Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1280
Revised: February 29, 2024
Accepted: April 10, 2024
Published online: May 27, 2024
Processing time: 169 Days and 8.1 Hours
Robotic surgery (RS) is gaining popularity; however, evidence for abdominoperineal resection (APR) of rectal cancer (RC) is scarce.
To compare the efficacy of RS and laparoscopic surgery (LS) in APR for RC.
We retrospectively identified patients with RC who underwent APR by RS or LS from April 2016 to June 2022. Data regarding short-term surgical outcomes were compared between the two groups. To reduce the effect of potential confounding factors, propensity score matching was used, with a 1:1 ratio between the RS and LS groups. A meta-analysis of seven trials was performed to compare the efficacy of robotic and laparoscopic APR for RC surgery.
Of 133 patients, after propensity score matching, there were 42 patients in each group. The postoperative complication rate was significantly lower in the RS group (17/42, 40.5%) than in the LS group (27/42, 64.3%) (P = 0.029). There was no significant difference in operative time (P = 0.564), intraoperative transfusion (P = 0.314), reoperation rate (P = 0.314), lymph nodes harvested (P = 0.309), or circumferential resection margin (CRM) positive rate (P = 0.314) bet
Our study shows that RS is a safe and effective approach for APR in RC and offers better short-term outcomes than LS.
Core Tip: This study compared the efficacy of robotic surgery (RS) and laparoscopic surgery (LS) in abdominoperineal resection (APR) for rectal cancer (RC). Our results showed that RS patients had fewer positive circumferential resection margins, less estimated blood loss, shorter postoperative hospital stays, and fewer postoperative complications than did LS patients. Our findings demonstrate that RS is a safe and effective approach for APR in RC and offers better short-term outcomes than LS. This study contributes to the existing evidence base and can assist surgeons and healthcare providers in making informed decisions on using RS in APR for RC.
- Citation: Song L, Xu WQ, Wei ZQ, Tang G. Robotic vs laparoscopic abdominoperineal resection for rectal cancer: A propensity score matching cohort study and meta-analysis. World J Gastrointest Surg 2024; 16(5): 1280-1290
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1280.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1280
Colorectal cancer is the third most common cancer and one of the most common causes of cancer-related deaths worldwide. Nearly 40% of colorectal cancers occur in the rectum[1]. Surgery is the primary treatment for rectal cancer (RC)[2]. Common surgical procedures for RC include intersphincteric resection, low anterior resection (LAR), anterior resection, and abdominoperineal resection (APR)[3]. APR, also known as Miles’s procedure, was first reported by Miles[4] in 1908. With the increasing use of LAR for lower RC, the application of APR has gradually declined. However, APR remains the best choice for RC cases in lower locations, cases with perianal muscle invasion, or cases where sphincter-preserving techniques are unsuitable for radical resection[5].
Laparoscopic minimally invasive surgery for colorectal cancer was first reported in the 1990s[5]. Compared with traditional open surgery, laparoscopic surgery (LS) has been widely used in RC surgery due to its advantages of shorter hospital stays, reduced blood loss, and faster postoperative recovery[6,7]. However, some limitations of LS, such as a two-dimensional field of view, amplification of operative tremors, and poor flexibility, may affect its efficacy in radical sur
Therefore, we conducted a retrospective cohort study to evaluate the effects of RS on postoperative complications, pathological findings, and postoperative recovery in RC patients undergoing APR. Propensity score matching (PSM) was performed to reduce the influence of imbalanced factors between the two groups. In addition, we performed a meta-analysis of all previous studies evaluating the efficacies of robotic and laparoscopic APR for RC surgery and combined the results of this trial.
This retrospective study included 133 patients with pathologically confirmed RC who underwent APR via RS or LS at the First Affiliated Hospital of Chongqing Medical University from April 2016 to June 2022. This study was ethically ap
Patient demographics [age, sex, body mass index (BMI), the American Society of Anesthesiologists physical status classification (ASA) scores, comorbidity, tumor distance from the anal verge, and neoadjuvant therapy], surgical in
All laparoscopic and robotic procedures were performed by the same experienced surgeon. Robotic and laparoscopic approaches were used only for abdominal procedures. The perineal portion of the procedure was performed manually by the surgeon. All surgical procedures were performed in accordance with the principle of total mesorectal excision, which included resection of the entire mesorectum to the pelvic floor, ligation of the inferior mesenteric artery at the origin of the inferior mesenteric artery, and lymph node dissection. Perineal resection involved the removal of the internal and external anal sphincters and a part of the levator ani muscle. Extended resection of the levator ani muscle, posterior va
The primary endpoint was postoperative complications within 30 postoperative days. The secondary endpoints included operative time, blood loss, time to first flatus and defecation, conversion rate, intensive care rate, histological exami
Data were presented as frequencies (percentages), means (standard deviation), or medians (interquartile range). Di
We conducted a meta-analysis of all published cohort studies, case-control studies, and randomized controlled trials (RCTs) following the PRISMA guidelines, comparing RS with LS in APR for RC. The PubMed, Embase, Web of Science, and Cochrane databases were searched from inception until December 7, 2022. Studies were included if they met the following criteria: (1) Patients undergoing APR for RC; (2) intervention with RS; (3) comparison with LS; (4) outcomes included postoperative complications, completeness of resection, operative time, length of hospital stay, mortality, con
In total, 133 patients (96 males and 37 females) who underwent APR for RC were included. The median (interquartile range: 25th-75th percentile) age and mean BMI of the patients were 63.0 (55.5-70.0) years and 22.53 ± 2.43 kg/m2, res
After matching, 42 patients were included in each group (Table 1). Operative times were similar between the two groups
| Group RS (n = 42) | Group LS (n = 42) | P value | |
| Age (yr)1 | 63.5 (55-69) | 65 (57.8-72.3) | 0.211 |
| Sex | 1.000 | ||
| Male | 32 (76.2) | 32 (76.2) | |
| Female | 10 (23.8) | 10 (23.8) | |
| BMI2 | 22.5 (2.03) | 22.6 (2.41) | 0.815 |
| COPD | 8 (19) | 8 (19) | 1.000 |
| Hypertension | 11 (26.2) | 9 (21.4) | 0.608 |
| Diabetes mellitus | 2 (4.8) | 4 (9.5) | 0.397 |
| Coronary artery disease | 1 (2.4) | 2 (4.8) | 0.557 |
| ASA Grade | 0.890 | ||
| 1 | 5 (11.9) | 4 (9.5) | |
| 2 | 22 (52.4) | 24 (57.1) | |
| 3 | 15 (35.7) | 14 (33.3) | |
| Neoadjuvant therapy received | 9 (21.4) | 8 (19) | 0.786 |
| Distance between tumor and AV (cm)1 | 3 (2-5) | 3 (2.5-4) | 0.996 |
| Stage | 0.969 | ||
| I | 10 (23.8) | 10 (23.8) | |
| II | 16 (38.1) | 15 (35.7) | |
| III | 16 (38.1) | 17 (40.5) |
| Group RS (n = 42) | Group LS (n = 42) | P value | |
| Duration of surgery (min)1 | 245 (191.5-295) | 230 (200-286.3) | 0.564 |
| Intraoperative blood loss (ml)1 | 60 (50-100) | 100 (50-200) | 0.012 |
| Transfusion | 1 (2.4) | 0 (0) | 0.314 |
| Days to first flatus1 | 2 (1-2) | 2 (2-3) | 0.023 |
| Days to first defecation1 | 3 (2.8-4.3) | 3 (2.8-4.3) | 0.679 |
| Reoperation | 0 (0) | 1 (2.4) | 0.314 |
| Mortality | 0 (0) | 0 (0) | - |
| Intensive care | 0 (0) | 2 (4.8) | 0.152 |
| Conventional open | 0 (0) | 0 (0) | - |
| Circumferential resection margin positive | 0 (0) | 1 (2.4) | 0.314 |
| Lymph nodes harvested1 | 15 (11-18) | 13 (9-18.3) | 0.309 |
| Perineural invasion | 1 (2.4) | 1 (2.4) | 1.000 |
| Lymphovascular invasion | 2 (4.8) | 2 (4.8) | 1.000 |
| Hospital stay (d)1 | 9 (7.8-13) | 11 (8-18) | 0.044 |
| Postoperative complications | 17 (40.5) | 27 (64.3) | 0.029 |
| Urinary infection | 2 (4.8) | 1 (2.4) | 0.557 |
| Pneumonia | 1 (2.4) | 1 (2.4) | 1.000 |
| Ileus | 3 (7.1) | 3 (7.1) | 1.000 |
| Wound infection | 5 (11.9) | 12 (28.6) | 0.057 |
| Intraabdominal infection | 5 (11.9) | 8 (19) | 0.365 |
| Urinary retention | 1 (2.4) | 2 (4.8) | 0.557 |
| Hospital charge (RMB)1 | 81886.5 (70540.5-109854.2) | 70102.8 (60308.6-109415.4) | 0.040 |
Postoperative complication rate was significantly lower in the RS group (17/42, 40.5%) than in the LS group (27/42, 64.3%) (P = 0.029). There were no significant differences observed in pneumonia (P = 1.000), urinary infection rate (P = 0.557), ileus rate (P = 1.000), wound infection rate (P = 0.057), abdominal infection rate (P = 0.365), reoperation rate (P = 0.314), urinary retention (P = 0.557), or intensive care rate (P = 0.152) between the two groups, and no deaths were re
Regarding intestinal function recovery, the time to first flatus in the robotic group (P = 0.023) was significantly shorter than that in the laparoscopic group. However, there was no significant difference in the time to first defecation between the two groups (P = 0.679). In addition, the median postoperative hospital stay was significantly shorter in the RS group (9.0 d) than in the LS group (11.0 d; P = 0.044).
Our literature search yielded 810 potential records, of which 11 published articles were completely reviewed. In addition to our study, six trials[10-12,15-17] published between 2015 and 2022 were included. Details of the seven eligible trials are summarized in Table 3. The risk of bias was low in all seven studies included in the review.
| Ref. | Country | Study design | Sample | Age | Gender (M/ F) | Outcomes | NOS |
| Moghadamyeghaneh et al[15], 2015 | United States | Retrospective cohort study | R: 872; L: 4737 | R: 64; L: 62 | R: 556/316; L: 2844/1893 | Hospital stay, postoperative complications, mortality | 7 |
| Kamali et al[16], 2017 | United Kingdom | Retrospective case-control study | R: 11; L: 11 | R: 71; L: 57 | R: 7/4; L: 9/2 | Postoperative complications, mortality, CRM, operating time, hospital stay, lymph nodes harvested, conversion rate | 8 |
| Gavrila et al[17], 2021 | Romania | Retrospective case-control study | R: 46; L: 63 | R: 62; L: 62 | R: 34/12; L: 32/31 | Postoperative complications, mortality, operating time, blood loss, hospital stay, conversion rate, reoperation rate | 8 |
| Kasai et al[11], 2022 | Japan | Retrospective cohort study | R: 33; L: 20 | R: 74; L: 78 | R: 20/13; L: 16/4 | Postoperative complications, CRM, operating time, blood loss, hospital stay, conversion rate, lymph nodes harvested | 8 |
| Feng et al[10], 2022 | China | Randomized controlled trial | R: 174; L: 173 | R: 58; L: 60 | R: 108/66; L: 113/60 | Postoperative complications, mortality, CRM, operating time, blood loss, hospital stay, conversion rate, reoperation rate, lymph nodes harvested | - |
| Gorgun et al[12], 2022 | United States | Retrospective PSM | R: 34; L: 34 | R: 66; L: 66 | R: 25/9; L: 25/9 | Postoperative complications, CRM, operating time, blood loss, hospital stay, conversion rate, reoperation rate, lymph nodes harvested | 9 |
| Current study, 2022 | China | Retrospective PSM | R: 34; L: 34 | R: 34; L: 34 | R: 34; L: 34 | Lymph nodes harvested, postoperative complications, mortality, CRM, operating time, blood loss, hospital stay, conversion rate, reoperation rate | 9 |
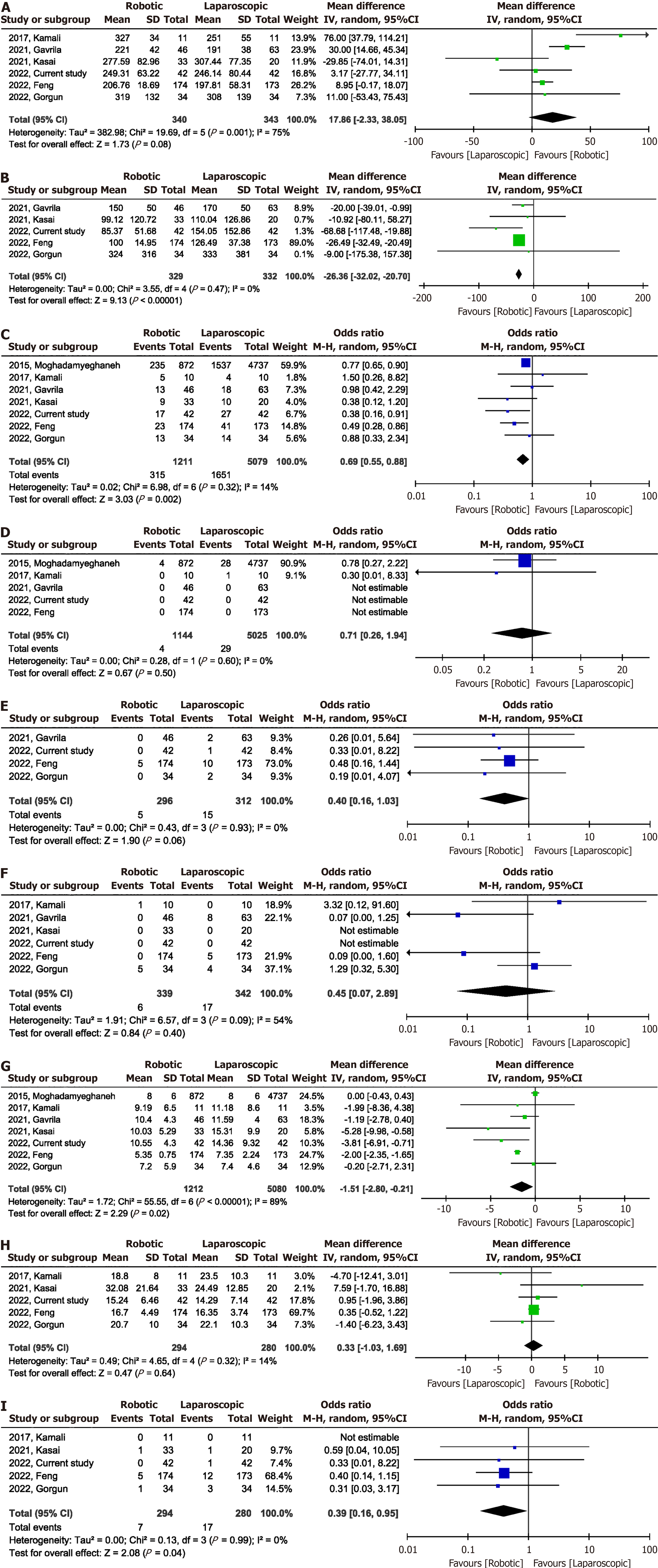
Meta-analysis of the six studies[10-12,16,17] showed no significant difference in operative time [MD = 17.86 min; 95%CI: -2.33 to 38.05; P = 0.08, with high heterogeneity (I2 = 75%)] (Figure 1A and Table 4). Intraoperative blood loss was significantly lower in the RS group than in the LS group (MD -26.36 mL, 95%CI: -32.02 to -20.70; I2 = 0%, P = 0.47) (Figure 1B). A total of 6290 participants in the seven studies[10-12,15-17] had postoperative complications. The incidence of postoperative complications was lower in the RS group than in the LS group (OR 0.69, 95%CI: 0.55-0.88; I2 = 14%, P = 0.32) (Figure 1C). There was no significant difference (OR, 0.71; 95%CI: 0.26-1.94; P = 0.50) in the postoperative mortality between the RS and LS groups, with low heterogeneity between studies (I2 = 0%, P = 0.60) (Figure 1D). Data on reope
| Indicators | No. of studies | Events for RS | Events for LS | Effect size | 95%CI |
| Operative time | 6 | - | - | 17.86 min | -2.33, 38.05 |
| Intraoperative blood loss | 5 | - | - | -26.36 mL | -32.02, -20.70 |
| Postoperative complications | 7 | 315/1211 | 1651/5079 | 0.69 | 0.55, 0.88 |
| Postoperative mortality | 5 | 4/1144 | 29/5025 | 0.71 | 0.26, 1.94 |
| Reoperation | 4 | 5/296 | 15/312 | 0.40 | 0.16, 1.03 |
| Conversion to open surgery | 6 | 6/339 | 17/342 | 0.45 | 0.07, 2.89 |
| The length of stay | 7 | - | - | -1.51 d | -2.80, -0.21 |
| Lymph nodes harvested | 5 | - | - | 0.33 | -1.03, 1.69 |
| Circumferential resection margin positive | 5 | 7/294 | 17/280 | 0.39 | 0.16, 0.95 |
The results of the sensitivity analysis showed that the total effect size of intraoperative blood loss, postoperative complications, postoperative mortality, reoperation rate, conversion to open surgery, and number of lymph nodes har
With advancements in technology, LS is gradually becoming the preferred technique for colorectal surgery. LS is safe and effective in the short and long term[9]. However, laparoscopic RC surgery has some inherent limitations, especially in patients with low RC[18]. In addition, neoadjuvant use can lead to pelvic tissue fibrosis, which increases the difficulty of surgery and affects the efficacy of LS[9]. RS is another surgical technique that is under development. Compared with LS, RS has several major advantages, including a wider surgical field, more flexible surgical instruments, and less fatigue for doctors[19]. In addition, LS is difficult to perform on the pelvic floor and requires a long learning curve, whereas RS has a shorter learning period, making this technique easier for younger doctors to learn[20,21]. A recently published meta-analysis[21] showed that robotic rectal surgery had similar long-term outcomes as LS, with shorter operative time, lower incidence of postoperative complications, shorter hospital stays, and lower conversion to open surgery rates. However, there are few related studies on RS for APR, and the efficacy is still controversial. Postoperative complications of mini
In addition, the advantages of the RS could theoretically bring benefits in terms of conversion to open surgery. A meta-analysis of 42 studies[21] showed that RS reduced the conversion rate. A recent large RCT[23] showed that robotics was associated with a lower conversion rate. However, in our study, there was no difference in the rate of conversion to open surgery between the RS and LS groups. After meta-analysis, conversion rates between RS and LS groups remained comparable. However, our analysis included a limited number of studies; more high-quality studies are needed to eva
In RC surgery, surgeons focus on the quality of tumor resection. The number of harvested lymph nodes is related to the accuracy of tumor staging and oncologic radicality. In addition, it affects the patient's oncologic prognosis[27]. Being CRM positive, defined as having a minimum distance between the tumor and the CRM of 1 mm or less[28], is associated with tumor recurrence and shorter survival[18]. Studies have reported that being CRM positive leads to a 1- to 5-fold increased risk of local recurrence and a 1- to 4-fold increased risk of distant metastasis[23,29-32]. In traditional LS, surgical instruments need to enter the pelvic cavity in a nearly vertical direction, and their operation in the horizontal direction is limited. In addition, the narrow space in the pelvic cavity can lead to interference between instruments. Lower rectal surgery requires the cooperation of experienced assistants[23]. These factors may affect the quality of LS. RS has better three-dimensional vision and more flexible tools. In addition, the operating arm of the robot can be controlled by the surgeon, which can replace the role of the assistant in LS. These factors allow the robot to perform precise surgical manipulations in a narrow space and improve the quality of tumor specimens[21,23]. Although there was no benefit of RS in terms of the number of lymph nodes harvested, our meta-analysis showed that RS significantly reduced the CRM positive rate. However, the sensitivity analysis showed that the total effect of the CRM positive rate was not robust. More studies are needed to explore the effect of RS on the quality of APR in the future.
Minimally invasive surgery is characterized by a rapid recovery of bowel function and a short hospital stay[19]. Post
Regarding safety, some researchers have expressed concerns that RS will lead to longer operation times[3]. However, our retrospective study and meta-analysis suggest that RS does not lead to longer operation times. This is similar to the results of several previous studies[23,34,35]. In addition, we found that intraoperative blood loss was significantly lower in the RS group than that in the LS group. This may be due to the technical advantages of the robotic system providing a better surgical field of view, clearer anatomy, and easier suture manipulation, helping to prevent more bleeding[15,18].
A significant limitation of RS is its high cost[23]. Moghadamyeghaneh et al[15] used the nationwide inpatient sample database from 2009 to 2012 and found that the average total hospitalization cost of robotic APR was 37% higher than that of laparoscopy. Similar to previous studies, in the present study, we found a 17% increase in median hospitalization costs in the RS group compared with that in the LS group. Recently, Gorgun et al[12] reported an increase in direct costs of robotic APR compared with those of laparoscopic APR (26% increase in mean cost and 43% increase in median cost); however, the difference was not significant. The increase in hospitalization costs is an important factor hindering the routine application of RS[12]. Increased complication rates and longer hospital stays are associated with increased treat
Our study had some limitations. First, our study was retrospective and may have been subject to some confounding factors. Therefore, we performed a PSM analysis, and the post-PSM RS and LS groups had similar underlying characteristics. Second, it was difficult to compare the effects of the two surgical techniques on long-term survival because the postoperative follow-up time was too short. However, given the concern about the impact of postoperative complications on survival and the lower incidence of postoperative complications in the RS group compared with that in the LS group, it is necessary to evaluate the long-term efficacy of the two surgical methods. Finally, this study was a single-center study, and all operations were performed by the same surgeon, which was not representative of the skill level of most colorectal surgeons. Therefore, we conducted a meta-analysis of data from other previous studies to further confirm the reliability of the results. To the best of our knowledge, this is the first meta-analysis comparing the short-term efficacy of robotic vs laparoscopic APR.
RS is a safe and effective treatment for APR in RC. Although RS is more expensive than LS, RS offers better short-term outcomes including fewer complications, fewer positive CRMs, less blood loss, and a faster postoperative recovery. More high-quality prospective studies are warranted to confirm the benefits of RS in APR.
We would like to express our gratitude to all those who contributed to the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Vyshka G, Albania S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64698] [Article Influence: 16174.5] [Reference Citation Analysis (177)] |
| 2. | Su WC, Huang CW, Ma CJ, Chen PJ, Tsai HL, Chang TK, Chen YC, Li CC, Yeh YS, Wang JY. Feasibility of robot-assisted surgery in elderly patients with rectal cancer. J Minim Access Surg. 2021;17:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg. 2018;267:1034-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z. Laparoscopic Versus Conventional Open Abdominoperineal Resection for Rectal Cancer: An Updated Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:526-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Tang B, Lei X, Ai J, Huang Z, Shi J, Li T. Comparison of robotic and laparoscopic rectal cancer surgery: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2021;19:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Liu Y, Han G, Yi B, Zhu S. The severity of postoperative complications after robotic versus laparoscopic surgery for rectal cancer: A systematic review, meta-analysis and meta-regression. PLoS One. 2020;15:e0239909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Liu G, Zhang S, Zhang Y, Fu X, Liu X. Robotic Surgery in Rectal Cancer: Potential, Challenges, and Opportunities. Curr Treat Options Oncol. 2022;23:961-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 9. | Qiu H, Yu D, Ye S, Shan R, Ai J, Shi J. Long-term oncological outcomes in robotic versus laparoscopic approach for rectal cancer: A systematic review and meta-analysis. Int J Surg. 2020;80:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Feng Q, Tang W, Zhang Z, Wei Y, Ren L, Chang W, Zhu D, Liang F, He G, Xu J. Robotic versus laparoscopic abdominoperineal resections for low rectal cancer: A single-center randomized controlled trial. J Surg Oncol. 2022;126:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 11. | Kasai S, Kagawa H, Shiomi A, Hino H, Manabe S, Yamaoka Y, Kato S, Hanaoka M, Kinugasa Y. Advantages of robotic abdominoperineal resection compared with laparoscopic surgery: a single-center retrospective study. Surg Today. 2022;52:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Gorgun E, Cengiz TB, Ozgur I, Dionigi B, Kalady MF, Steele SR. Outcomes and Cost Analysis of Robotic Versus Laparoscopic Abdominoperineal Resection for Rectal Cancer: A Case-Matched Study. Dis Colon Rectum. 2022;65:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25814] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 14. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470712184. |
| 15. | Moghadamyeghaneh Z, Phelan M, Smith BR, Stamos MJ. Outcomes of Open, Laparoscopic, and Robotic Abdominoperineal Resections in Patients With Rectal Cancer. Dis Colon Rectum. 2015;58:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Kamali D, Reddy A, Imam S, Omar K, Jha A, Jha M. Short-term surgical outcomes and patient quality of life between robotic and laparoscopic extralevator abdominoperineal excision for adenocarcinoma of the rectum. Ann R Coll Surg Engl. 2017;99:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Gavrila D, Bitere O, Droc G, Lacatus M, Minciuna C, Ilie V, Trandafir B, Herlea V, Tudor S, Vasilescu C. Abdominoperineal Resection for Rectal Cancer: Open, Laparoscopic or Robotic Approach. Chirurgia (Bucur). 2021;116:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Wang X, Cao G, Mao W, Lao W, He C. Robot-assisted versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2020;16:979-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Cheong C, Kim NK. Minimally Invasive Surgery for Rectal Cancer: Current Status and Future Perspectives. Indian J Surg Oncol. 2017;8:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Araujo SE, Seid VE, Klajner S. Robotic surgery for rectal cancer: current immediate clinical and oncological outcomes. World J Gastroenterol. 2014;20:14359-14370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Safiejko K, Tarkowski R, Koselak M, Juchimiuk M, Tarasik A, Pruc M, Smereka J, Szarpak L. Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection: A Systematic Review and Meta-Analysis of 19,731 Patients. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Wang XT, Li DG, Li L, Kong FB, Pang LM, Mai W. Meta-analysis of oncological outcome after abdominoperineal resection or low anterior resection for lower rectal cancer. Pathol Oncol Res. 2015;21:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, Zhang W, Zhao R, Zhang C, Cheng L, Zhang X, Liang F, He G, Wei Y, Xu J; REAL Study Group. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 24. | Tang G, Pi F, Zhang DH, Qiu YH, Wei ZQ. Novel surgical procedure for preventing anastomotic leakage following colorectal cancer surgery: A propensity score matching study. Front Oncol. 2022;12:1023529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | van Kooten RT, Elske van den Akker-Marle M, Putter H, Meershoek-Klein Kranenbarg E, van de Velde CJH, Wouters MWJM, Tollenaar RAEM, Peeters KCMJ. The Impact of Postoperative Complications on Short- and Long-Term Health-Related Quality of Life After Total Mesorectal Excision for Rectal Cancer. Clin Colorectal Cancer. 2022;21:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Gamboa AC, Lee RM, Turgeon MK, Varlamos C, Regenbogen SE, Hrebinko KA, Holder-Murray J, Wiseman JT, Ejaz A, Feng MP, Hawkins AT, Bauer P, Silviera M, Maithel SK, Balch GC. Impact of Postoperative Complications on Oncologic Outcomes After Rectal Cancer Surgery: An Analysis of the US Rectal Cancer Consortium. Ann Surg Oncol. 2021;28:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Tonini V, Birindelli A, Bianchini S, Cervellera M, Bacchi Reggiani ML, Wheeler J, Di Saverio S. Factors affecting the number of lymph nodes retrieved after colo-rectal cancer surgery: A prospective single-centre study. Surgeon. 2020;18:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Baik SH, Kim NK, Lee YC, Kim H, Lee KY, Sohn SK, Cho CH. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2007;14:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 745] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 30. | Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, Abbott CR, Scott N, Finan PJ, Johnston D, Quirke P. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 540] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 31. | Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 32. | Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J; Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators. Disease-free Survival and Local Recurrence After Laparoscopic-assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial. Ann Surg. 2019;269:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 33. | Tang G, Huang W, Tao J, Wei Z. Prophylactic effects of probiotics or synbiotics on postoperative ileus after gastrointestinal cancer surgery: A meta-analysis of randomized controlled trials. PLoS One. 2022;17:e0264759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 34. | Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, Chi HS, Cho CH. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Tang B, Gao GM, Zou Z, Liu DN, Tang C, Jiang QG, Lei X, Li TY. [Efficacy comparison between robot-assisted and laparoscopic surgery for mid-low rectal cancer: a prospective randomized controlled trial]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |