Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1311
Revised: January 18, 2024
Accepted: April 3, 2024
Published online: May 27, 2024
Processing time: 156 Days and 8.2 Hours
Laparoscopic gastrectomy for esophagogastric junction (EGJ) carcinoma enables the removal of the carcinoma at the junction between the stomach and esophagus while preserving the gastric function, thereby providing patients with better treatment outcomes and quality of life. Nonetheless, this surgical technique also presents some challenges and limitations. Therefore, three-dimensional recon
To discuss the application and advantages of 3D RVT in precise laparoscopic resection of EGJ carcinomas.
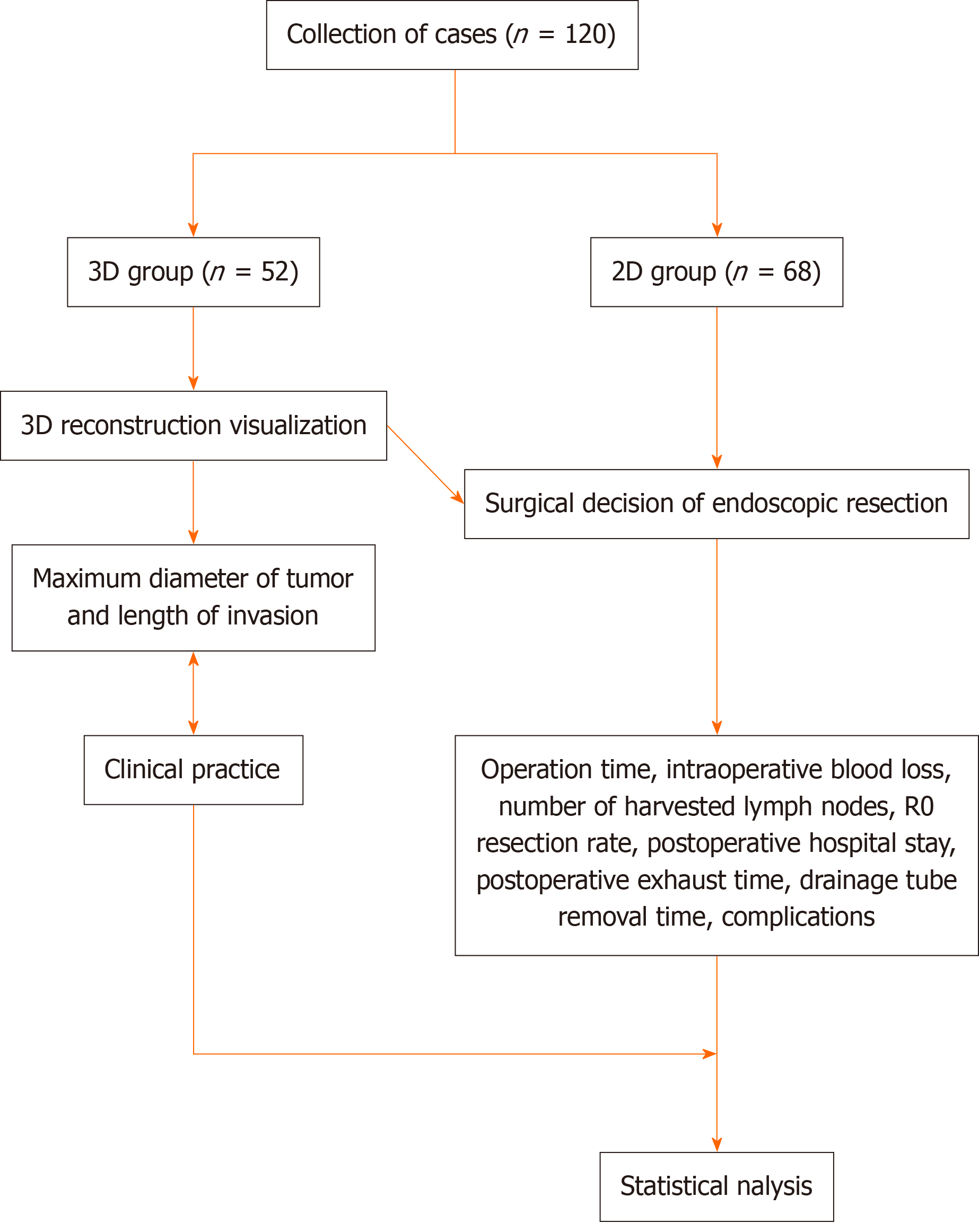
Data were obtained from the electronic or paper-based medical records at The First Affiliated Hospital of Hebei North University from January 2020 to June 2022. A total of 120 patients diagnosed with EGJ carcinoma were included in the study. Of these, 68 underwent laparoscopic resection after computed tomography (CT)-enhanced scanning and were categorized into the 2D group, whereas 52 underwent laparoscopic resection after CT-enhanced scanning and 3D RVT and were categorized into the 3D group. This study had two outcome measures: the deviation between tumor-related factors (such as maximum tumor diameter and infiltration length) in 3D RVT and clinical reality, and surgical outcome indicators (such as operative time, intraoperative blood loss, number of lymph node dissections, R0 resection rate, post
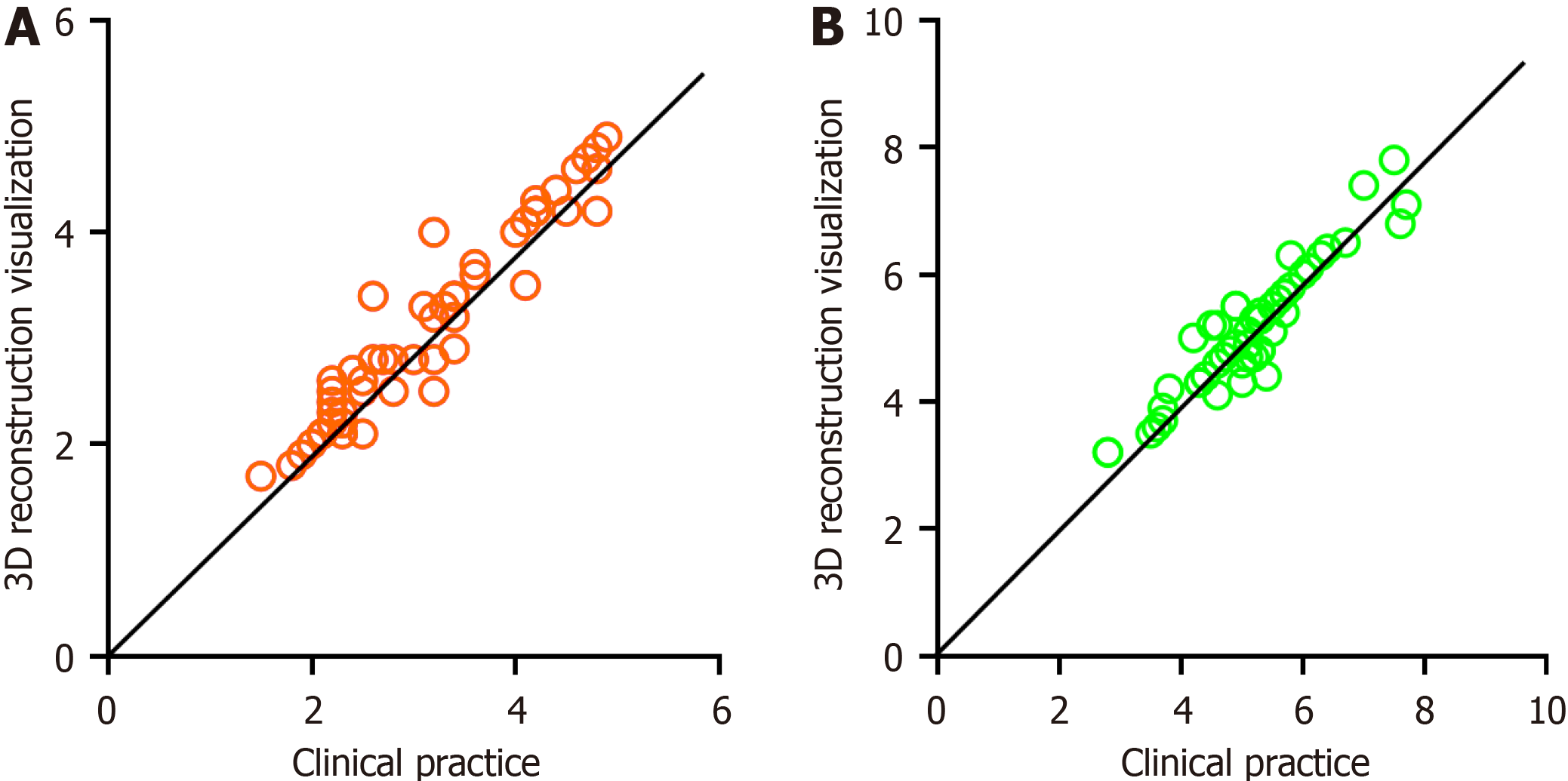
Among patients included in the 3D group, 27 had a maximum tumor diameter of less than 3 cm, whereas 25 had a diameter of 3 cm or more. In actual surgical observations, 24 had a diameter of less than 3 cm, whereas 28 had a diameter of 3 cm or more. The findings were consistent between the two methods (χ2 = 0.346, P = 0.556), with a kappa consistency coefficient of 0.808. With respect to infiltration length, in the 3D group, 23 patients had a length of less than 5 cm, whereas 29 had a length of 5 cm or more. In actual surgical observations, 20 cases had a length of less than 5 cm, whereas 32 had a length of 5 cm or more. The findings were consistent between the two methods (χ2 = 0.357, P = 0.550), with a kappa consistency coefficient of 0.486. Pearson correlation analysis showed that the maximum tumor diameter and infiltration length measured using 3D RVT were positively correlated with clinical observations during surgery (r = 0.814 and 0.490, both P < 0.05). The 3D group had a shorter operative time (157.02 ± 8.38 vs 183.16 ± 23.87), less intraoperative blood loss (83.65 ± 14.22 vs 110.94 ± 22.05), and higher number of lymph node dissections (28.98 ± 2.82 vs 23.56 ± 2.77) and R0 resection rate (80.77% vs 61.64%) than the 2D group. Fur
Using 3D RVT, doctors can gain a more comprehensive and intuitive understanding of the anatomy and related lesions of EGJ carcinomas, thus enabling more accurate surgical planning.
Core Tip: Three-dimensional reconstruction visualization technology (3D RVT) provides a more comprehensive and intuitive understanding of the anatomy and related lesions at the gastroesophageal junction. This study compared the 2D group, which underwent laparoscopic resection surgery after computed tomography (CT)-enhanced scanning, with the 3D group, which underwent laparoscopic resection surgery after CT-enhanced scanning and 3D RVT. Our findings highlight the benefits of using 3D RVT to improve surgical outcomes and reduce complications in patients with gastroesophageal junction cancer.
- Citation: Guo D, Zhu XY, Han S, Liu YS, Cui DP. Evaluating the use of three-dimensional reconstruction visualization technology for precise laparoscopic resection in gastroesophageal junction cancer. World J Gastrointest Surg 2024; 16(5): 1311-1319
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1311.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1311
Esophagogastric junction (EGJ) carcinoma is a malignant tumor that occurs at the junction of the esophagus and stomach[1]. Current research has indicated a global increase in the incidence of EGJ carcinoma[2], with a nearly 2.5-fold increase since the early 1970s[3]. The development of EGJ carcinoma is complex and associated with various factors, such as diet, smoking, alcohol consumption, chronic gastritis, and Helicobacter pylori infection.
Due to its unique anatomical location and highly invasive nature, it is the primary choice for most patients with EGJ carcinomas to achieve long-term survival. Compared with traditional open surgery, laparoscopic resection offers ad
With the advancement of medical imaging technology, three-dimensional reconstruction visualization technology (3D RVT) has become increasingly important for accurately assessing tumors before surgery, planning individualized surgical approaches, and facilitating the application of minimally invasive laparoscopic techniques in the treatment of EGJ car
With the emergence of clinical techniques and the widespread use of 3D RVT, studies have explored their application in preoperative evaluation, treatment planning, intraoperative complication guidance, and postoperative recovery for various diseases such as liver[6] and gastric cancers[7]. However, to the best of our knowledge, research on the use of 3D RVT in the context of EGJ carcinomas is limited. Comprehensive research on the application of this technology in the preoperative planning of EGJs is crucial. Therefore, this study aimed to evaluate the application and advantages of 3D RVT in precise laparoscopic resection of EGJ carcinomas.
This study enrolled patients with EGJ carcinoma who were hospitalized at The First Affiliated Hospital of Hebei North University from January 2020 to June 2022. Data were obtained from electronic or paper-based medical records.
The inclusion criteria were as follows: Age between 18 and 75 years; preoperative diagnosis of Siewert type II or III EGJ carcinoma confirmed by gastroscopy, abdominal plain and enhanced computed tomography (CT) scan, and magnetic resonance imaging (MRI) with preoperative gastroscopic biopsy[8]; postoperative pathological confirmation of EGJ car
The exclusion criteria were as follows: severe comorbidities, such as severe heart disease and liver or kidney failure; advanced or distant metastasis before surgery; a tumor diameter of 10 cm or more; severe adhesions in the abdominal cavity discovered during surgery, with tumor infiltration into other organs; patients who received other treatment moda
According to different preoperative imaging guidelines for surgical decision-making, the 3D group (n = 52) underwent laparoscopic resection surgery based on 3D RVT after CT-enhanced scanning, while the 2D group (n = 68) underwent laparoscopic resection surgery after CT-enhanced scanning.
2D group: Before surgery, patients underwent comprehensive physical examinations and evaluations, including gas
3D group: 3D reconstruction was performed using Mimics software (Materialise Corp., Belgium), which is an interactive medical image-control system. The CT-enhanced scan data of the EGJ were saved as “DICOM” format files and imported into the 3D visualization software for editing and optimization of the 3D model. In the 3D visualized model of re
Observation indicators included the deviation between tumor-related information based on 3D RVT and clinical practice. Indicators included the maximum tumor diameter and infiltration length. It also included a comparison of surgical outcomes between the 3D and 2D groups.
Intraoperative indicators were the operative time, intraoperative blood loss, number of lymph node dissections, and R0 resection rate. Postoperative indicators were the postoperative hospital stay, postoperative gas evacuation time, drainage tube removal time, and complications such as incision infection, anastomotic fistula, lung infection, adhesive intestinal obstruction, and anastomotic bleeding.
Statistical analyses were performed using SPSS software version 32.0 (IBM Corp., New York, United States). Normally distributed continuous data were presented as mean ± SD; a t-test was used for analysis when comparing two groups. Non-normally distributed continuous data were expressed as interquartile ranges (IQRs); the Mann-Whitney U test was used for analysis when comparing two groups. Categorical data were reported as frequencies and percentages, and the χ2 test was used for analysis when comparing two or more groups. The kappa test was used for consistency analysis, with higher values indicating better consistency. In this study, a Pearson’s correlation analysis was employed. A P value of less than 0.05 was considered statistically significant.
This study consisted of two parts: the deviation between tumor-related information based on 3D RVT and clinical practice and the surgical outcome indices between the 3D and 2D groups (Figure 1).
A total of 112 patients were enrolled in the study. There were no statistically significant differences between the clinical characteristics of the two groups (all P > 0.05), suggesting that the data were well-balanced and the study results were reliable and comparable (Table 1).
| Variables | 3D group (n = 52) | 2D group (n = 68) | χ2/t value | P value |
| Age (yr, mean ± SD) | 51.96 ± 8.30 | 50.28 ± 9.10 | 1.042 | 0.300 |
| BMI (kg/m2, mean ± SD) | 21.68 (19.62, 24.92) | 22.06 (20.45, 23.92) | -0.172 | 0.863 |
| Sex | 2.631 | 0.105 | ||
| Male | 38 (73.08) | 40 (58.82) | ||
| Female | 14 (26.92) | 28 (41.18) | ||
| Pathological pattern | 0.502 | 0.778 | ||
| Squamous cell carcinoma | 15 (28.85) | 18 (26.47) | ||
| Adenocarcinoma | 24 (46.15) | 29 (42.65) | ||
| Other | 13 (25.00) | 21 (30.88) | ||
| Helicobacter pylori infection | 0.900 | 0.343 | ||
| Yes | 12 (23.08) | 21 (30.88) | ||
| No | 40 (76.92) | 47 (69.12) | ||
| Siewert type | 1.222 | 0.269 | ||
| II | 23 (44.23) | 37 (54.41) | ||
| III | 29 (55.77) | 31 (45.59) | ||
| Scope of resection | 0.351 | 0.554 | ||
| Whole stomach | 27 (51.92) | 39 (57.35) | ||
| Proximal stomach | 25 (48.08) | 29 (42.65) | ||
| Operative route | 0.2115 | 0.643 | ||
| Transthoracic approach | 35 (67.31) | 43 (63.24) | ||
| Transabdominal | 17 (32.69) | 25 (36.76) | ||
| Maximum tumor diameter | 0.543 | 0.461 | ||
| < 3 cm | 24 (46.15) | 36 (52.94) | ||
| ≥ 3 cm | 28 (53.85) | 32 (47.06) | ||
| Infiltration length | 1.211 | 0.271 | ||
| < 5 cm | 20 (38.46) | 33 (48.53) | ||
| ≥ 5 cm | 32 (61.54) | 35 (51.47) |
Among 52 patients who underwent 3D RVT, 27 had a maximum tumor diameter of less than 3 cm, whereas 25 had a maximum diameter of 3 cm or more. In actual surgical observations, 24 patients had a maximum tumor diameter of less than 3 cm, whereas 28 had a maximum diameter of 3 cm or more. For the infiltration length in the 3D RVT, 23 patients had a length of less than 5 cm, while 29 had a length of 5 cm or more. In the actual surgical observations, 20 had a length of less than 5 cm, while 32 had a length of 5 cm or more. These findings were consistent between the two methods (P > 0.05). The kappa consistency coefficients were 0.808 and 0.486, respectively. Pearson’s correlation analysis showed a positive correlation, with a coefficient of 0.814 for one set and 0.490 for the other set (Table 2 and Figure 2).
| 3D (n = 52) | Clinical practice (n = 52) | χ2/t value | P value | |
| Maximum tumor diameter | 0.346 | 0.556 | ||
| < 3 cm | 27 (51.92) | 24 (46.15) | ||
| ≥ 3 cm | 25 (48.08) | 28 (53.85) | ||
| Infiltration length | 0.357 | 0.550 | ||
| < 5 cm | 23 (44.23) | 20 (38.46) | ||
| ≥ 5 cm | 29 (55.77) | 32 (61.54) |
The 3D model group had a shorter operative time and less intraoperative blood loss than the 2D model group; however, the number of lymph node dissections and the R0 resection rate were higher in the 3D model group (P < 0.05) (Table 3).
| Variables | 3D group (n = 52) | 2D group (n = 68) | χ2/t value | P value |
| Operative time (min) | 157.02 ± 8.38 | 183.16 ± 23.87 | -8.381 | < 0.001 |
| Intraoperative blood loss (mL) | 83.65 ± 14.22 | 110.94 ± 22.05 | -8.212 | < 0.001 |
| Number of lymph node dissections (n) | 28.98 ± 2.82 | 23.56 ± 2.77 | 10.539 | < 0.001 |
| R0 resection rate (%) | 80.77% (42/52) | 61.64% (42/68) | 5.068 | 0.024 |
The 3D group had a significantly shorter hospitalization time, exhaust time, and drainage tube removal time than the 2D group (P < 0.05) (Table 4).
| Variables | 3D group (n = 52) | 2D group (n = 68) | Z value | P value |
| Postoperative hospital stay (d, IQR) | 8 (8, 9) | 13 (14, 16) | -9.341 | < 0.001 |
| Postoperative gas evacuation time (d, IQR) | 3 (3, 4) | 4 (5, 5) | -7.402 | < 0.001 |
| Drainage tube removal time (d, IQR) | 4 (4, 5) | 6 (6, 7) | -8.413 | < 0.001 |
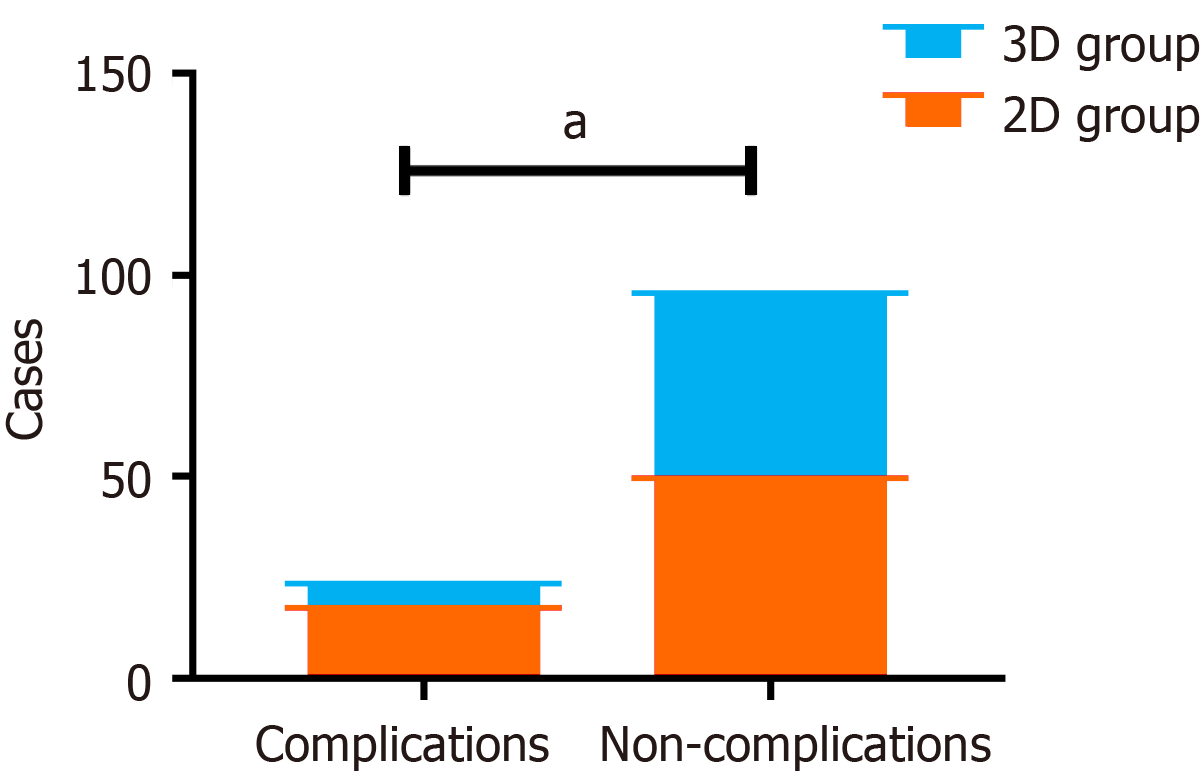
A total of 18 patients in the 2D group experienced complications such as incision infection, anastomotic fistula, lung infection, adhesive intestinal obstruction, and anastomotic bleeding. Six patients in the 3D group developed post
The factors influencing radical resection of EGJ carcinomas can be summarized as anatomical, physiological, and biological characteristics of the tumor and choice of surgical approach. The anatomical location of the EGJ determines the difficulty and feasibility of the surgery. The biological properties of the tumor such as tumor size, extent of invasion, and lymph node metastasis also influence the feasibility of surgery. Moreover, the choice of surgical approach and the specific location of the tumor has a significant impact on its complete resection, radical lymph node clearance, and gastro
Since the 1970s, new imaging technologies have provided effective methods for observing tissue and organ function. Traditional 2 imaging methods lack the ability to provide precise preoperative assessment and planning, limiting their use in qualitative disease analysis. In contrast, 3D reconstruction utilizes computer image processing technology to analyze, calculate, segment, extract, and merge data from 2D imaging. This advanced technology offers accurate and visually intuitive data support, thereby enabling improved surgical planning and evaluation[5,9,10]. 3D reconstruction uses computer image-processing technology to analyze, calculate, segment, extract, and fuse traditional 2D imaging data. More importantly, this technology provides virtual surgical demonstration and 3D image measurement capabilities, allowing surgeons to use medical 3D surgical simulations, intraoperative navigation systems, and other operating sys
The results of this study indicate that 3D RVT provides accurate measurements of tumor characteristics and extent of infiltration, which is consistent with actual surgical observations. This is in line with previous studies[13] suggesting that 3D RVT have high reliability and accuracy in tumor diagnosis and surgical planning. Zeng et al[14] demonstrated that preoperative 3D RVT can shorten surgical time and reduce intraoperative blood loss. These positive outcomes can be attributed to the improved localization of tumors and enhanced understanding of their interactions with the surrounding tissues provided by 3D reconstruction[14,15]. We observed similar results in this study, with the 3D group exhibiting a shorter operative time, less intraoperative blood loss, a higher R0 resection rate, and a lower incidence of complications. This confirms the guiding and predictive capabilities of 3D RVT. Possible reasons for these findings include the fact that 3D RVT can provide more comprehensive anatomical information and allow for virtual surgical rehearsals preope
A meta-analysis conducted on the use of 3D RVT in tumor surgery demonstrated significant reductions in operative time, intraoperative blood loss, and incidence of complications[16]. In addition, previous studies on liver cancer and hilar cholangiocarcinoma showed that 3D RVT improved the accuracy and safety of surgery while reducing intraoperative blood loss and complications, shortening the postoperative intestinal gas discharge time, and increasing the rates of R0 resection and lymph node dissection[17,18]. In our study, the incidence of complications was lower in the 3D group than in the 2D group (11.54% vs 26.47%). This finding may be attributed to the clearer and more stereoscopic views of anatomical structures provided by 3D RVT, which aided the surgeons to more accurately evaluate the surgical area prior to surgery and formulate more precise surgical plans, thereby reducing intraoperative accidents and complications. Additionally, 3D RVT enhanced the accuracy of surgery, potentially shortening the operative time, reducing surgical trauma, and lowering the risk of infection and complications. Patients recovered faster, leading to a shorter hospital stay, improved quality of life, and reduced postoperative discomfort and long-term health problems. Nevertheless, other studies have reported inconsistent results. A study on the repair of total extraperitoneal inguinal hernia reported that the use of 3D RVT did not significantly reduce the operative time, hospital stay, or pain score; however, there were fewer peritoneal tears[19]. Another study on complex lower lobe resection showed that the use of 3D RVT did not significantly affect intraoperative blood loss, postoperative drainage volume, postoperative hospital stay, pneumonia/pulmonary atelectasis, and hemoptysis but shortened the operative time[20]. This may be due to differences in tumor types and surgical procedures. We believe that the application prospects of 3D RVT in tumor surgery are very broad but require more time and financial investment. Moreover, for some complex surgeries, the effect of 3D RVT may not be as expected. Further research is required to confirm its application in surgeries of different types and complexities. Simultaneously, we need to explore more surgical techniques and methods to further improve the safety and accuracy of surgery.
This study had certain limitations that should be acknowledged. First, the retrospective design restricted the explo
The findings of this study align with those of previous studies and provide additional evidence for the efficacy of 3D RVT in surgery for EGJ carcinomas.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Guilford P, New Zealand S-Editor: Wang JL L-Editor: A P-Editor: Xu ZH
| 1. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 914] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1797] [Article Influence: 449.3] [Reference Citation Analysis (1)] |
| 3. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4901] [Article Influence: 700.1] [Reference Citation Analysis (1)] |
| 4. | Jung MK, Schmidt T, Chon SH, Chevallay M, Berlth F, Akiyama J, Gutschow CA, Mönig SP. Current surgical treatment standards for esophageal and esophagogastric junction cancer. Ann N Y Acad Sci. 2020;1482:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Safhi AY. Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 6. | Bonomi AM, Kersik A, Bracchetti G, Cotsoglou C. 3D reconstruction in complex parenchymal sparing liver surgery. Heliyon. 2023;9:e13857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Liu H, Wang F, Liu B, Zheng Z, Zhao J, Zhang J. Application of three-dimensional reconstruction with a Hisense computer-assisted system in upper pancreatic lymph node dissection during laparoscopic-assisted radical gastrectomy. Asian J Surg. 2021;44:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Li Y, Li J. Two updates on oesophagogastric junction adenocarcinoma from the fifth WHO classification: Alteration of definition and emphasis on HER2 test. Histol Histopathol. 2021;36:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Rocha-Júnior E, Pêgo-Fernandes PM. Three-dimensional computed tomography reconstruction in the era of digital personalized medicine. Sao Paulo Med J. 2023;141:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Vervoorn MT, Wulfse M, Mohamed Hoesein FAA, Stellingwerf M, van der Kaaij NP, de Heer LM. Application of three-dimensional computed tomography imaging and reconstructive techniques in lung surgery: A mini-review. Front Surg. 2022;9:1079857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Ujiie H, Yamaguchi A, Gregor A, Chan H, Kato T, Hida Y, Kaga K, Wakasa S, Eitel C, Clapp TR, Yasufuku K. Developing a virtual reality simulation system for preoperative planning of thoracoscopic thoracic surgery. J Thorac Dis. 2021;13:778-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Liu J, Li X, Leng X, Zhong B, Liu Y, Liu L. Effect of 3D Slicer Preoperative Planning and Intraoperative Guidance with Mobile Phone Virtual Reality Technology on Brain Glioma Surgery. Contrast Media Mol Imaging. 2022;2022:9627663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Lin C, Gao J, Zheng H, Zhao J, Yang H, Lin G, Li H, Pan H, Liao Q, Zhao Y. Three-Dimensional Visualization Technology Used in Pancreatic Surgery: a Valuable Tool for Surgical Trainees. J Gastrointest Surg. 2020;24:866-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Zeng N, Tao H, Fang C, Fan Y, Xiang N, Yang J, Zhu W, Liu J, Guan T, Xiang F. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type III hilar cholangiocarcinoma. World J Surg Oncol. 2016;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 15. | Chen Y, Zhang J, Chen Q, Li T, Chen K, Yu Q, Lin X. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: a quasi-randomised clinical trial. World J Surg Oncol. 2020;18:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Zhang S, Huang Z, Cai L, Zhang W, Ding H, Zhang L, Chen Y. Three-dimensional versus two-dimensional video-assisted hepatectomy for liver disease: a meta-analysis of clinical data. Wideochir Inne Tech Maloinwazyjne. 2021;16:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Wang Q, Du B, Wang X, Xue Q, Gao W. A meta-analysis of the three-dimensional reconstruction visualization technology for hepatectomy. Asian J Surg. 2023;46:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Cui DP, Fan S, Guo YX, Zhao QW, Qiao YX, Fei JD. Accurate resection of hilar cholangiocarcinoma using eOrganmap 3D reconstruction and full quantization technique. World J Gastrointest Surg. 2023;15:1693-1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kim AY, Choi SI, Yeom JH. Short-term comparative study of three-dimensional and two-dimensional laparoscopic surgery for total extraperitoneal primary inguinal hernia repair. J Minim Invasive Surg. 2021;24:98-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Wang X, Wang Q, Zhang X, Yin H, Fu Y, Cao M, Zhao X. Application of three-dimensional (3D) reconstruction in the treatment of video-assisted thoracoscopic complex segmentectomy of the lower lung lobe: A retrospective study. Front Surg. 2022;9:968199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |