Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1301
Revised: April 21, 2024
Accepted: April 26, 2024
Published online: May 27, 2024
Processing time: 126 Days and 19.4 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) is a cause of acute-on-chronic liver failure (ACLF).
To investigate the risk factors of ACLF within 1 year after TIPS in patients with cirrhosis and construct a prediction model.
In total, 379 patients with decompensated cirrhosis treated with TIPS at Nanjing Drum Tower Hospital from 2017 to 2020 were selected as the training cohort, and 123 patients from Nanfang Hospital were included in the external validation cohort. Univariate and multivariate logistic regression analyses were performed to identify independent predictors. The prediction model was established based on the Akaike information criterion. Internal and external validation were con
Age and total bilirubin (TBil) were independent risk factors for the incidence of ACLF within 1 year after TIPS. We developed a prediction model comprising age, TBil, and serum sodium, which demonstrated good discrimination and calibration in both the training cohort and the external validation cohort.
Age and TBil are independent risk factors for the incidence of ACLF within 1 year after TIPS in patients with decompensated cirrhosis. Our model showed satisfying predictive value.
Core Tip: Previous studies have proposed several models for predicting the prognosis of patients with acute-on-chronic liver failure (ACLF). However, to date, no such prediction model exists for forecasting the occurrence of ACLF following transjugular intrahepatic portosystemic shunt (TIPS). This study provides an internally and externally validated nomogram model, as well as an easy-to-use risk score scale for predicting the risk of ACLF within 1 year after TIPS. This information could enable physicians to effectively communicate the risks and benefits of the procedure to patients, facilitating shared decision-making.
- Citation: Zhang W, Jin YN, Sun C, Zhang XF, Li RQ, Yin Q, Chen JJ, Zhuge YZ. Development and validation of a predictive model for acute-on-chronic liver failure after transjugular intrahepatic portosystemic shunt. World J Gastrointest Surg 2024; 16(5): 1301-1310
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1301.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1301
Acute-on-chronic liver failure (ACLF) is a clinical syndrome characterized by acute liver failure on underlying chronic liver disease. It manifests as jaundice, coagulation dysfunction, and hepatic encephalopathy. Currently, medical therapy, including pharmacological treatment and artificial liver therapy, is the main treatment for patients with ACLF. For those who do not respond to routine medical treatment, liver transplantation is the only curative treatment[1]. However, the use of liver transplantation is limited by the number of donors and the high cost of the procedure. Despite aggressive therapy, the short-term mortality remains very high among patients with ACLF, usually over 30%[2,3]. Therefore, it is crucial to identify the risk factors of ACLF, identify high-risk patients in the early stages, and improve the management of high-risk patients to delay or prevent the progression of liver damage to ACLF[4]. In recent years, several risk score models have been developed to predict the prognosis of patients with ACLF, such as the model for end-stage liver disease (MELD) score, chronic liver failure-sequential organ failure assessment score, and the Asian Pacific Association for the Study of the Liver (APASL) ACLF research consortium score[5]; however, studies predicting the incidence of ACLF are relatively rare.
Transjugular intrahepatic portosystemic shunt (TIPS) is an interventional strategy that reduces portal venous pressure. It is mostly used to treat the complications of portal hypertension, such as esophagogastric variceal bleeding and re
Currently, there are no studies on ACLF after TIPS. Predicting the risk of ACLF after TIPS in patients with cirrhosis may help clinicians make more accurate treatment decisions. Therefore, we investigated independent predictors of ACLF within 1 year after TIPS and developed an effective predictive model to predict the risk of ACLF after TIPS. This pre
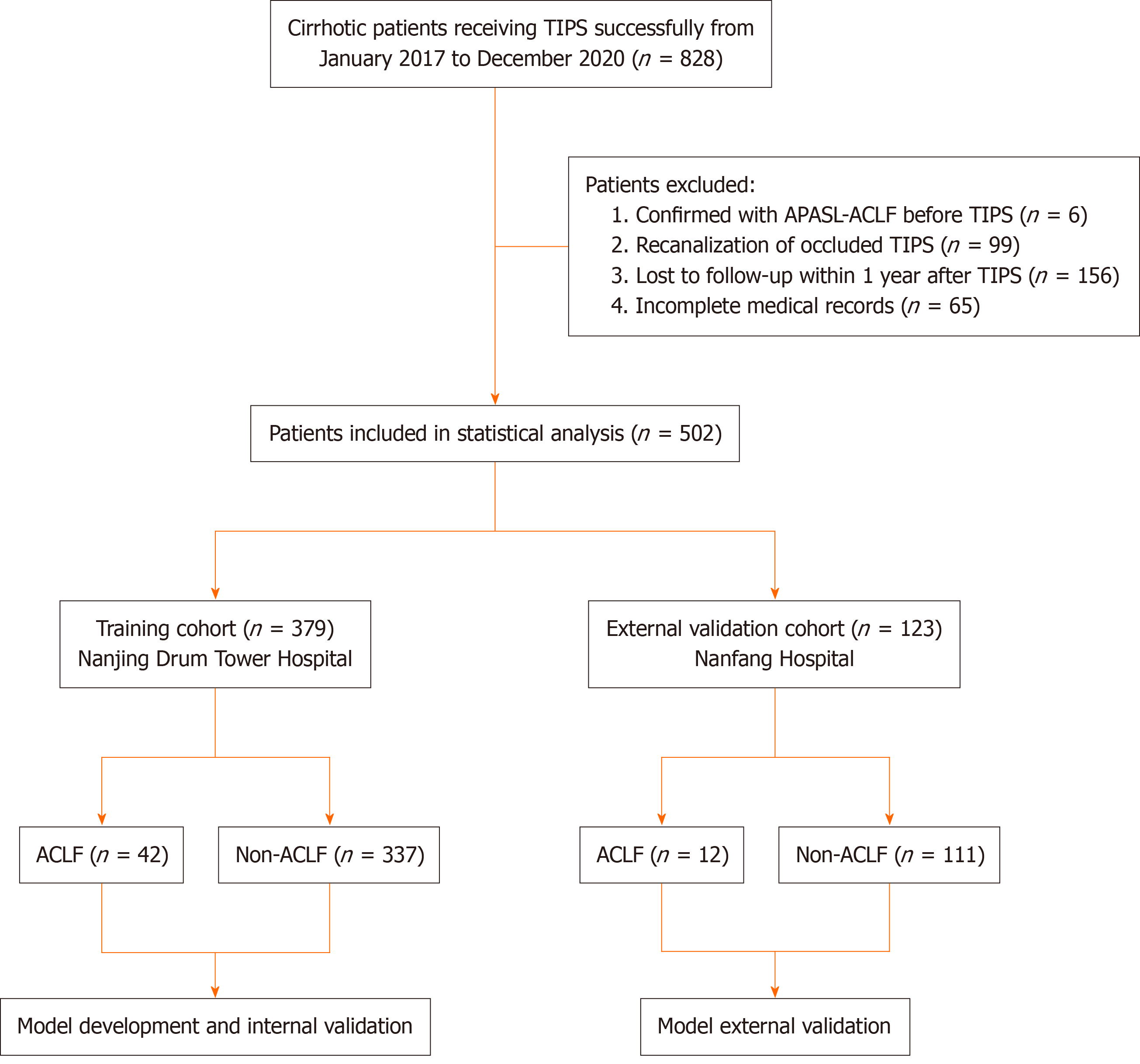
In total, 828 consecutive patients with cirrhosis who underwent TIPS at Nanjing Drum Tower Hospital and Nanfang Hospital, Southern Medical University, between January 2017 and December 2020, were screened based on the inclusion and exclusion criteria. Inclusion criteria were as follows: (1) Age more than or equal to 18 years; (2) meeting the diag
Preoperative and intraoperative variables were recorded for each patient. Preoperative variables included gender, age, history of diabetes mellitus, history of portal vein thrombosis, history of splenectomy, etiology of cirrhosis, and TIPS indication. Four liver function scores, including the Child-Pugh score, MELD score, MELD-Na score, and CLIF-C acute decompensation (AD) score, were recorded. Preoperative laboratory indicators included white blood cell (WBC) count, platelet count, alanine transaminase (ALT), serum total bilirubin (TBil), serum albumin (Alb), serum creatinine, serum sodium (Na), international normalized ratio (INR), and fibrinogen, which were measured within 1 wk before surgery. Intraoperative variables included stent diameter and puncture site.
The primary endpoint was the incidence of ACLF within 1 year after TIPS. Postoperative death, postoperative rebleeding, and postoperative stent stenosis were secondary endpoints. All patients were followed in clinic or via telephone at 1, 3, and 6 months after TIPS and every 6 months thereafter until August 31, 2022.
There is no universal definition of ACLF globally; therefore, we used the definition of APASL because its study popu
R 4.2.1 was used for data processing and development and validation of the nomogram model. Continuous variables are expressed as mean ± SD or median (interquartile range), and statistical differences were estimated using the independent sample t-test or the Mann-Whitney U test. Count data are expressed as the number of cases and percentage (%). We used the χ2 test or Fisher’s exact probability method to compare groups. Univariate regression analysis was conducted using binary logistic regression, and variables with P < 0.10 in univariate analysis were included in multivariate logistic regression analysis. We used the Akaike Information Criterion (AIC) as a stopping criterion and selected the model with the lowest AIC using the backward stepwise method. The nomogram model was then plotted. We performed a bootstrap internal validation procedure with 1000 bootstrap resamples. Additionally, a geographically independent cohort was used for external validation. Model performance was measured using discrimination and calibration. The model’s dis
In the entire cohort, 828 patients were successfully treated with TIPS from January 2017 to December 2020. Of them, 6 patients who were diagnosed with APASL-ACLF before TIPS procedure, 99 patients who underwent TIPS for shunt dysfunction, 156 patients who were lost to follow-up within 1 year after TIPS, and 65 patients with notable missing data were excluded based on the exclusion criteria. In total, 502 patients were enrolled in the final analysis, including 379 patients in the training cohort and 123 patients in the external validation cohort. The training cohort consisted of 219 men and 160 women, with a median age of 59 years (range: 18-86 years). The median follow-up time was 629 d in the ACLF group and 722 d in the non-ACLF group. The external validation cohort consisted of 100 men and 23 women, with a me
Overall, 54 patients (10.76%) developed ACLF within 1 year after TIPS. Among those who developed ACLF within 1 year after TIPS, 23 (42.60%) developed ACLF within 28 d and 17 (31.50%) developed ACLF between day 29 and day 180. In the entire cohort, the incidence of ACLF within 28 d, 180 d, and 1 year after the TIPS procedure was 4.68%, 7.97%, and 10.76%, respectively. The baseline characteristics of the training cohort and the external validation cohort are shown in Supplementary Table 1.
Univariate and multivariate analysis for the incidence of ACLF within 1 year after the TIPS procedure. In total, 17 variables were included in a univariate regression analysis to investigate the predictors of ACLF within 1 year after TIPS. Variables with a P value of less than 0.10 in the univariate analysis were selected for multivariate logistic regression analysis. The model was built using a backward conditional method, which identified age [age ≥ 65 years, odds ratio (OR): 2.649, 95% confidence interval (95%CI): 1.263-5.558, P = 0.010] and TBil (TB: 17.1-34.2 umol/L, OR: 2.944, 95%CI: 1.151-7.528, P = 0.024; TB: 34.2-51.2 umol/L, OR: 11.632, 95%CI: 4.068-33.259, P < 0.001; TB ≥ 51.3 umol/L, OR: 28.746, 95%CI: 6.969-118.579, P < 0.001) as independent risk factors for ACLF within 1 year after TIPS procedure (Table 1).
| Variables | OR comparison | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Gender | Male vs female | 0.922 | 0.471-1.802 | 0.811 | |||
| Age | ≥ 65 yr vs < 65 yr | 2.086 | 1.074-4.055 | 0.030 | 2.649 | 1.263-5.558 | 0.010a |
| DM | Yes vs No | 1.847 | 0.905-3.769 | 0.092 | |||
| Portal vein thrombosis | Yes vs No | 0.809 | 0.411-1.590 | 0.539 | |||
| Splenectomy | Yes vs No | 0.426 | 0.146-1.240 | 0.117 | |||
| Etiology of cirrhosis | Viral hepatitis vs other | 1.174 | 0.609-2.265 | 0.631 | |||
| TIPS indication | EGVB vs refractory ascites | 1.304 | 0.477-3.563 | 0.605 | |||
| WBC | < 4.0 × 109/L vs ≥ 4.0 × 109/L | 0.578 | 0.297-1.123 | 0.106 | |||
| PLT | < 100 × 109/L vs ≥ 100 × 109/L | 2.085 | 0.930-4.674 | 0.075 | |||
| ALT | > 40 U/L vs ≤ 40 U/L | 2.000 | 0.856-4.673 | 0.109 | |||
| TBil | 17.1-34.1 μmol/L vs < 17.1 μmol/L | 3.064 | 1.212-7.742 | 0.018 | 2.944 | 1.151-7.528 | 0.024a |
| 34.2-51.2 μmol/L vs < 17.1 μmol/L | 12.357 | 4.433-34.443 | 0 | 11.632 | 4.068-33.259 | < 0.001a | |
| ≥ 51.3 μmol/L vs < 17.1 μmol/L | 24.714 | 6.342-96.315 | 0 | 28.746 | 6.969-118.579 | < 0.001a | |
| Alb | ≥ 35.0 g/L vs < 35.0 g/L | 1.651 | 0.758-3.596 | 0.206 | |||
| Na | < 135 mmol/L vs ≥ 135 mmol/L | 3.606 | 1.402-9.275 | 0.008 | 2.741 | 0.960-7.821 | 0.0601 |
| Scr | ≥ 133 μmol/L vs < 133 μmol/L | 1.368 | 0.295-6.349 | 0.689 | |||
| INR | < 1.5 vs ≥ 1.5 | 2.363 | 1.001-5.575 | 0.050 | |||
| FIB | < 2.0 g/L vs ≥ 2.0 g/L | 1.542 | 0.726-3.273 | 0.259 | |||
| Stent diameter | ≥ 8 mm vs < 8 mm | 0.787 | 0.392-1.581 | 0.501 | |||
| Puncture site | Right branch vs left branch | 1.000 | 0.373-2.680 | 1.000 | |||
| Bifurcation vs left branch | 1.067 | 0.437-2.602 | 0.887 | ||||
| Trunk vs left branch | 0.889 | 0.352-2.246 | 0.803 | ||||
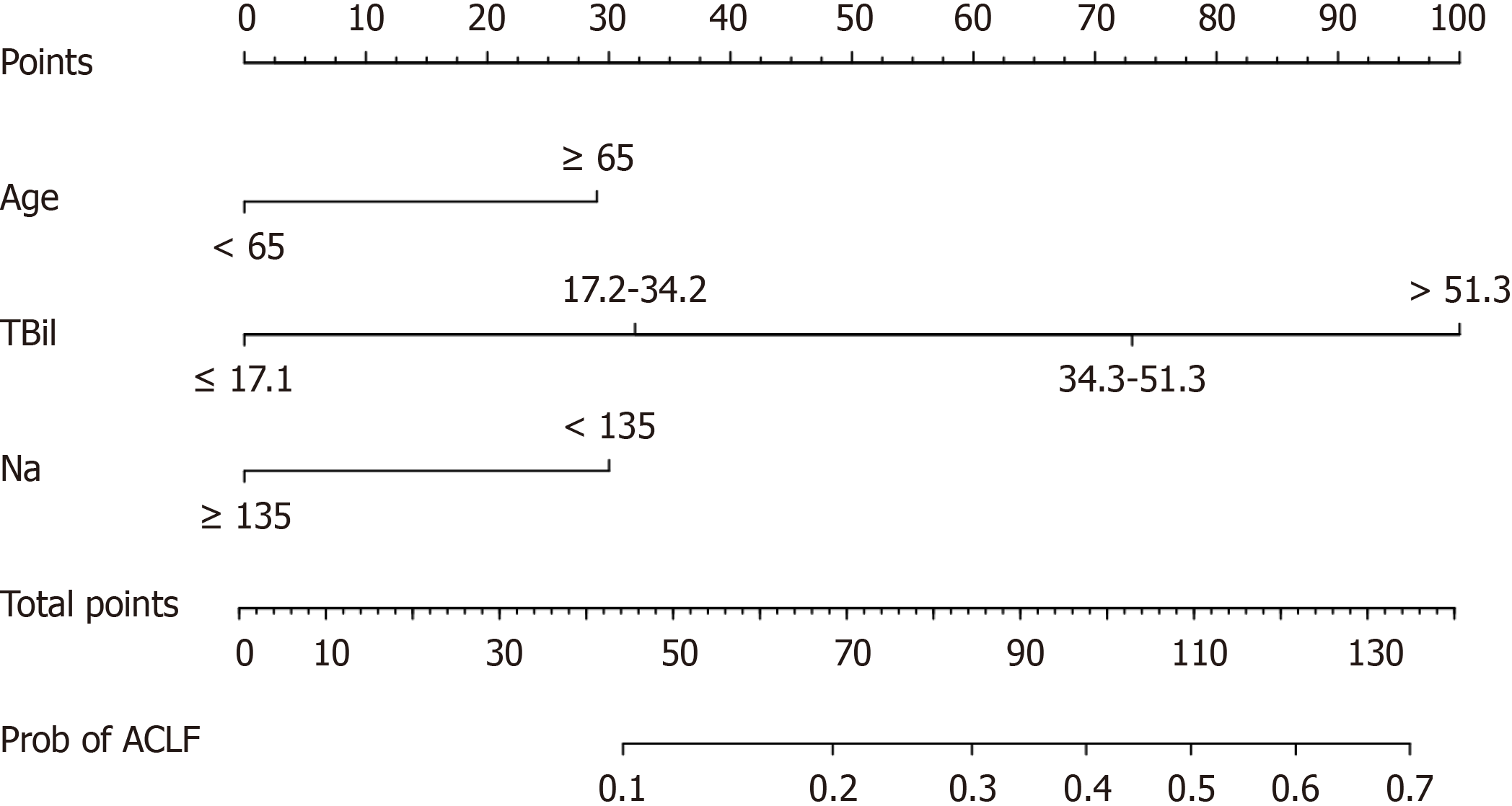
Serum sodium level was included in the final prediction model based on the minimum AIC. Therefore, we developed a predictive model including three variables: Age, TBil, and serum sodium. We compared the area of the curve (AUC) of this model with other models using data from the entire cohort (Supplementary Table 2). Considering the simplicity and predictive power of the model, it was determined to be the optimal choice compared to other models. Figure 2 displays a predictive nomogram based on the three variables. In addition, the nomogram is available through a free browser-based online calculator at https://jyn1212.shinyapps.io/DynNomapp/. Using this calculator, the risk of developing ACLF within 1 year after TIPS can be estimated and displayed.
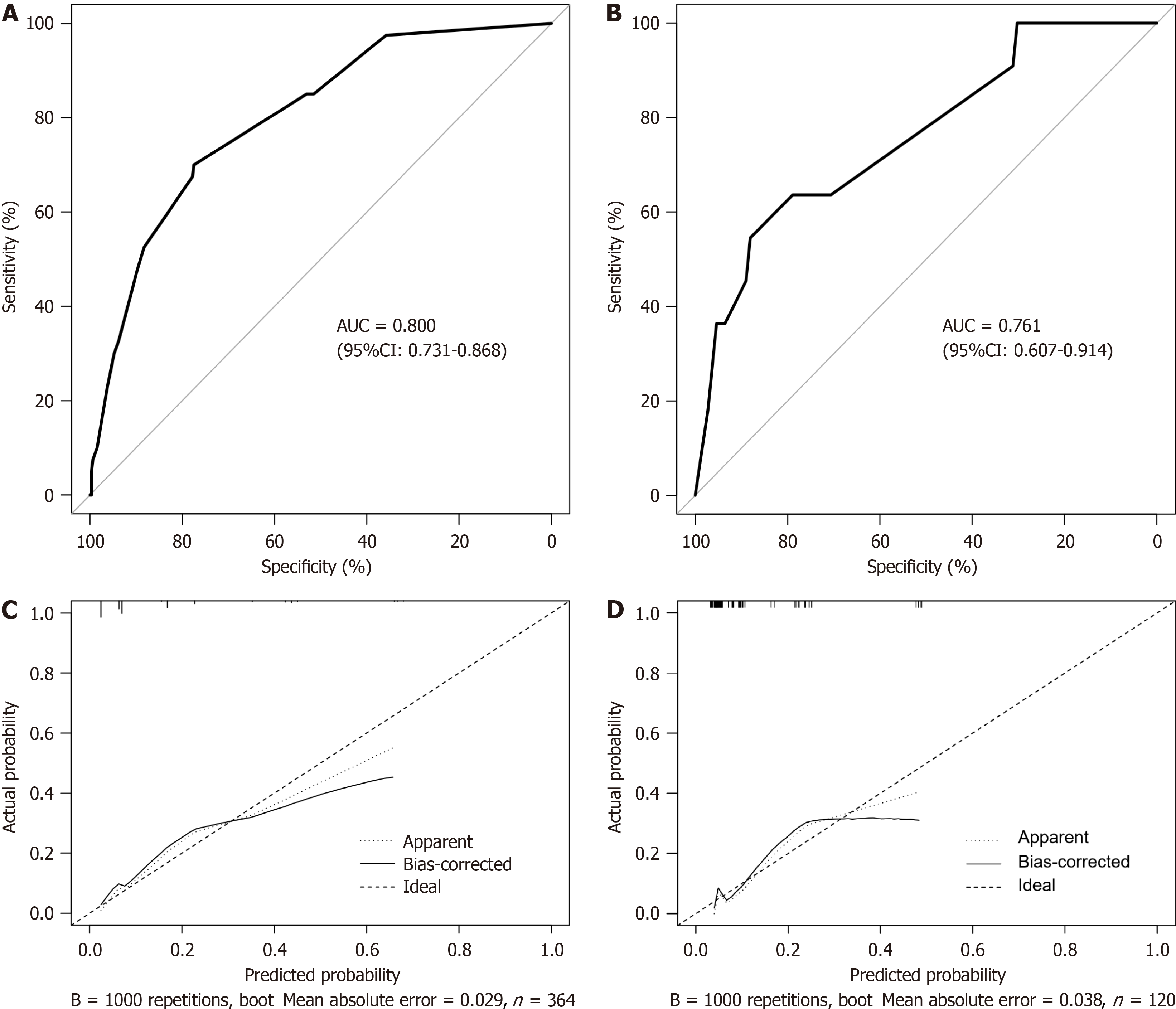
In the training cohort, the AUC of this prediction model was 0.800 (95%CI: 0.731-0.868) with a cutoff value of 0.112, corresponding to a sensitivity and specificity of 0.700 and 0.775, respectively (Figure 3A). We conducted a bootstrap in
We also assigned scores to each variable based on the regression coefficients of the variables in the model and compiled a risk score (Table 2). The total score was 8, with a cutoff value of 4.5. In the entire cohort, the AUC of the risk score was 0.787 (95%CI: 0.729-0.845) (Figure 4), which was comparable to that of the nomogram model (P = 0.645) (Supplementary Table 2). The probability of ACLF for each score is shown in Supplementary Table 3. Patients were then stratified into three groups based on their scores: Low risk (total score of 3-4), medium risk (total score of 5-6), and high risk (total score of 7-8) group. In the whole cohort, the actual incidence of ACLF was 4.7% among patients with a total score of 3-4, 24.1% among patients with a total score of 5-6, and 50.0% among patients with a total score of 7-8.
| Variable | 1 point | 2 points | 3 points | 4 points |
| Age (yr) | < 65.0 | ≥ 65.0 | ||
| TBil (μmol/L) | ≤ 17.1 | 17.2-34.2 | 34.3-51.3 | > 51.3 |
| Na (mmol/L) | ≥ 135.0 | < 135.0 |
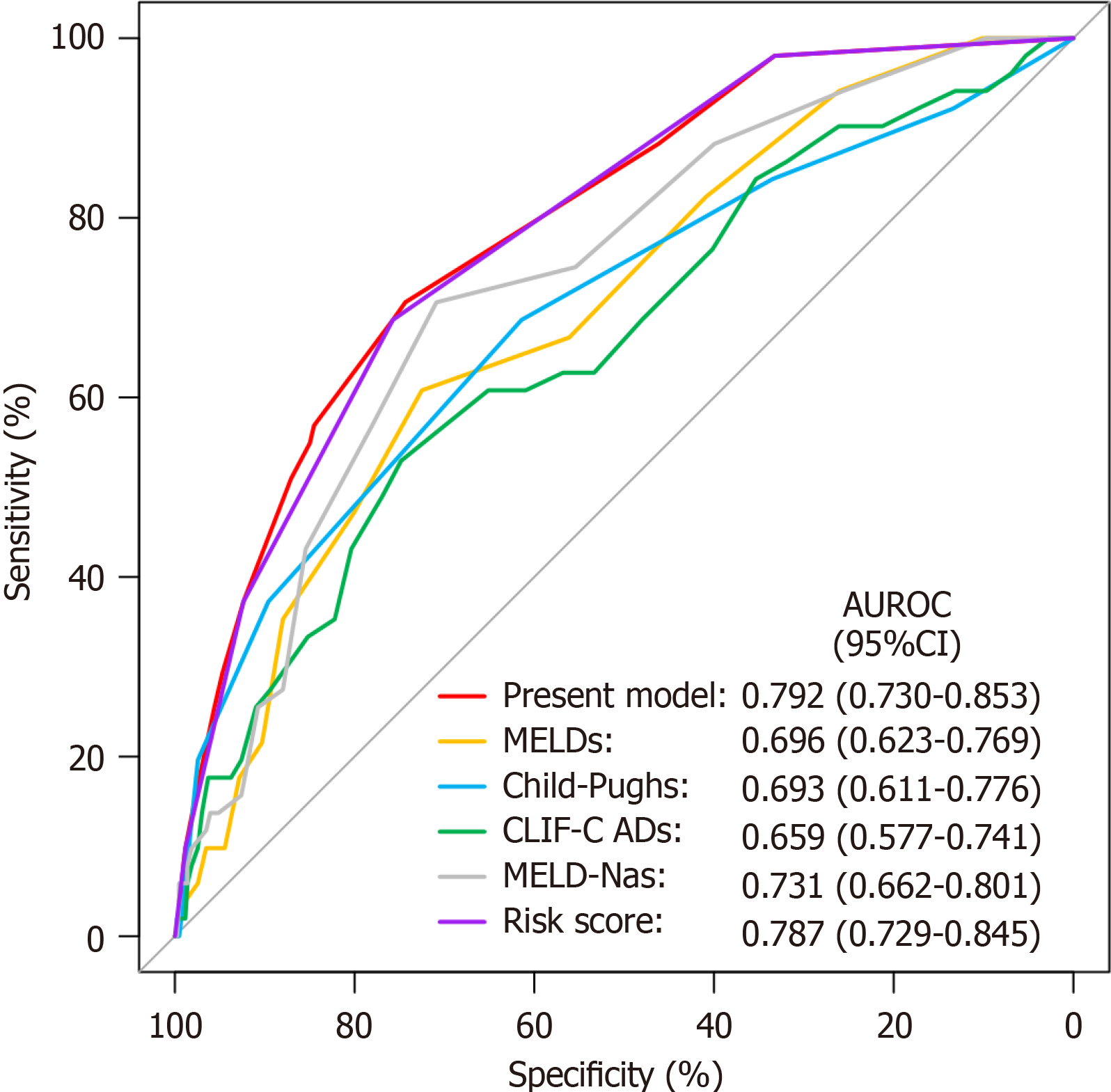
The predictive ability of the proposed nomogram model was compared with that of four commonly used liver function scoring systems using ROC curve analysis (Figure 4) for the entire cohort of 502 patients. The discriminatory ability of the nomogram model had an AUC of 0.792 (95%CI: 0.730-0.853), which was superior to MELD score (AUC: 0.696, 95%CI: 0.623-0.769, P = 0.002), Child-Pugh score (AUC: 0.693, 95%CI: 0.611-0.776, P = 0.018), CLIF-C AD score (AUC: 0.659, 95%CI: 0.577-0.741, P = 0.002), and MELD-Na score (AUC: 0.731, 95%CI: 0.662-0.801, P = 0.022).
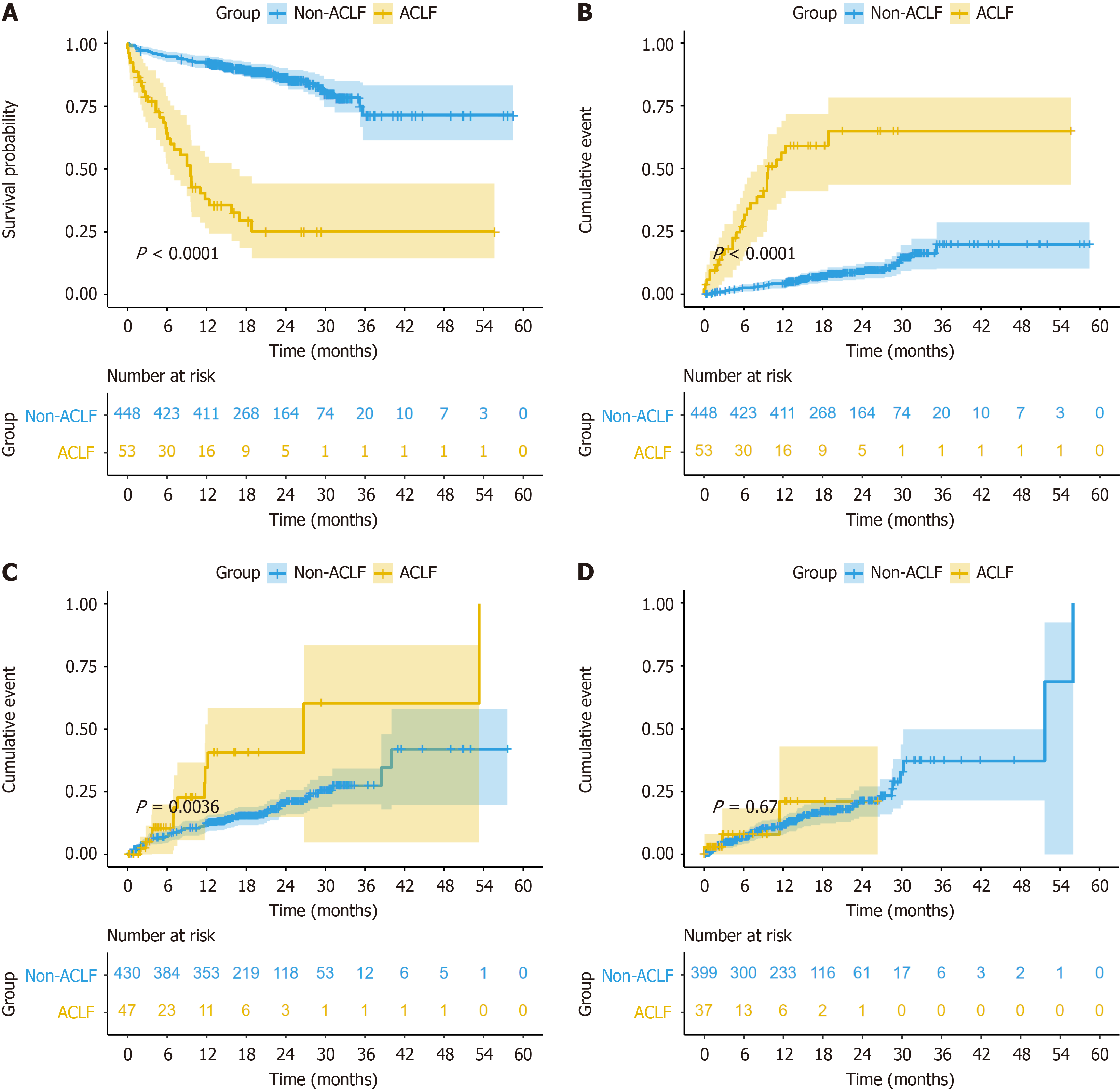
During the follow-up period, 34 deaths occurred in the ACLF group, of which 27 were associated with liver disease. The cumulative survival rates at 28 d, 180 d, and 1 year were 88.7%, 64.2%, and 38.0%, respectively. The Kaplan-Meier sur
ACLF is an acute deterioration of chronic liver disease characterized by high short-term mortality. Early diagnosis and treatment of potential precipitating events are crucial in preventing ACLF[9]. These precipitating events include hepatitis B infection, acute viral hepatitis, alcohol consumption, hepatotoxic drugs, and acute variceal bleeding[1]. Based on the CANONIC study in 2011, the TIPS procedure, as an invasive transhepatic treatment strategy, may increase the risk of ACLF[3]. After the TIPS procedure, the portal blood flow enters directly into the systemic circulation without passing through the liver, reducing blood flow to the liver. In addition, the puncture process causes direct mechanical damage to the liver. In addition, the stent compresses the surrounding liver tissue and affects bile excretion, impairing liver function.
Considering these mechanisms, the main purpose of this study was to establish a predictive model based on the risk factors of ACLF after TIPS to help clinicians select appropriate patients for TIPS procedures and reduce the risk of ACLF after TIPS. To date, several studies have been conducted to predict ACLF, but none of them were conducted on post-TIPS patients; thus, their results are not suitable for predicting ACLF after TIPS. Xiao et al[10] proposed the first prediction model of APASL-ACLF based on outpatients with compensated cirrhosis. The independent predictors included ha
Similar to previous studies, in our study, TBil and age were found to be independent predictors of ACLF after TIPS. TBil is often used to assess the severity of liver impairment, and higher levels of TBil indicate more severe hepatic da
Previous studies have confirmed that a severe systemic inflammatory state is the major driver of extensive tissue and organ damage in patients with acute decompensated cirrhosis who develop ACLF[16]. Compared to cirrhotic patients without ACLF, patients with ACLF have higher WBC counts and plasma levels of C-reactive protein[3]. However, our study did not find a correlation between WBC count and the development of ACLF. This could be because our data were the baseline data of patients, which were collected before TIPS, and patients did not exhibit a severe systemic inflammatory state before developing ACLF.
Based on the minimum AIC, we developed a risk prediction model that can predict the incidence of ACLF within 1 year after the TIPS procedure. The model showed good discrimination and calibration in both the training cohort and the external validation cohort. Furthermore, the discriminatory ability of the nomogram model was superior to that of the MELD score, Child-Pugh score, CLIF-C AD score, and MELD-Na score. Based on this model, we also constructed other models, and after comparison, we found that there was no significant improvement in the AUC. Therefore, we finally chose the first model as it was simpler. To facilitate its clinical application, we also transformed the nomogram model into a risk score. The predictive ability of the risk score was comparable to that of the nomogram model. Patients with a score of 3-4 are at low risk of ACLF, and for such patients, TIPS can be actively performed in the presence of an indication for TIPS. In contrast, patients with a score of 7-8 are at higher risk of ACLF, and clinicians should be cautious and adopt active treatment to reduce patients’ scores before the TIPS procedure. Other treatments or liver transplantation should be considered if patients’ risk scores remain high. For patients with a score of 5-6, we should assess the benefit-risk of TIPS and fully consider patients’ willingness to undergo the procedure.
Limitations: First, selection bias may exist as this study was a retrospective study with a limited sample size. Addi
In conclusion, the incidence of ACLF within 1 year after TIPS was independently associated with age and TBil. Our model and risk score can help predict the incidence of ACLF after TIPS, providing a reference for clinical decision-making. Prospective validation in larger cohorts is needed to assess the generalizability of our findings.
The authors would like to thank all the study participants for their voluntary participation and extend special thanks to Tai-shun Li for his guidance in biostatistics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Kumar R, India S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH
| 1. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 579] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2162] [Article Influence: 180.2] [Reference Citation Analysis (5)] |
| 4. | Chen EQ, Zeng F, Zhou LY, Tang H. Early warning and clinical outcome prediction of acute-on-chronic hepatitis B liver failure. World J Gastroenterol. 2015;21:11964-11973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, Mahtab M, Rahman S, Chawla YK, Taneja S, Tan SS, Devarbhavi H, Duan Z, Yu C, Ning Q, Jia JD, Amarapurkar D, Eapen CE, Goel A, Hamid SS, Butt AS, Jafri W, Kim DJ, Ghazinian H, Lee GH, Sood A, Lesmana LA, Abbas Z, Shiha G, Payawal DA, Dokmeci AK, Sollano JD, Carpio G, Lau GK, Karim F, Rao PN, Moreau R, Jain P, Bhatia P, Kumar G, Sarin SK; APASL ACLF Working Party. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 7. | Trebicka J. Emergency TIPS in a Child-Pugh B patient: When does the window of opportunity open and close? J Hepatol. 2017;66:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Luca A, Miraglia R, Maruzzelli L, D’Amico M, Tuzzolino F. Early Liver Failure after Transjugular Intrahepatic Portosystemic Shunt in Patients with Cirrhosis with Model for End-Stage Liver Disease Score of 12 or Less: Incidence, Outcome, and Prognostic Factors. Radiology. 2016;280:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Xiao KY, Hubbard RA, Kaplan DE, Taddei TH, Goldberg DS, Mahmud N. Models for acute on chronic liver failure development and mortality in a veterans affairs cohort. Hepatol Int. 2020;14:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Yu M, Li X, Lu Y, Jie Y, Shi X, Zhong S, Wu Y, Xu W, Liu Z, Chong Y. Development and Validation of a Novel Risk Prediction Model Using Recursive Feature Elimination Algorithm for Acute-on-Chronic Liver Failure in Chronic Hepatitis B Patients With Severe Acute Exacerbation. Front Med (Lausanne). 2021;8:748915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Tajiri K, Shimizu Y. Liver physiology and liver diseases in the elderly. World J Gastroenterol. 2013;19:8459-8467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Pereira G, Baldin C, Piedade J, Reis V, Valdeolivas T, Victor L, Guimarães L, Duarte J, Veiga Z, Alcântara C, Fernandes F, Pereira JL. Combination and sequential evaluation of acute-on-chronic liver failure (ACLF) and hyponatremia and prognosis in cirrhotic patients. Dig Liver Dis. 2020;52:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Vizzutti F, Celsa C, Calvaruso V, Enea M, Battaglia S, Turco L, Senzolo M, Nardelli S, Miraglia R, Roccarina D, Campani C, Saltini D, Caporali C, Indulti F, Gitto S, Zanetto A, Di Maria G, Bianchini M, Pecchini M, Aspite S, Di Bonaventura C, Citone M, Guasconi T, Di Benedetto F, Arena U, Fanelli F, Maruzzelli L, Riggio O, Burra P, Colecchia A, Villa E, Marra F, Cammà C, Schepis F. Mortality after transjugular intrahepatic portosystemic shunt in older adult patients with cirrhosis: A validated prediction model. Hepatology. 2023;77:476-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Lopes-Secundo TM, Sevá-Pereira T, Correa BR, Silva NCM, Imbrizi MR, Cunha-Silva M, Soares EC, Almeida JRS. Serum sodium, model for end-stage liver disease, and a recent invasive procedure are risk factors for severe acute-on-chronic liver failure and death in cirrhotic patients hospitalized with bacterial infection. Eur J Gastroenterol Hepatol. 2018;30:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Casulleras M, Zhang IW, López-Vicario C, Clària J. Leukocytes, Systemic Inflammation and Immunopathology in Acute-on-Chronic Liver Failure. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |