Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1121
Peer-review started: January 19, 2024
First decision: February 5, 2024
Revised: February 12, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: April 27, 2024
Processing time: 94 Days and 7.6 Hours
Surgical site infection (SSI) is a common complication of colorectal surgery. Mi
To compare the incidences of SSI after RACS and LACS, and to analyze the risk factors associated with SSI after minimally invasive colorectal surgery.
Clinical data derived from patients who underwent minimally invasive colorectal surgery between October 2020 and October 2022 at the First Affiliated Hospital of Soochow University were collated. Differences in clinical characteristics and sur
A total of 246 patients (112 LACS and 134 RACS) were included in the study. Fortythree (17.5%) developed SSI. The proportions of patients who developed SSI were similar in the two groups (17.9% vs 17.2%, P = 0.887). Diabetes mellitus, in
There was no difference in SSI incidence in the RACS and LACS groups. Diabetes mellitus, intraoperative blood loss ≥ 100 mL, and incision length were indepen
Core Tip: The application of robotic surgery in colorectal surgery is becoming increasingly widespread. While it brings convenience of operation, it is still unclear whether it increases the risk of surgical site infection (SSI). The current study compared the incidences of SSI in robot-assisted colorectal surgery and laparoscopic-assisted colorectal surgery, and analyzed potential risk factors associated with SSI after minimally invasive colorectal surgery, to provide guidance for clinical practice.
- Citation: Ni LT, Zhao R, Ye YR, Ouyang YM, Chen X. Incidence of surgical site infection in minimally invasive colorectal surgery. World J Gastrointest Surg 2024; 16(4): 1121-1129
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1121.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1121
According to World Health Organization (WHO) guidelines, surgical site infection (SSI) is defined as an infection that occurs within 30 d after an operation and involves the skin and subcutaneous tissue of the incision (superficial incisional) and/or the deep soft tissue (for example fascia and muscle) of the incision (deep incisional) and/or any part of the anatomy (for example organs and spaces) other than the incision that was opened or manipulated during an operation[1]. Common risk factors for SSI include preoperative diabetes mellitus, contaminative incision, excess subcutaneous fat, advanced age, obesity, and emergency surgery[2,3].
Colorectal surgical incision is a type II incision. Pathogens that often colonize the digestive tract may cause SSI. According to relevant studies, patients undergoing colorectal surgery are particularly at risk of SSI, with the infection rate as high as 26%[4,5]. Therefore, SSI is one of the most common early complications after colorectal surgery, which often leads to an increase in costs and hospitalization and even affects oncologic outcomes[6-8].
The application of robotic surgery is currently increasing, but it is expensive and involves issues such as installing and removing machines, resulting in longer surgery times. Robotic surgery brings convenience, but it is not clear whether it increases the risk of incision infections. The current study compared the incidences of SSI after robot-assisted colorectal surgery (RACS) and laparoscopic assisted colorectal surgery (LACS), and analyzed potential risk factors associated with SSI after minimally invasive colorectal surgery.
This retrospective study included 246 patients who underwent minimally invasive colorectal surgery (LACS or RACS) at the General Surgery Department of the First Affiliated Hospital of Suzhou University from October 2020 to October 2022. The inclusion criteria were: (1) Patients scheduled to undergo elective radical surgery, and preoperative preparation was complete; (2) the minimally invasive surgery undergone was the first operation since admission; (3) age range 18-90 years; and (4) the operation was performed by the same general surgeon with experience in robotic surgery and laparoscopic surgery. The exclusion criteria were: (1) Patients who underwent open surgery or emergency surgery; (2) patients with active infection or a purulent cavity in the operation area before surgery; and (3) patients who were lost to follow-up.
According to existing guidelines, the patients all met the scope of minimally invasive colorectal surgery. The choice of surgical method was based on the patients’ wishes. The study was approved by the Ethics Committee of the First Affi
Age, gender, body mass index (BMI), American Society of Anesthesiologist (ASA) grade, history of past illness, pa
All patients were administered cefazolin (1 g) or cefathiamidine (1 g) via intravenous drip 0.5-1.0 h before the ope
The main outcome measure was the incidence of SSI within 30 d after surgery. SSI was diagnosed based on WHO guidelines. Observation index information was mainly obtained via telephone calls and outpatient follow-up.
All statistical analyses were performed using SPSS software (version 26.0). The twotailed t-test was used for continuous variables, unless the data were non-normally distributed. In such cases the Mann-Whitney U test was used to assess comparisons. The χ2 test or Fisher’s exact test were used for categorical variables, which were summarized as frequencies and percentages. All collected variables were analyzed using univariate logistic analysis, and those with P < 0.15 were selected for inclusion in multivariable logistic analysis. All statistical analyses were two-sided, and P < 0.05 was con
A total of 246 patients were included in the study, 112 who underwent LACS and 134 who underwent RACS. The clinical characteristics of the patients are shown in Table 1. The age of the patients in the LACS and RACS groups was similar (64 years vs 66 years, P = 0.105), and most were male. Twenty-eight (11.4%) had a history of smoking, 97 (39.4%) had a history of hypertension, and 31 (12.6%) had diabetes mellitus. More patients in the LACS group received neoadjuvant therapy before surgery (9.8% vs 3.7%, P = 0.054) and there was more intraoperative blood loss in the LACS group (44.6% vs 14.2%, P < 0.001). The mean operation time was longer in the RACS group (281 min vs 243 min, P = 0.004). The anastomosis method was similar in the two groups. The incidences of SSI were similar in the two groups (17.9% vs 17.2%, P = 0.887), and the overall incidence of SSI was 17.5% (Table 2).
| LACS (n = 112) | RACS (n = 134) | P value | |
| Age (yr) | 64 (56, 70) | 66 (58, 72) | 0.105 |
| Sex | 0.590 | ||
| Male | 78 (69.6) | 89 (66.4) | |
| Female | 34 (30.4) | 45 (33.6) | |
| BMI | 0.334 | ||
| < 24 | 76 (67.9) | 83 (61.9) | |
| ≥ 24 | 36 (32.1) | 51 (38.1) | |
| Lesion site | 0.925 | ||
| Colon | 57 (50.9) | 69 (51.5) | |
| Rectum | 55 (49.1) | 65 (48.5) | |
| ASA | 0.188 | ||
| Ⅰ–Ⅱ | 98 (87.5) | 109 (81.3) | |
| Ⅲ–Ⅳ | 14 (12.5) | 25 (18.7) | |
| Hypertension | 0.407 | ||
| No | 71 (63.4) | 78 (558.2) | |
| Yes | 41 (36.6) | 56 (41.8) | |
| Diabetes mellitus | 0.415 | ||
| No | 100 (89.3) | 115 (85.8) | |
| Yes | 12 (10.7) | 19 (14.2) | |
| Hyperlipidemia | 0.291 | ||
| No | 79 (70.5) | 86 (64.2) | |
| Yes | 33 (29.5) | 48 (35.8) | |
| Smoking history | 0.919 | ||
| No | 99 (88.4) | 119 (88.8) | |
| Yes | 13 (11.6) | 15 (11.2) | |
| History of abdomen surgery | 0.534 | ||
| No | 84 (75.0) | 105 (78.4) | |
| Yes | 28 (25.0) | 29 (21.6) | |
| Previous operation except abdomen surgery | 0.801 | ||
| No | 82 (73.2) | 100 (74.6) | |
| Yes | 30 (26.8) | 34 (25.4) | |
| Tumor marker | 0.730 | ||
| Normal | 93 (83.0 | 109 (81.3) | |
| Abnormal | 19 (17.0) | 25 (18.7) | |
| Liver function | 0.599 | ||
| Normal | 107 (95.5) | 126 (94.0) | |
| Abnormal | 5 (4.5) | 8 (6.0) | |
| Albumin | 0.366 | ||
| ≥ 40 | 63 (56.3) | 83 (61.9) | |
| < 40 | 49 (43.8) | 51 (38.1) | |
| Preoperative CRP | 0.687 | ||
| Normal | 82 (73.2) | 95 (70.9) | |
| Abnormal | 30 (26.8) | 39 (29.1) | |
| Uric acid | 0.109 | ||
| Normal | 98 (87.5) | 107 (79.9) | |
| Abnormal | 14 (12.5) | 27 (20.1) | |
| Recent weight loss | 0.378 | ||
| No | 85 (75.9) | 95 (70.9) | |
| Yes | 27 (24.1) | 39 (29.1) | |
| Neoadjuvant therapy | 0.054 | ||
| No | 101 (90.2) | 129 (96.3) | |
| Yes | 11 (9.8) | 5 (3.7) |
| LACS (n = 112) | RACS (n = 134) | P value | |
| Pathology | |||
| Benign | 49 (43.8) | 47 (35.1) | 0.165 |
| Malignant | 63 (56.3) | 87 (64.9) | |
| Method of anastomosis | 0.848 | ||
| Intracorporeal | 63 (56.2) | 77 (57.5) | |
| Extracorporeal | 49 (43.8) | 57 (42.5) | |
| Incision length | (66, 6) | 6 (5, 6) | 0.549 |
| Intraoperative blood loss | < 0.001 | ||
| < 100 mL | 62 (55.4) | 115 (85.8) | |
| ≥ 100 mL | 50 (44.6) | 19 (14.2) | |
| Operation time | 243 (212, 305) | 281 (239, 317) | 0.004 |
| Time of postoperative intake (d) | 2 (2, 3) | 2 (2, 3) | 0.783 |
| SSI | 0.887 | ||
| No | 92 (82.1) | 111 (82.8) | |
| Yes | 20 (17.9) | 23 (17.2) |
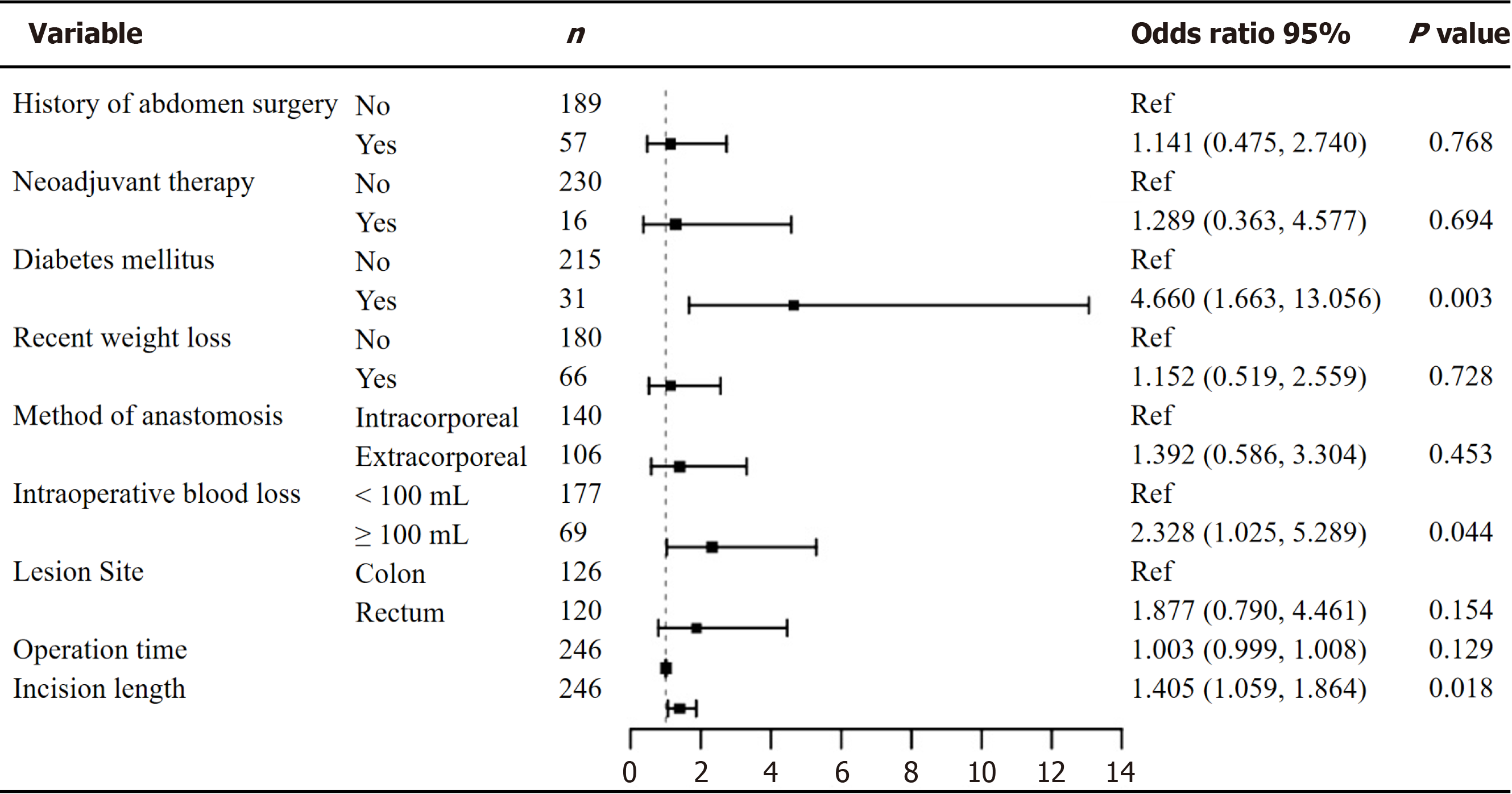
Demographic and operative information of the cohort by the occurrence or otherwise of SSI is shown in Table 3. In logistic analysis lesion site [odds ratio (OR) 1.996, 95% confidence interval (CI) 1.014-3.929, P = 0.045], diabetes mellitus (OR 3.749, 95%CI 1.656-8.484, P = 0.002), neoadjuvant therapy (OR 3.130, 95%CI 1.072-9.138, P = 0.037), incision length (OR 1.429, 95%CI 1.126-1.815, P = 0.003), intraoperative blood loss ≥ 100 mL (OR 3.082, 95%CI 1.562-6.084, P = 0.001), and long operation time (OR 1.005, 95%CI 1.001-1.009, P = 0.006) were predictors of SSI. There was no significant difference in the incidences of SSI in the LACS and RACS groups (OR 0.953, 95%CI 0.493-1.844, P = 0.887). Variables with P < 0.15 in the univariate analysis were then included in a multivariate analysis (Figure 1). Independent risk factors for SSI after minimally invasive colorectal surgery indicated in that analysis were diabetes mellitus (OR 4.660, 95%CI 1.663-13.056, P = 0.003), intraoperative blood loss ≥ 100 mL (OR 2.328, 95%CI 1.025-5.289, P = 0.044), and incision length (OR 1.405, 95%CI 1.059-1.864, P = 0.018).
| Non-SSI (n = 203) | SSI (n = 43) | OR | 95%CI | P value | |
| Sex | |||||
| Male | 136 (76.0) | 31 (72.1) | Ref | ||
| Female | 6 (33.0) | 12 (27.9) | 0.786 | 0.380-1.627 | 0.516 |
| Age | 65 (57, 71) | 64 (57, 72) | 1.006 | 0.976-1.037 | 0.697 |
| BMI | |||||
| < 24 | 134 (66.0) | 25 (58.1) | Ref | ||
| ≥ 24 | 69 (34.0) | 18 (41.9) | 1.398 | 0.714-2.738 | 0.328 |
| Pathology | |||||
| Benign | 80 (39.4) | 16 (37.2) | Ref | ||
| Malignant | 123 (60.6) | 27 (62.8) | 1.098 | 0.556-2.165 | 0.788 |
| Lesion site | |||||
| Colon | 110 (54.2) | 16 (37.2) | Ref | ||
| Rectum | 93 (45.8) | 27 (62.8) | 1.996 | 1.014-3.929 | 0.045 |
| ASA | |||||
| Ⅰ–Ⅱ | 172 (84.7) | 35 (81.4) | Ref | ||
| Ⅲ–Ⅳ | 31 (15.3) | 8 (18.6) | 1.268 | 0.538-2.991 | 0.587 |
| Hypertension | |||||
| No | 125 (61.6) | 24 (55.8) | Ref | ||
| Yes | 78 (38.4) | 19 (44.2) | 1.269 | 0.652-2.467 | 0.483 |
| Diabetes mellitus | |||||
| No | 184 (90.6) | 31 (72.1) | Ref | ||
| Yes | 19 (9.4) | 12 (27.9) | 3.749 | 1.656-8.484 | 0.002 |
| Hyperlipidemia | |||||
| No | 140 (69.0) | 25 (58.1) | Ref | ||
| Yes | 63 (31.0) | 18 (41.9) | 1.600 | 0.815-3.142 | 0.172 |
| Smoking history | |||||
| No | 178 (87.7) | 40 (93.0) | Ref | ||
| Yes | 25 (12.3) | 3 (7.0) | 0.534 | 0.154-1.856 | 0.324 |
| History of abdomen surgery | |||||
| No | 160 (78.8) | 29 (67.4) | Ref | ||
| Yes | 43 (21.2) | 14 (32.6) | 1.796 | 0.873-3.695 | 0.111 |
| Previous operation except abdomen surgery | |||||
| No | 151 (74.4) | 31 (72.1) | Ref | ||
| Yes | 52 (25.6) | 12 (27.9) | 1.124 | 0.538-2.349 | 0.756 |
| Tumor marker | |||||
| Normal | 167 (82.3) | 35 (81.4) | Ref | ||
| Abnormal | 36 (17.7) | 8 (18.6) | 1.060 | 0.454-2.477 | 0.892 |
| Liver function | |||||
| Normal | 194 (95.6) | 39 (90.7) | Ref | ||
| Abnormal | 9 (4.4) | 4 (9.3) | 2.211 | 0.648-7.541 | 0.205 |
| Preoperative albumin | |||||
| ≥ 40 | 124 (61.1) | 22 (51.2) | Ref | ||
| < 40 | 79 (38.9) | 21 (48.8) | 1.498 | 0.773-2.902 | 0.231 |
| Uric acid | |||||
| Normal | 166 (81.8) | 39 (90.7) | Ref | ||
| Abnormal | 37 (18.2) | 4 (9.3) | 0.460 | 0.155-1.367 | 0.162 |
| Recent weight loss | |||||
| No | 153 (75.4) | 27 (62.8) | Ref | ||
| Yes | 50 (24.6) | 16 (37.2) | 1.813 | 0.904-3.637 | 0.094 |
| Neoadjuvant therapy | |||||
| No | 193 (95.1) | 37 (86.0) | Ref | ||
| Yes | 10 (4.9) | 6 (14.0) | 3.130 | 1.072-9.138 | 0.037 |
| Method of anastomosis | |||||
| Intracorporeal | 120 (59.1) | 20 (46.5) | Ref | ||
| Extracorporeal | 83 (40.9) | 23 (53.5) | 1.663 | 0.858-3.221 | 0.132 |
| Incision length | 6 (5, 7) | 6 (6, 6) | 1.429 | 1.126-1.815 | 0.003 |
| Intraoperative blood loss | |||||
| < 100 mL | 155 (76.4) | 22 (51.2) | Ref | ||
| ≥ 100 mL | 48 (23.6) | 21 (48.8) | 3.082 | 1.562-6.084 | 0.001 |
| Operation time | 260 (215, 312) | 301 (243, 334) | 1.005 | 1.001-1.009 | 0.006 |
| Time of postoperative intake (d) | 2 (2, 3) | 2 (2, 3) | 1.241 | 0.737-2.091 | 0.416 |
| Preoperative CRP | |||||
| Normal | 147 (72.4) | 30 (69.8) | Ref | ||
| Abnormal | 56 (27.6) | 13 (30.2) | 1.137 | 0.554-2.337 | 0.726 |
| Surgery approach | |||||
| LACS | 92 (45.3) | 20 (46.5) | Ref | ||
| RACS | 111 (54.7) | 23 (53.5) | 0.953 | 0.493-1.844 | 0.887 |
SSIs are a common complication of colorectal surgery. In previous studies the overall incidence of SSI after colorectal surgery has ranged from approximately 7% to 26%[4,5,9,10]. The high incidence and various adverse effects of SSI have attracted the attention of surgeons. With advances in minimally invasive surgery the incidence of SSI has decreased significantly. In an analysis of a large database in the United States, minimally invasive surgery was associated with a lower incidence of SSI after surgery than open surgery. This was verified at different surgical sites in another study based on the prospective database of the National Surgical Quality Improvement Program for major surgical procedures[11]. Compared with open surgery, laparoscopic surgery can result in a smaller incision length, clearer surgical vision, and a milder systemic inflammatory reaction, which helps to reduce the occurrence of SSI[12].
Since the launch of the da Vinci surgical robot in 2000, minimally invasive surgery has undergone significant changes. It was initially approved for use in general surgeries. Over the past 20 years robotic surgery has undergone further de
The total incidence of SSI in the current study was 17.5%, which is within the range of previously reported incidences of SSI mentioned above. There was also no significant difference in the incidences of postoperative SSI in the LACS and RACS groups (P = 0.887). Robotic surgery does not seem to reduce the incidence of postoperative SSI. This may be due to the methods of anastomosis and specimen extraction in robotic surgery being similar to those used in laparoscopic-assisted surgery at present. In a sense, the advantage of robotic surgery lies in optimizing the surgeon’s senses and ope
The independent risk factors for SSI identified in the current study were diabetes mellitus (OR 4.660, 95%CI 1.663-13.056, P = 0.003), intraoperative blood loss ≥ 100 mL (OR 2.328, 95%CI 1.025-5.289, P = 0.044) and incision length (OR 1.405, 95%CI 1.059-1.864, P = 0.018), which was similar to the results of previous studies[18]. The occurrence of SSI in patients with diabetes mellitus may be due to the fact that hyperglycemia interferes with the normal metabolism of cells and produces excessive reactive oxygen species, leading to blocked blood circulation and reduced tissue perfusion. In addition, hyperglycemia can activate the inflammatory pathway, reduce immune function, facilitate the growth of bacte
The study had some limitations. It was retrospective, the sample size was relatively small, and there was some bias. There are also differences between colonic surgery and rectal surgery. Considering the fact that both the colon and the rectum belong to the digestive tract, they were included in the analysis and discussion; but this is another limitation of the study. Moreover, due to insufficient medical records there were few clinical indicators. A prospective study incor
In the present study there was no difference in SSI incidences after RACS and LACS. RACS involved less bleeding, but required longer operation times. In logistic regression analysis diabetes mellitus, intraoperative blood loss, and incision length were independent risk factors for SSI.
The authors sincerely thank the First Affiliated Hospital of Soochow University for the provision of clinical data, which enabled the research to be performed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Augustin G, Croatia; Shah OJ, India S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2018. [PubMed] |
| 2. | Teppa R, Sude NS, Karanam VPK, Mallipudi BVP. Relevance of Subcutaneous Fat Thickness as a Risk Factor for Surgical Site Infections in Abdominal Surgeries. Cureus. 2022;14:e20946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Alkaaki A, Al-Radi OO, Khoja A, Alnawawi A, Maghrabi A, Altaf A, Aljiffry M. Surgical site infection following abdominal surgery: a prospective cohort study. Can J Surg. 2019;62:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Bennett-Guerrero E, Pappas TN, Koltun WA, Fleshman JW, Lin M, Garg J, Mark DB, Marcet JE, Remzi FH, George VV, Newland K, Corey GR; SWIPE 2 Trial Group. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med. 2010;363:1038-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Itani KM, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med. 2006;355:2640-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Sugamata N, Okuyama T, Takeshita E, Oi H, Hakozaki Y, Miyazaki S, Takada M, Mitsui T, Noro T, Yoshitomi H, Oya M. Surgical site infection after laparoscopic resection of colorectal cancer is associated with compromised long-term oncological outcome. World J Surg Oncol. 2022;20:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Katsumata K, Enomoto M, Ishizaki T, Fujita S, Kanemitsu Y, Ito M, Shiomi A, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bando H, Sekimoto M, Kobatake T, Machida R, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Risk factors for surgical site infection and association of surgical site infection with survival of lower rectal cancer patients without clinical lateral pelvic lymph node metastasis (clinical Stage II/III): Analysis of data from JCOG0212. Clin Exp Metastasis. 2021;38:459-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014;149:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, Pinta T, Scheinin T, Sallinen V. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet. 2019;394:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for Antibiotic Administration for Bowel Preparation Among Patients Undergoing Elective Colorectal Surgery: A Network Meta-analysis. JAMA Surg. 2022;157:34-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Gandaglia G, Ghani KR, Sood A, Meyers JR, Sammon JD, Schmid M, Varda B, Briganti A, Montorsi F, Sun M, Menon M, Kibel AS, Trinh QD. Effect of minimally invasive surgery on the risk for surgical site infections: results from the National Surgical Quality Improvement Program (NSQIP) Database. JAMA Surg. 2014;149:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Delgado S, Lacy AM, Filella X, Castells A, García-Valdecasas JC, Pique JM, Momblán D, Visa J. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum. 2001;44:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Krummel TM. Surgical simulation and virtual reality: the coming revolution. Ann Surg. 1998;228:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Fruscione M, Pickens R, Baker EH, Cochran A, Khan A, Ocuin L, Iannitti DA, Vrochides D, Martinie JB. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB (Oxford). 2019;21:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Chen K, Pan Y, Zhang B, Maher H, Wang XF, Cai XJ. Robotic versus laparoscopic Gastrectomy for gastric cancer: a systematic review and updated meta-analysis. BMC Surg. 2017;17:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Sheng S, Zhao T, Wang X. Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer: A network meta-analysis. Medicine (Baltimore). 2018;97:e11817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Kamarajah SK, Sutandi N, Robinson SR, French JJ, White SA. Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB (Oxford). 2019;21:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Cai W, Wang L, Wang W, Zhou T. Systematic review and meta-analysis of the risk factors of surgical site infection in patients with colorectal cancer. Transl Cancer Res. 2022;11:857-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |