Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1176
Peer-review started: October 12, 2023
First decision: January 12, 2024
Revised: February 2, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: April 27, 2024
Processing time: 192 Days and 21.5 Hours
Chronic myelomonocytic leukemia (CMML) complicated with Sweet syndrome (SS) is a rare hematological neoplasm. However, cases of concomitant develop
We report a case of a 49-year-old male patient who underwent sequential procedures for hemorrhoids and perianal abscess. He developed postoperative incision infection and was referred to the department where the authors work. Initially, perianal necrotizing fasciitis secondary to incision infection after perianal abscess surgery was suspected. Despite receiving antibiotic therapy and under
CMML with perianal NSS is a rare condition, often misdiagnosed as perianal abscess or perianal necrotizing fasciitis. Conventional antibiotic therapy and surgical debridement are ineffective in managing this condition.
Core Tip: Chronic myelomonocytic leukemia with Sweet syndrome (SS) is uncommon. Distinguishing between necrotizing SS (NSS) and necrotizing fasciitis can be challenging, despite significant differences in their treatment approaches. While prompt surgical debridement and antimicrobial therapy are crucial for patients with necrotizing fasciitis, antimicrobial therapy is ineffective in NSS, and surgical debridement may exacerbate the condition. Cases presenting with initial manifestations in the perianal region have not been reported previously. Therefore, our case study contributes to a better under
- Citation: Yu KQ, Li HX, Wu J. Suspected coexistence of perianal necrotizing sweet syndrome in chronic myelomonocytic leukemia: A case report. World J Gastrointest Surg 2024; 16(4): 1176-1183
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1176.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1176
Chronic myelomonocytic leukemia (CMML) is a rare hematological neoplasm that primarily affects the elderly population and lacks specific clinical features. We here report a 49-year-old male patient with CMML who was highly suspected to have perianal necrotizing Sweet syndrome (SS). SS, also known as acute febrile neutrophilic dermatosis, can occur as a cause of fever, and about 20% of SS cases are associated with malignancies, most of which are hematological tumors[1]. Skin lesions in SS typically appear on the upper limbs, face and neck[2]. However, up to date, there have been no reported cases of perianal SS associated with hematological diseases in public databases such as PubMed. In recent years, a no50-63vel necrotizing variant of SS, known as acute necrotizing neutrophilic dermatosis, has emerged. This variant involves the fascia and deep tissue, often leading to misdiagnosis as necrotizing fasciitis[3]. Therefore, it is crucial to differentiate this new disease from necrotizing fasciitis.
The patient underwent hemorrhoid surgery at a local hospital 20 d previously due to the protrusion of a perianal swelling accompanied by fresh blood during bowel movements. Ten days after the procedure, he experienced perianal redness, swelling, and pain accompanied by fever. Perianal abscess was diagnosed, and incision and drainage of were performed. Following surgery, he received antimicrobial therapy and underwent regular dressing changes. However, the perianal incision failed to heal, and he experienced recurrent episodes of fever. Consequently, the patient was referred to the hospital where the authors work.
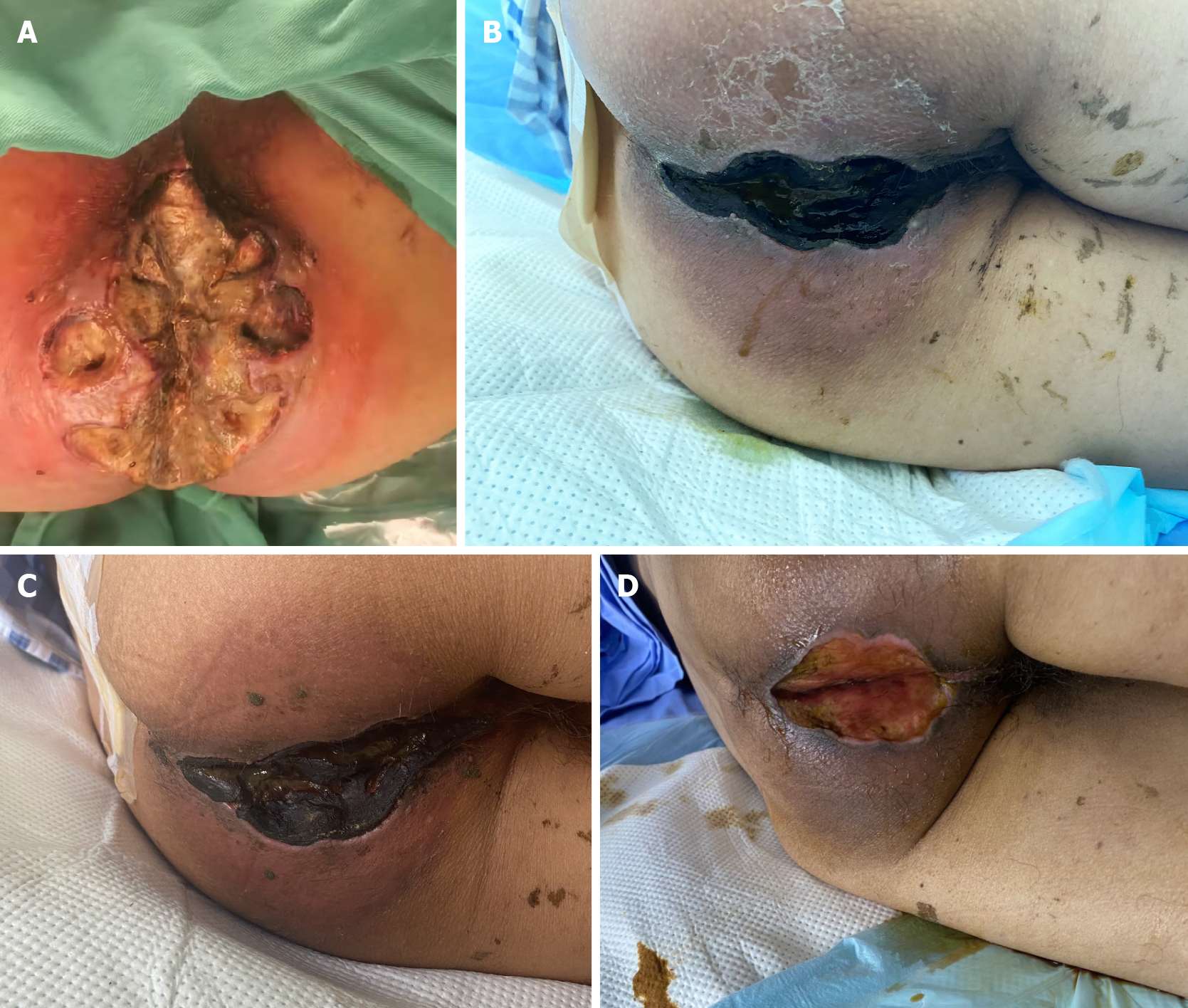
The patient was promptly treated with intravenous cefoxitin sodium antibiotics upon admission, and the dressing was changed daily. The wound exudate culture revealed the growth of carbapenem-resistant Acinetobacter baumannii, which was found to be resistant to cefoxitin. Consequently, tigecycline was chosen based on the results of drug sensitivity testing, but it failed to alleviate wound secretion and improve the surrounding tissue necrosis. Two days later, the patient developed a fever, with the highest recorded temperature being 38.5 °C. Due to the lack of improvement in the infection with the current treatment approach, surgical debridement was performed under general anesthesia. During the operation, a significant area of perianal skin necrotic ulceration was identified, reaching the myofascial layer. The wound exhibited gray-white appearance with flocculent fascial necrosis, while the local skin displayed black gangrene. The skin surrounding the lesion was red and enveloped in subcutaneous purulent secretion, with foul-smelling necrotic tissue fluid and purulent discharge emanating from the squeezed wound cavity (Figure 1A). Necrosis and blackening of the mucosa in the anal canal area was observed.
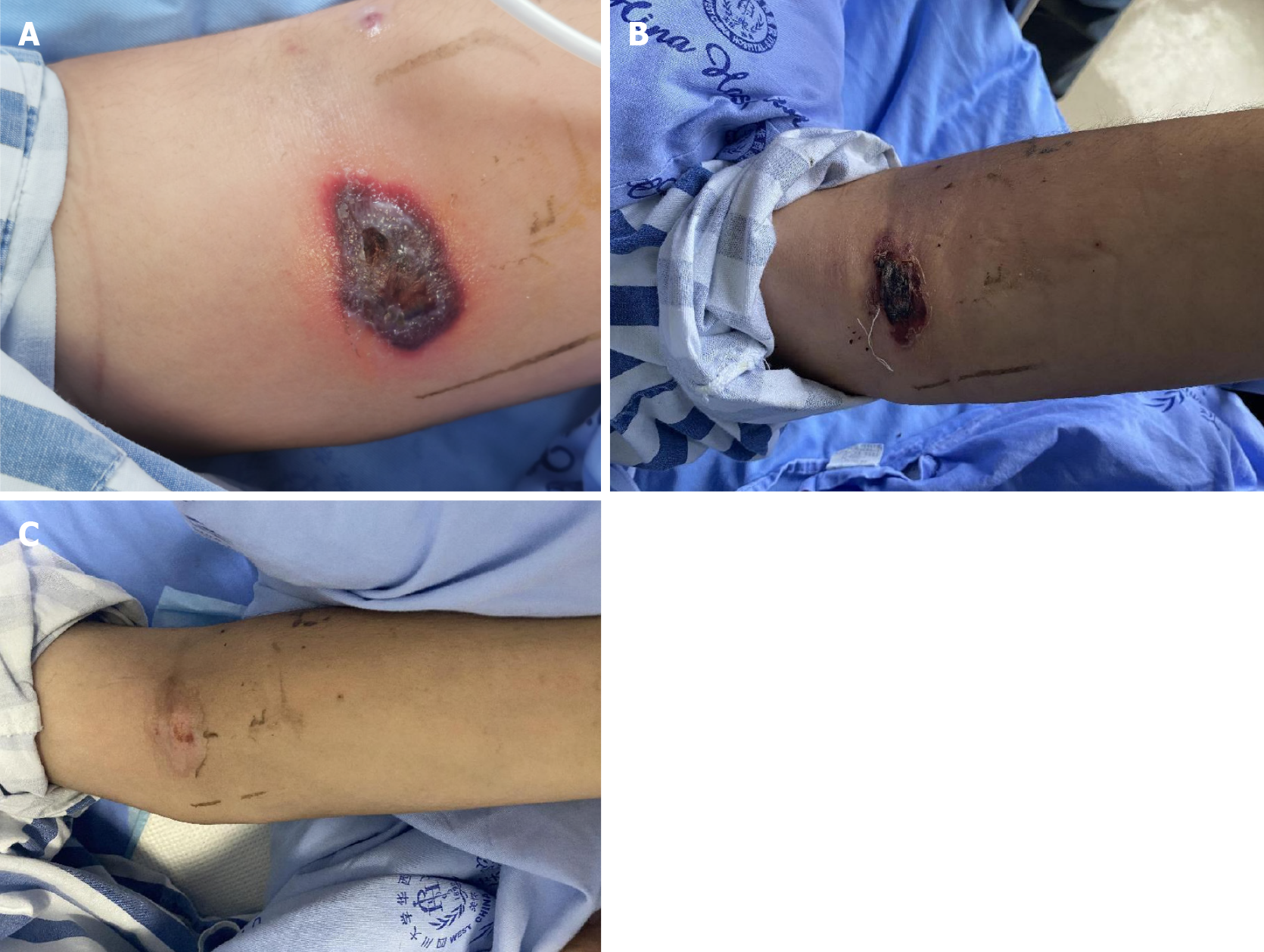
After surgery, body temperature returned to normal. However, after 3 d, the patient experienced recurrence of fever, with the temperature reaching 38.8 °C. The perianal surgical wound displayed gangrenous changes and turned black (Figure 1B). Simultaneously, the patient developed several hemorrhagic blisters, some of which appeared at the venipuncture sites in the left cubital fossa and right hip (Figure 2).
The patient’s past medical history was non-contributory.
The patient had no history of malignancy and no family history of genetic diseases.
Upon admission, a perianal examination revealed a perianal incision that extended radially, accompanied by swelling around and in the proximity of the anus. The incision wound was observed to be covered with necrotic tissue.
Complete blood count showed: Hemoglobin 75 g/L (normal range: 115-150 g/L), white blood cell counts 4.49 × 109/L (normal range: 3.5-9.5 × 109/L), with differential count revealing 43.5% (normal range: 40%-75%) neutrophils, 30.6% (Normal range: 20%-50%) lymphocytes, and 25.3% (normal range: 3.0%-10.0%) monocytes. The absolute monocyte count was 1.13 × 109/L (normal range: 0.1-0.6 × 109/L). The platelet count was 47 × 109/L (normal range: 100-300 × 109/L). Biochemical tests indicated an albumin level of 31.8 g/L (normal range: 40-50 g/L) and a blood glucose level of 6.76 mmol/L (normal range: 3.90-6.1 mmol/L).
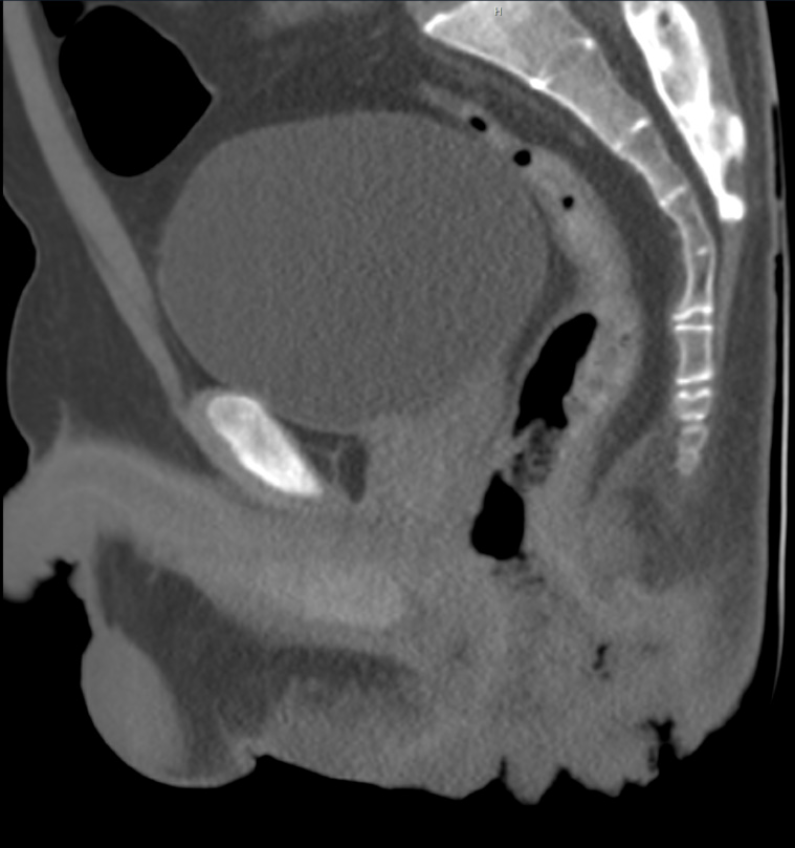
Enhanced pelvic computed tomography scan demonstrated thickening and swelling of the perianal skin with contrast enhancement. Multiple small air cavities were identified. The mucosa in the posterior aspect of the anal canal exhibited partial discontinuity, suggestive of an infectious lesion and the formation of an anal fistula (Figure 3). Abdominal ultrasound revealed splenomegaly with a splenic thickness of 6.4 cm.
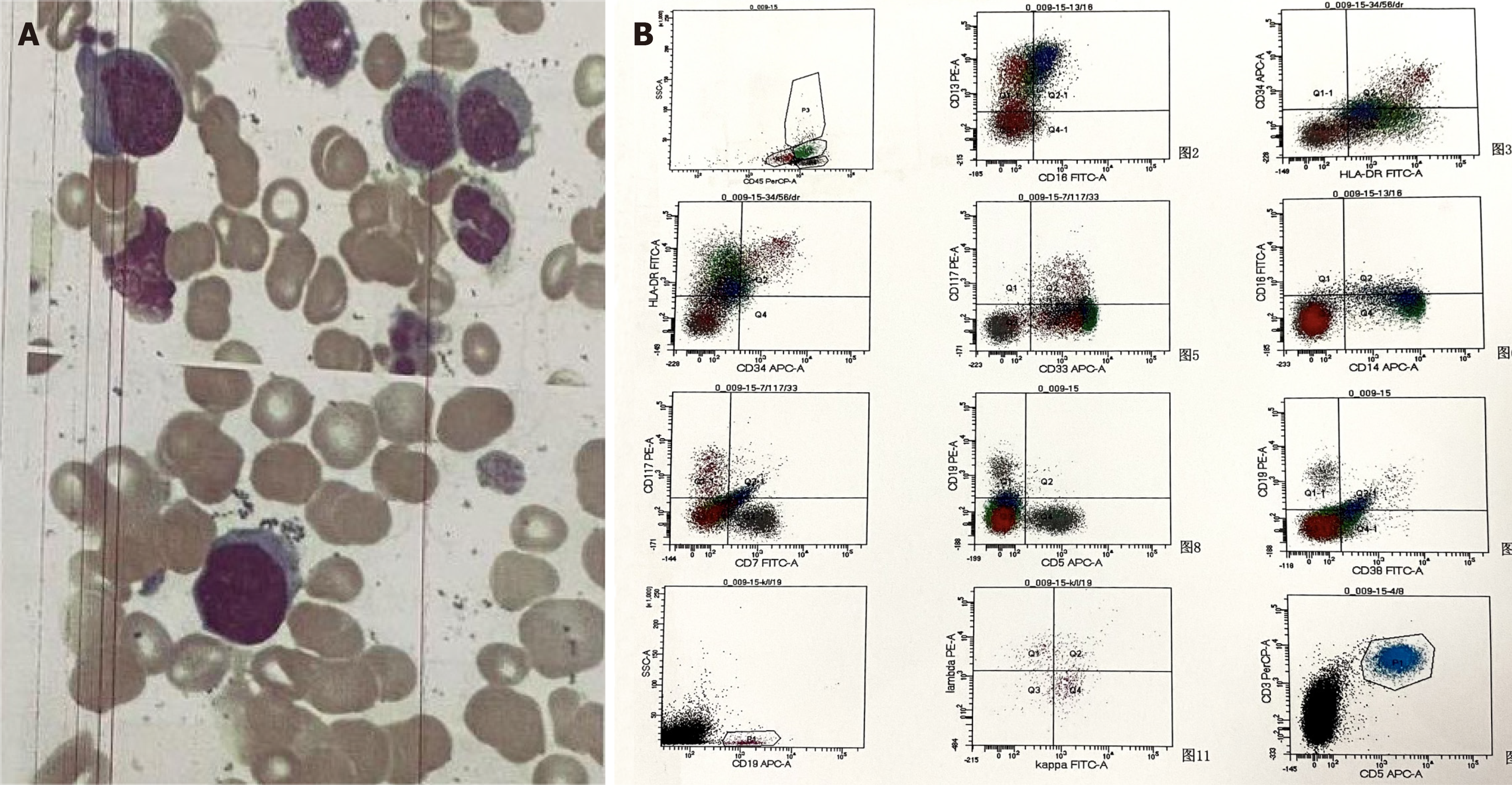
Perianal histopathological diagnosis revealed patchy necrosis and inflammatory exudation. Bone marrow smears demonstrated 4.5% myeloblasts and 45% monocytes (Figure 4A). Bone marrow flow cytometry showed 5.2% abnormal myeloblasts, which expressed CD34+, human leukocyte antigen (HLA)-DR+, CD117+, CD13+, CD33+, CD5-, CD7-, CD56-, CD19- and CD38-, as well as 34% monocytes. Monocytes were partially positive for HLA-DR and positive for CD13, CD14 and CD64, but negative for CD16. Among the monocytes, 95.5% expressed CD14+CD16-. The Side Scatter value of lower-stage granulocytes displayed a slight decrease (Figure 4B). In consideration of perianal infections, infection-induced monocytosis needed to be ruled out. Cytometric analysis revealed 95.5% nonclassical monocytes, supporting the diagnosis of CMML.
During hospitalization, multiple hemorrhagic blister lesions developed at the venipuncture site in the left cubital fossa, the bone marrow puncture site in the left buttock, and the right hip joint. Based on the patient’s medical history and clinical examination, SS was suspected.
After the diagnosis of CMML, the patient was referred to the hematology department. Azacitidine at a doe of 100 mg/d was administered for 7 consecutive days, and the dexamethasone dose was gradually reduced. The hemorrhagic blisters on the upper limbs showed significant improvement. Bone marrow suppression occurred as a result of the treatment, leading to the administration of red blood cell transfusion, platelet transfusion, and granulocyte-stimulating factor injection. During this period, the patient developed a pulmonary infection, which improved following treatment with imipenem and voriconazole. Concurrently, the perianal area and the perianal wound gradually formed crusts (Figure 1C).
After 3 wk of treatment with azacitidine, the patient exhibited normalization of the white cell count, significant improvement in the platelet count and hemoglobin level, gradual shedding of scabs in the perianal region and perianal wounds, and exposure of newly formed granulation tissue (Figure 1D). The patient expressed a wish to be transferred back to a local hospital for further management. Unfortunately, after a follow-up period of 6 months, the patient succumbed.
A review of the patient’s condition revealed that the patient was diagnosed with perianal necrotizing fasciitis based on the manifestations of local necrosis and the results of a pathological biopsy (inflammatory granulation tissue and necrotic tissue) conducted at another hospital. The patient underwent surgery to remove the necrotic tissue and was administered broad-spectrum antibiotics. However, the perianal wound progressed to a deeper necrotic wound. The patient also experienced recurrent high fever after surgery, with negative results of blood cultures and fungal cultures. The pathology report described patchy necrosis and inflammatory exudation. Following joint discussions among dermatologists, hematologists, and other multidisciplinary physicians, it was concluded that there was no evidence of CMML infiltration based on the pathological examination. Meanwhile, the patient developed multiple hemorrhagic blister lesions during hospitalization at the left cubital fossa venipuncture site, the left hip bone marrow puncture site, and the right hip joint. Based on these findings, the most likely diagnosis was necrotizing SS (NSS). After multidisciplinary consultation, dexamethasone treatment was initiated, resulted in significant improvement in the patient’s skin and perianal wounds. We believe that the rarity of CMML and the atypical nature of its skin lesions, coupled with the potential impact of early repeated surgical intervention on the skin lesions, significantly complicate the diagnosis of the patient’s condition, increasing the possibility of misdiagnosis.
CMML is a type of leukemia that is characterized by a combination of myelodysplastic syndromes and myeloproliferative neoplasms. In 2016, the World Health Organization revised the classification of myeloid tumors and acute leukemia, and proposed the latest diagnostic criteria for CMML[4] (Table 1). The clinical manifestations of the disease may include night sweats, splenomegaly, weight loss, and other, nonspecific symptoms[5]. Niemeyer et al[6] summarized its clinical features as fever, pallor, hemorrhage, hepatosplenomegaly and lymphadenopathy. Most cases of the disease progress slowly, with initial symptoms being mild and hematological manifestations not being distinct, making timely diagnosis challenging. The skin lesions of CMML can manifest as specific skin lesions caused by leukemia cells directly invading the epidermis, dermis, or subcutaneous tissue. These may include purple or reddish-brown nodules, papules, plaques of different sizes, and papular rashes. Additionally, CMML skin lesions can present as non-specific manifestations, resembling conditions such as sarcoidosis and SS[7].
| Diagnostic criteria for CMML |
| Persistent PB monocytosis ≥ 1 × 109/L, with monocytes accounting for ≥ 10% of the WBC count |
| Not meeting WHO criteria for BCR-ABL1+ CML, PMF, PV, or ET |
| No evidence of PDGFRA, PDGFRB, or FGFR1 rearrangement or PCM1-JAK2 (should be specifically excluded in cases of eosinophilia) |
| < 20% blasts in the blood and BM |
| Dysplasia in 1 or more myeloid lineages. If myelodysplasia is absent or minimal, the diagnosis of CMML may still be made if the other requirements are met |
| An acquired clonal cytogenetic or molecular genetic abnormality is present in hemopoietic cells |
| The monocytosis (as previously defined) has persisted for at least 3 months |
| All other causes of monocytosis have been excluded |
SS was first described in 1964 by Sweet[8] as an acute febrile neutrophilic dermatosis. It is characterized by a sudden onset of fever, leukocytosis, and tender, erythematous, and well-defined papules and plaques. It can occur after infection or in association with pregnancy, inflammatory bowel disease, drug treatment, or malignancy[9].
To diagnose SS or acute febrile neutrophilic dermatosis, the major criterion and at least two of the four minor criteria[10] must be met (Table 2). However, the patient’s elevated risk of skin lesions induced by venipuncture and surgical trauma, coupled with the refusal of the patient’s wife (an authorized surrogate) to undergo an invasive skin biopsy, limited our ability to unequivocally confirm the diagnosis of SS. Following discussions among multidisciplinary physicians, including hematologists and dermatologists, and considering clinical symptoms along with the swift remission of skin lesions after the administration of glucocorticoids, the presence of SS was considered.
| Major | (1) Abrupt onset of tender or painful erythematous plaques or nodules occasionally with vesicles, pustules or bullae |
| (2) Predominantly neutrophilic infiltration in the dermis without leukocytoclastic vasculitis | |
| Minor | (1) Preceded by a nonspecific respiratory or gastrointestinal tract infection or vaccination or associated with: |
| Inflammatory diseases such as chronic autoimmune disorders, infections | |
| Hemoproliferative disorders or solid malignant tumors | |
| Pregnancy | |
| (2) Accompanied by periods of general malaise and fever (> 38 °C) | |
| (3) Laboratory values during onset: ESR > 20 mm; C-reactive protein positive; segmented-nuclear neutrophils and stabs > 70% in peripheral blood smear; leukocytosis > 8000 (three of four of these values necessary) | |
| (4) Excellent response to treatment with systemic corticosteroids or potassium iodide |
Typically, SS manifests as painful erythematous plaques or nodules on the upper limbs, trunk, head and neck. The lesions can be clear or vesicular, but are usually solid[11]. However, in cases associated with malignancy, atypical skin lesions may occur, such as bullous SS[12], giant cellulitis-like SS[13], or NSS. Several cases of NSS in different body parts have been reported in the literature[14-17]. These cases were initially misdiagnosed. In all reports, the authors emphasize the need to distinguish NSS from necrotizing fasciitis, as the surgical debridement of NSS can lead to further exacerbation of SS and involvement of additional tissue.
Perianal necrotizing fasciitis, also known as Fournier’s gangrene, is a rapidly progressing bacterial infection that affects the subcutaneous fascia and parts of the deep fascia[18]. However, it does not involve the muscles of the scrotum, perianal, and perineal regions. The treatment of necrotizing fasciitis requires extensive debridement, antibiotic therapy, and nutritional support. Although timely surgical intervention can reduce mortality associated with necrotizing fasciitis, surgical intervention may worsen NSS. Therefore, early identification of necrotizing fasciitis and NSS is crucial[19].
To achieve early identification, collaboration between different medical specialties is essential. By working together, doctors and medical teams from various specialties can collect and analyze the clinical manifestations, laboratory test results, and imaging data of patients to comprehensively evaluate their condition. Moreover, multidisciplinary physicians can develop treatment strategies together to ensure that patients receive the appropriate treatment as early as possible.
This case contributes to a better understanding of the etiology and symptomatic features of CMML presenting with perianal NSS, which is a rare occurrence. Further understanding of the various aspects of this disease will facilitate more precise diagnoses.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Augustin G, Croatia; Handra-Luca A, France; Kupeli S, Turkey S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Fazili T, Duncan D, Wani L. Sweet's syndrome. Am J Med. 2010;123:694-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Paydas S. Sweet's syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kroshinsky D, Alloo A, Rothschild B, Cummins J, Tan J, Montecino R, Hoang MP, Duncan L, Mihm M, Sepehr A. Necrotizing Sweet syndrome: a new variant of neutrophilic dermatosis mimicking necrotizing fasciitis. J Am Acad Dermatol. 2012;67:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6745] [Article Influence: 749.4] [Reference Citation Analysis (0)] |
| 5. | Liu XZ, Z Q, Zhai YZ, Chen X, Miao YY, Zhao CH, Xiao HJ, Liu G. Chronic Fever-Characterized Chronic Myelomonocytic Leukemia Accompanying Sweet’s Syndrome: A Case Report. Jiefangjun Yixueyuan Xuebao. 2013;34:1128-1130+1134. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Niemeyer CM, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Haas O, Harbott J, Hasle H, Kerndrup G, Locatelli F, Mann G, Stollmann-Gibbels B, van't Veer-Korthof ET, van Wering E, Zimmermann M. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS). Blood. 1997;89:3534-3543. [PubMed] |
| 7. | Qiao Y, Jian J, Deng L, Tian H, Liu B. Leukaemia cutis as a specific skin involvement in chronic myelomonocytic leukaemia and review of the literature: Acknowledgments. Transl Cancer Res. 2020;9:4988-4998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Sweet RD. An Acute Febrile Neutrophilic Dermatosis. Br J Dermatol. 1964;76:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 693] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | So JK, Carlos CA, Frucht CS, Cohen PR. Histiocytoid giant cellulitis-like Sweet's syndrome: case report and review of the literature. Dermatol Online J. 2015;21. [PubMed] |
| 10. | von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-56; quiz 557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 470] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Joshi TP, Friske SK, Hsiou DA, Duvic M. New Practical Aspects of Sweet Syndrome. Am J Clin Dermatol. 2022;23:301-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Giannoni M, Rizzetto G, Sapigni C, Paolinelli M, Tagliati C, Diotallevi F, Campanati A, Mandolesi A, Pepi L, Offidani A. Bullous Sweet's syndrome in a patient with ulcerative colitis: a rare case report. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29:153-155. [PubMed] |
| 13. | Mitaka H, Jammal R, Saabiye J, Yancovitz S, Perlman DC. Giant cellulitis-like Sweet syndrome: An underrecognized clinical variant mimicking skin and soft tissue infection. IDCases. 2020;21:e00874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sanchez IM, Lowenstein S, Johnson KA, Babik J, Haag C, Keller JJ, Ortega-Loayza AG, Cohen J, McCalmont TH, Demer AM, Mansh MD, Hylwa SA, Liu J, Shinkai K. Clinical Features of Neutrophilic Dermatosis Variants Resembling Necrotizing Fasciitis. JAMA Dermatol. 2019;155:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Hradil E, Jeppsson C, Hamnerius N, Svensson Å. The diagnosis you wish you had never operated on: Pyoderma gangrenosum misdiagnosed as necrotizing fasciitis-a case report. Acta Orthop. 2017;88:231-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Gowda A, Christensen L, Polly S, Barlev D. Necrotizing neutrophilic dermatosis: A diagnostic challenge with a need for multi-disciplinary recognition, a case report. Ann Med Surg (Lond). 2020;57:299-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Nakanishi K, Kinjo M. Mimicker of necrotising fasciitis with systemic inflammatory response syndrome: recurrent necrotising Sweet's syndrome associated with chronic myelogenous leukaemia. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Bruketa T, Majerovic M, Augustin G. Rectal cancer and Fournier's gangrene - current knowledge and therapeutic options. World J Gastroenterol. 2015;21:9002-9020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Anand A, Gentile T, Kato H, Wang Q. Recurrent soft tissue inflammation, necrotizing fascitis or Sweet syndrome, diagnostic dilemma. Clin Case Rep. 2019;7:2483-2487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |