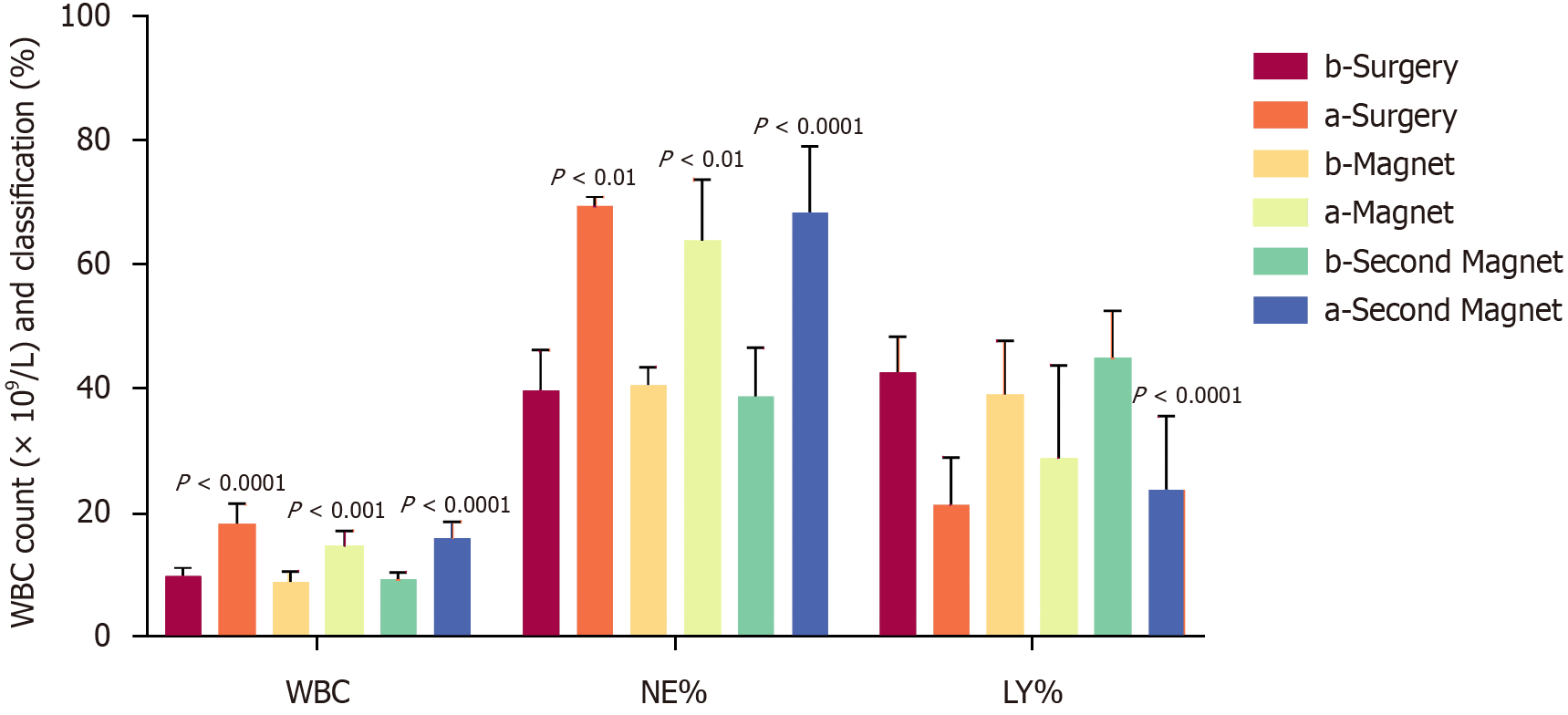
Three days after successful establishment of the model, blood analysis revealed no significant differences in the white blood cell (WBC) count or WBC composition between the two groups. However, both groups exhibited significant diffe
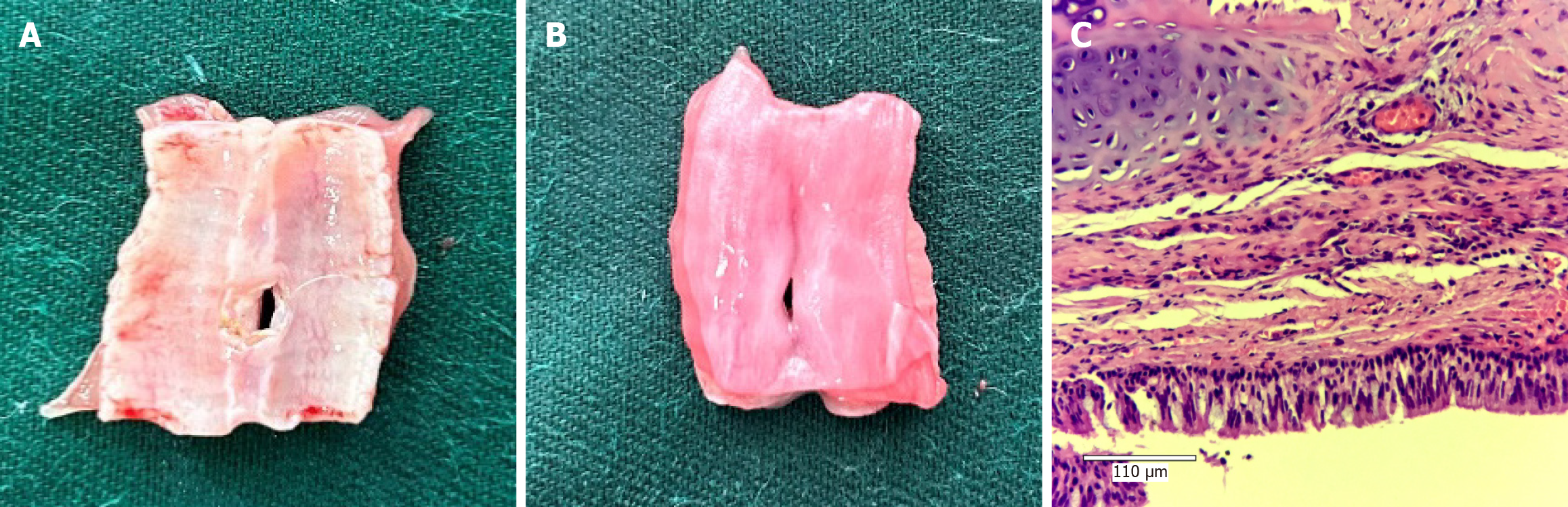
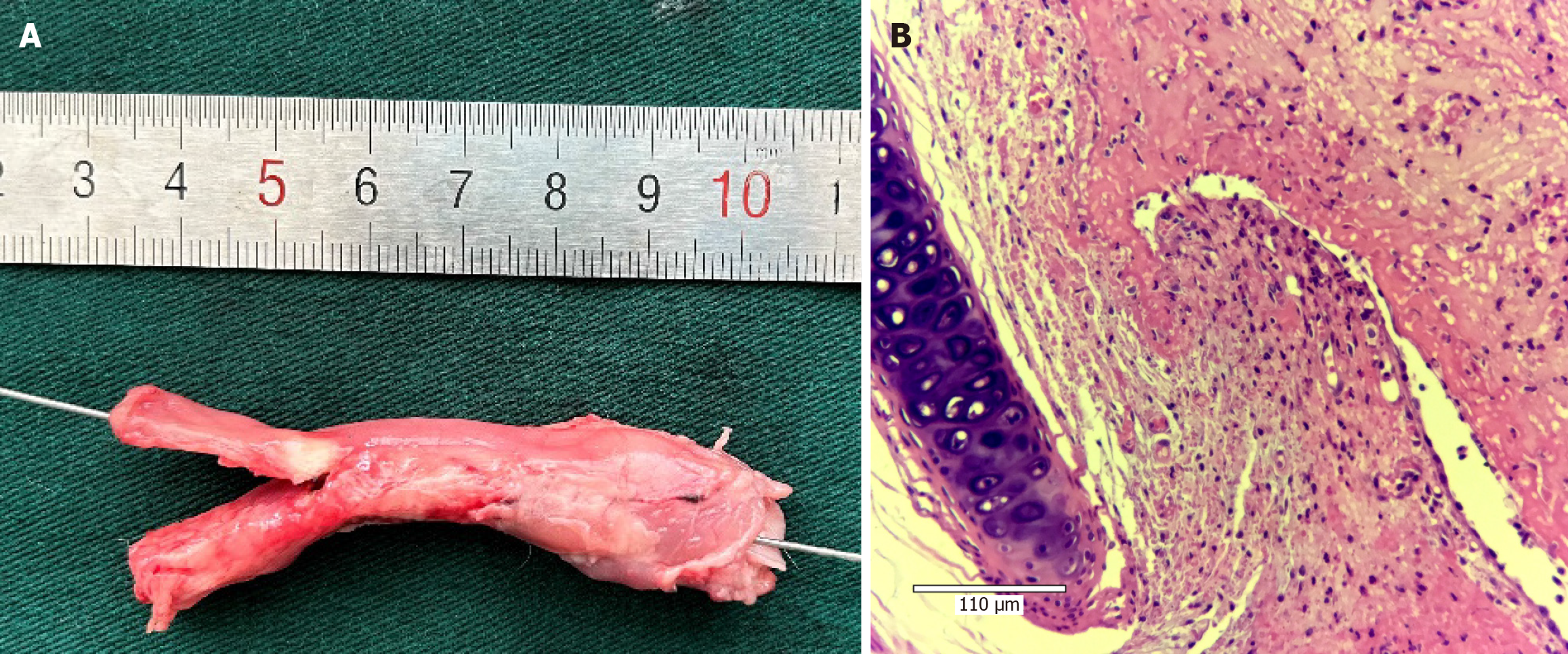
Gross and histological observations: In the MMC group, the tracheal mucosa of the rabbits appeared pale red, and the mucosa around the fistula was smooth. The anatomical structure of the esophagus and trachea was intact, with no signs of adhesions or scar formation. Pathologically, the tracheal mucosa displayed a clear structure with neutrophil infiltration, and the inflammation score of the lung tissue was 2.7 ± 0.7 (Figures 3B, C and 4; Supplementary Figure 4).
Conversely, in the SM group, the tracheal mucosa appeared congested and rough. The junction of the esophagus and trachea exhibited adhesions, and a dirty moss-like appearance was observed on the surface of the fistula. Pathologically, the tracheal mucosa exhibited swelling, a disrupted anatomical structure, and significant neutrophil infiltration. The inflammation score of the lung tissue was 3.6 ± 0.5, which was significantly greater than that of the MMC group (bP < 0.01; Figures 3D and 5; Supplementary Figure 5).
In the second round of MMC modeling, 15 rabbits were employed, with an average weight of 3.458 ± 0.221 kg. The process of model establishment was smoother, averaging 2.1 ± 0.4 min, which was significantly shorter than that of the first round (cP < 0.001). After placing the magnets, some rabbits exhibited reduced food and water intake. One rabbit died on the 6th day, with no apparent changes in the tracheal or esophageal mucosa, lung or spleen. Two rabbits experienced magnet detachment into the trachea; the magnet was successfully removed using an external magnet in one of the rabbits, and the other rabbit died. The model success rate was 86.7%, and the time to magnet detachment averaged 6.2 ± 1.3 d, which was significantly less than that of the first round (dP < 0.05); however, the magnet detachment time was not significantly correlated with body weight, model establishment time, or other factors.
On the 2nd day after successful establishment of the model, one rabbit died; this rabbit exhibited normal during model establishment but displayed decreased food and water intake and lethargy after magnet detachment. Pathological sections revealed tracheal mucosa edema and extensive neutrophil infiltration (Supplementary Figure 6A-C), and pulmonary pathological sections revealed significant neutrophil infiltration, with near complete obstruction of the small and tiny bronchi. The inflammation score of the lung tissue was 2.9 ± 0.8 (Supplementary Figure 6D-F).
On the 5th day after successful establishment of the model, the average body weight of the rabbits was 3.419 ± 0.276 kg, and there were no significant differences in body weight between before and after establishment of the model. Tracheal pathological biopsies revealed a clear mucosal structure with neutrophil infiltration (Supplementary Figure 7).
After modeling, the WBC and neutrophil% increased, while the lymphocyte% decreased; these values were significantly different from those at baseline but were not significantly different from those of the first round (Supplementary Table 2 and Figure 6).
Gao et al[16,17] successfully created TEF models in New Zealand rabbits and beagles using the magnetic compression technique[16,17]. They achieved this by utilizing external anchoring magnets to pinpoint the target location and employed a transportation magnet to introduce the DM and PM into the body. After model establishment, X-rays were taken every other day. As the diameter of the trachea and esophagus in rabbits is relatively small, prolonged modeling procedures could affect ventilation, posing a risk to animal safety. Therefore, we aimed to shorten the model establi
The SM technique is considered one of the most convenient methods for constructing TEF models, and it has been successfully applied to large animals such as small pigs and beagles[18,19]. In 2003, Kiyan et al[15] reported the only known case of a TEF model in rabbits in which a surgical approach was used[15]. The experimental results indicate that the success rate of SM is only 33.3%, which is half that of MMC. Although 2 rabbits successfully underwent the SM procedure, significant weight loss occurred, and one of the rabbits may not have been able to be used for subsequent experiments due to its short survival time. Moreover, SM requires a high level of surgical expertise. While the use of a puncher may ensure a consistent fistula size between the trachea and esophagus, the suturing process might alter the original anatomical relationship of the two structures and the size of the fistula. Surgical procedures can be highly stressful for experimental animals, affecting their postoperative food and water intake. Since providing long-term nutritional support for rabbits can be challenging, this approach increases the experimental difficulty. If SM is chosen, it is recommended that rabbits with greater starting weights be selected because these rabbits may have greater stress tolerance.
The MMC technique has a minimal impact on food and water intake, causes only mild local inflammatory reactions, and is suitable for conducting follow-up research. However, unexpected deaths occurred during the initial round of model establishment, which has not been reported before. This incident might be related to corrosion of the magnets within the body, which the organism could not tolerate. NdFeB magnets are prone to oxidation and have poor corrosion resistance. Although the magnets are zinc-plated, they may still experience varying degrees of corrosion in complex biological environments. Research has shown that magnets coated with epoxy resin, a highly polymeric material, exhibit the highest resistance to gastric juice corrosion[20].
Additionally, rabbits typically use all four limbs for movement, and their trachea is situated below the esophagus when in a normal position. When the DM and PM are dislodged from their target location, certain movements, such as neck extension and jumping, could lead to a misalignment or tilting of the PM. This may cause it to slide out of the esophageal smooth muscle tissue and enter the trachea under the influence of gravity, causing airway obstruction. The combined thickness of the DM and PM is 6 mm, and when combined with secretions and necrotic mucosal tissue, there is a potential for complete blockage of the airway, leading to rapid asphyxiation. Although the rabbits were promptly identified when they were experiencing respiratory distress, the unforeseeable nature of this situation delayed intervention and resulted in death.
WBC count analysis for both methods revealed that both techniques could lead to infectious diseases associated with TEF, such as pneumonia. However, the surgical group cannot avoid the infection or inflammation associated with the surgical procedure. In summary, compared to the SM method, the MMC method is simpler, more practical, and induces less stress response. The MMC technique is conducive to the experimental exploration of treatment methods for TEF in animals.
Due to the number of failed cases and to further verify the repeatability and stability of the MMC technique, we increased the sample size and continued our research. Nevertheless, the success rate of the second round of model establishment still did not reach 100%, and similar incidents were encountered. However, based on the experience gained from the first round of modeling, we were fortunate to save one rabbit by removing the magnets that had fallen into the trachea, and it subsequently remained in good condition. Unfortunately, another rabbit suffocated at midnight, as its respiratory issues were not detected in time. Upon necropsy of the rabbits that did not survive the establishment of the model, no significant pathological changes were observed, and the tracheal and esophageal mucosa appeared normal. The exact cause of death remains unclear. The analysis indicated that the modeling success rate was not significantly correlated with the baseline weight or weight changes. The incidences of unexpected death or entry of the magnets into the trachea seemed to be random. A rabbit’s weight gradually increases with age, and the diameter of the trachea and esophagus also increases accordingly. This allows for placement of a larger PM, increasing the diameter difference between the DM and PM, which may reduce the occurrence of airway obstruction.
Our work showcases the potential of the MMC technique as a reliable and efficient method for creating TEF models, which can facilitate research on therapeutic interventions, airway management, and the development of novel endoscopic procedures. In clinical practice, patients with TEF often remain asymptomatic following the formation of a fistula. These patients cannot seek immediate medical attention, and many present with symptoms of pneumonia, such as cough, sputum production, and fever, after food or drink enters the airway. Diagnosis typically requires a chest computed tomography scan, gastrointestinal contrast, or bronchoscopy. Among various treatment options, the use of anti-infective therapy is essential[12]. During the second round, it is understandable that one rabbit died on the 2nd day after successful model establishment. Due to the presence of the fistula, food and water can enter the trachea, leading to pneumonia and, in severe cases, systemic inflammatory responses that can be life-threatening. Although the magnets were successfully removed from the target site, the rabbit could not be used for subsequent experiments. Specifically, the success rate of the second round of MMC model established was 80%. Analysis of the WBC count and classification indicated that the rabbits developed infections related to TEF, with no significant difference compared to the first round of modeling. Therefore, it is recommended that animals be observed for several days after successful model establishment before the intervention treatment is employed to simulate the actual clinical diagnosis and treatment.
Acquired malignant TEF is more common in the clinic and significantly impacts the survival and quality of life of patients with esophageal cancer and other malignancies. Using a transplantable tumor animal model to establish a fistula may involve the local injection of tumor cells to simulate the response of a TEF to various treatment methods in the tumor microenvironment. Nevertheless, this method still has several limitations. First, animals are not immune deficient, and their immune system may eliminate the tumor cells. Second, the time required for tumor growth is relatively long, and the criteria for assessing the success of a malignant TEF model need further clarification. Third, the timing of tumor and fistula model establishment raises questions. If the fistula is created first, the waiting time for tumor growth may be extended, potentially increasing mortality without therapeutic intervention. Conversely, if the tumor model is established first, inserting the magnets may be more difficult. Tumor invasion can render the esophageal mucosa fragile and prone to ulceration, increasing the occurrence of adverse reactions such as magnets falling into the trachea. Therefore, further theoretical exploration and practical research are needed to address these issues.
In summary, the MMC method offers the advantages of being noninvasive, precise, and simple, making it more suitable than the SM technique for the establishment of TEF models in small animals. However, it is important to be aware of potential complications that may arise during the magnetic compression procedure, such as magnet blockage in the trachea. These complications can be managed by adjusting the diameter difference between the DM and PM and promptly addressing any issues that arise, which can help improve the model success rate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Ciarambino T, Italy; El-Arabey AA, Egypt S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | Singleton AO, Knight MD. Congenital Atresia of the Esophagus with Tracheo-Esophageal Fistulae: Transpleural Operative Approach. Ann Surg. 1944;119:556-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Jung E. Minimally invasive management of combined esophageal atresia with tracheoesophageal fistula and duodenal atresia: a comprehensive case report. Front Pediatr. 2023;11:1252660. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Altokhais T. Magnet Ingestion in Children Management Guidelines and Prevention. Front Pediatr. 2021;9:727988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Santosham R. Management of Acquired Benign Tracheoesophageal Fistulae. Thorac Surg Clin. 2018;28:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Krom H, Visser M, Hulst JM, Wolters VM, Van den Neucker AM, de Meij T, van der Doef HPJ, Norbruis OF, Benninga MA, Smit MJM, Kindermann A. Serious complications after button battery ingestion in children. Eur J Pediatr. 2018;177:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Shamji FM, Inculet R. Management of Malignant Tracheoesophageal Fistula. Thorac Surg Clin. 2018;28:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Hagens ERC, Anderegg MCJ, van Berge Henegouwen MI, Gisbertz SS. International Survey on the Management of Anastomotic Leakage After Esophageal Resection. Ann Thorac Surg. 2018;106:1702-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008;34:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Diddee R, Shaw IH. Acquired tracheo-oesophageal fistula in adults. BJA Educ. 2006;6:105-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Couraud L, Ballester MJ, Delaisement C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg. 1996;8:392-399. [PubMed] |
| 11. | Burt M, Diehl W, Martini N, Bains MS, Ginsberg RJ, McCormack PM, Rusch VW. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg. 1991;52:1222-8; discussion 1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 137] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Wang H, Li W, Wang Z, Chen L, Lai G, Jin F, Ke M, Sun J, Zhang J, Xie B, Zhang N, Zhou H, Wang X, Lin D, Zhou Y, Zhang H, Li D, Wang C, Song X, Wang J, Wu S, Yang J, Zhang L, Tao M, Zeng Y, Zou H, Li H, Song F, Sha Z, Tan Q, Cong M, Shi H, Han X, Luo L, Ma H, Wu G, Liu X, Wu W, Ye Y, Zhu G. Chinese expert consensus on interventional diagnosis and management of acquired digestive-respiratory tract fistulas (second edition). Clin Respir J. 2023;17:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Bibas BJ, Cardoso PFG, Minamoto H, Pêgo-Fernandes PM. Surgery for intrathoracic tracheoesophageal and bronchoesophageal fistula. Ann Transl Med. 2018;6:210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Zhou C, Hu Y, Xiao Y, Yin W. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis. 2017;11:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Kiyan G, Dağli TE, Tuğtepe H, Kodalli N. Double balloon esophageal catheter for diagnosis of tracheo-esophageal fistula. Eur Radiol. 2003;13:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Gao HM, Qiu ML, Zhang YC, Liu H, Ma SJ, Fu S, Lv Y, Yan XP. Establishment of acquired tracheoesophageal fistula by ultra-minimally invasive magnetic compression technique in rabbits. Zhongguo Linchuang Jiepouxue Zazhi. 2019;37:223-227. [DOI] [Full Text] |
| 17. | Gao Y, Wu RQ, Lv Y, Yan XP. Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula. World J Gastroenterol. 2019;25:4213-4221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 18. | Yang G, Zhou YA, Bai GZ, Han Y, Li WM, Wang J, Li XF, Yan XL. Effect of cauterizing esophageal mucosa in "double-patch" treatment for acquired benign tracheoesophageal fistula/bronchogastric stump fistula. J Surg Res. 2017;209:1-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Wagner HJ, Stinner B, Barth P, Klose KJ. Are covered stents really effective at closing esophagotracheal fistulas? Results of an animal study. Cardiovasc Intervent Radiol. 2000;23:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Tian BY, Liu H, Fu S, Ma SJ, Zhang YC, Qiu ML, Deng B, Lei L, Ye D, Gao HM, Li YX, Lv Y, Yan XP. In Vitro Study on Corrosion Resistance of Different Surface Modified NdFeB Magnets in Gastric Juice. Zhongguo Yieliao Shebei. 2019;34:5-8. [DOI] [Full Text] |