Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1066
Peer-review started: November 21, 2023
First decision: January 19, 2024
Revised: January 29, 2024
Accepted: March 15, 2024
Article in press: March 15, 2024
Published online: April 27, 2024
Processing time: 152 Days and 21.6 Hours
The management of hepatoblastoma (HB) becomes challenging when the tumor remains in close proximity to the major liver vasculature (PMV) even after a full course of neoadjuvant chemotherapy (NAC). In such cases, extreme liver resection can be considered a potential option.
To explore whether computer-assisted three-dimensional individualized extreme liver resection is safe and feasible for children with HB who still have PMV after a full course of NAC.
We retrospectively collected data from children with HB who underwent surgical resection at our center from June 2013 to June 2023. We then analyzed the detailed clinical and three-dimensional characteristics of children with HB who still had PMV after a full course of NAC.
Sixty-seven children diagnosed with HB underwent surgical resection. The age at diagnosis was 21.4 ± 18.8 mon
Extreme liver resection for HB that is still in close PMV after a full course of NAC is both safe and feasible. This approach not only reduces the necessity for liver transplantation but also results in a favorable prognosis. Individualized three-dimensional surgical planning is beneficial for accurate and complete resection of HB, particularly for assessing vascular involvement, remnant liver volume and anatomical variations.
Core Tip: Children with difficult hepatoblastoma (HB), characterized by a large size and complex location, pose a clinical challenge, particularly when the tumor remains in close proximity to the major liver vasculature (PMV) even after a full course of neoadjuvant chemotherapy (NAC). We retrospectively collected data from 67 children with HB who underwent surgical resection at our center from June 2013 to June 2023. Sixteen patients still had close PMV after a full course NAC and underwent extreme liver resection. In this process, the use of individualized three-dimensional surgical planning is beneficial for achieving safe and complete resection.
- Citation: Xiu WL, Liu J, Zhang JL, Wang JM, Wang XF, Wang FF, Mi J, Hao XW, Xia N, Dong Q. Computer-assisted three-dimensional individualized extreme liver resection for hepatoblastoma in proximity to the major liver vasculature. World J Gastrointest Surg 2024; 16(4): 1066-1077
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1066.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1066
Hepatoblastoma (HB) is the most common primary malignant liver tumor in children, and its incidence is increasing[1,2]. The combination of surgery and chemotherapy, particularly neoadjuvant chemotherapy (NAC), has allowed for the delayed resection of many children initially considered unresectable at diagnosis, resulting in a significant improvement in the survival rate of HB patients from approximately 30%[3,4]. Surgery remains a crucial treatment for HB, as it in
Clinically, HB is commonly a large tumor when discovered. Over 50% of HB patients cannot be surgically removed at initial diagnosis according to the PRE-TEXT staging system[6]. These patients require preoperative NAC. After 2 to 4 cycles of NAC, the response to treatment was evaluated using the POST-TEXT staging system. The tumor size of large HB significantly decreases due to its high chemotherapeutic sensitivity, allowing for sufficient future liver remnant volume (FLV) for surgery[7]. At this point, the evaluation of vascular involvement becomes crucial in determining the feasibility of tumor resection.
In fact, approximately 25% of HB patients remain in close proximity to the major liver vasculature (PMV), such as the inferior vena cava (IVC), the main portal vein (MPV), the bifurcation of the portal vein (BPV), and the hepatic veins (HVs), even after undergoing a full course of NAC. This poses a clinical challenge when planning further treatment. While liver transplantation can achieve complete tumor resection, there is increased risk due to the use of living donor livers and the need for lifelong immunosuppressive treatment[8,9]. In recent years, aggressive extreme liver resection has emerged as another viable option, as tumor involvement in blood vessels is no longer considered a contraindication for surgical resection.
The computer-assisted surgery system (Hisense CAS) is capable of performing individualized three-dimensional (3D) reconstruction using traditional two-dimensional (2D) computed tomography (CT) images. This system can provide objective and comparable 3D information about the liver, tumors and blood vessels, overcoming the limitations of 2D images. Additionally, it can be used to efficiently analyze the complex features and anatomical variations of the liver[10,11]. In this study, we present a case series of HB from a single center and aim to explore the safety of computer-assisted 3D individualized extreme liver resection in HB patients where major liver vasculature is still in close proximity after a full course of NAC.
We retrospectively collected data from children with HB who underwent surgical resection at The Affiliated Hospital of Qingdao University from June 2013 to June 2023. We subsequently analyzed the detailed clinical characteristics and treatment process of HB patients who still had major blood vessels close to the tumor after a full course of NAC. The alpha-fetoprotein (AFP) level and imaging features were monitored during follow-up in all the children. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY-WZLL-25776).
The PMV is defined according to COG (AHEP0731). In this study, the major liver vasculature of “V” refers to the IVC, all HVs, or both. “P” refers to the MPV, the BPV, or both. The proximity classification was based on the tumor's distance to these vessels: (1) Within 1 cm (V0 or P0); (2) touching (V1 or P1); (3) compressing, or encasing (V2 or P2); and (4) presence of a tumor thrombus or cavernous transformation of the MPV (V3 or P3)[2,3].
Patients who could not undergo upfront resection during the initial diagnosis underwent needle biopsy to obtain a clear pathological diagnosis of HB. Subsequently, they were administered a standardized chemotherapy regimen, which was extensively described in our previous study[11]. After 2 cycles of chemotherapy, contrast-enhanced CT (CECT) and 3D reconstruction were performed to evaluate the resectability of the tumor. If the tumor remains unresectable, further cycles of chemotherapy and imaging evaluation are continued. The completion of four or more cycles of NAC or tumor en
Computer-assisted surgery system (Hisense CAS, Qingdao, China) was utilized to conduct 3D reconstruction of the CECT DICOM file. This process allowed for the acquisition of individualized 3D information, including the relationship between the tumor and major blood vessels, as well as the volume of the liver and tumor. The specific steps for this reconstruction have been thoroughly explained in previous reports by our team[9,10]. The PRE-TEXT staging system designed by SIOPEL was used in combination with the individualized 3D model for staging and assessing tumor resectability.
Simulating tumor resection on the 3D model has three modes: (1) Anatomical liver resection, which involves resecting the liver segment invaded by the tumor based on the intrahepatic blood vessel arrangement and vascular domain ana
The incision was made in the upper abdomen, below the costal margin, to access the ligaments around the liver, and the size, location, and relationship of the tumor with nearby organs were examined. The first porta hepatis was dissected, and the intermittent Pringle method was subsequently used to temporarily block hepatic blood flow. Typically, the flow was blocked for 15 to 20 min and then opened for 5 min. The cutting line was determined using electrosurgery based on preoperative planning. During the blocking period, the liver was dissected and separated using cavitational ultrasonic surgical aspiration and vascular forceps to prioritize liver parenchyma resection. Compression and electrocoagulation were used during the opening period to minimize bleeding. The tumor-involved major blood vessels were carefully separated, ligated, and sutured if necessary.
The SPSS 26.0 software package was used to organize and analyze the data. Normally distributed data are expressed as the mean ± SD, and nonnormally distributed data are expressed as the median and quartile. The paired-sample t test was used for comparisons before and after treatment. A P value < 0.05 was considered to indicate statistical significance.
Over a 10-year period, a total of 67 children diagnosed with HB underwent surgical resection. The age at diagnosis was 21.4 ± 18.8 months, and 40 boys and 27 girls were included in the study. Of the 67 patients, 52 (77.6%) presented with upper abdominal masses, and the AFP level was 231109.5 [interquartile range (IQR), 39904.8-434407.8] ng/mL. Fifty-nine (88.1%) patients had a single tumor, 39 (58.2%) had a single tumor located in the right lobe of the liver, and 8 (11.9%) had multiple tumors. The majority of patients (47/67, 70.1%) were classified as PRE-TEXT III or IV. The maximum diameter of the tumor was 10.8 (IQR, 8.3-13.1) cm. Following evaluation and planning using Hisense CAS, 28 children with HB un
Children who received a full course of NAC were evaluated preoperatively. Among the 67 patients, 16 (23.9%) still had tumors in the PMV. The detailed clinical characteristics before and after NAC are presented in Table 1. An equal number of boys and girls were included, with a median age at diagnosis of 30.5 (IQR, 17.0-48.5) months. The median AFP level was 414968.5 (IQR, 271680.5-842240.0) ng/mL. There were 10 and 6 patients in PRE-TEXT III and IV, respectively. The maximum diameter of the tumor was 10.9 (IQR, 9.3-14.3) cm, with a tumor volume of 650.1 (IQR, 326.8-1014.9) cm3. The percentage of tumor-free livers was 40.5% (IQR, 31.0%-45.0%).
| No. | Sex | Age (m) | Before NAC | NAC regimen (cycle) | After NAC | TGR | ||||||||||
| AFP (ng/mL) | PRE-TEXT | MTD (cm) | TV (cm3) | LV (cm3) | LV/TLTV | AFP (ng/mL) | POST-TEXT | MT (cm) | TV (cm3) | LV (cm3) | LV/TLTV | |||||
| 1 | M | 73 | 782230 | III | 10.8 | 1109.9 | 360.1 | 24% | 4 | 35428 | III | 7 | 88.4 | 597.2 | 87% | -92% |
| 2 | F | 16 | 420190 | III | 8.5 | 288.3 | 248.8 | 46% | 4 | 47 | II | 3.9 | 40.3 | 328.7 | 89% | -86% |
| 3 | F | 2 | 330590 | III | 9 | 365.3 | 137.8 | 27% | 4 | 27901 | II | 5.2 | 27.3 | 212.3 | 89% | -93% |
| 4 | M | 18 | 238592 | III | 6.5 | 149.4 | 412.2 | 73% | 4 | 27 | II | 4 | 11.4 | 380.3 | 97% | -92% |
| 5 | M | 29 | 439147 | IV | 14.1 | 732.1 | 536.2 | 42% | 4 | 46981 | III | 7.4 | 126.5 | 513.4 | 80% | -83% |
| 6 | M | 5 | 326550 | III | 9.5 | 287.3 | 292 | 50% | 4 | 276 | II | 4.7 | 58.9 | 351.6 | 86% | -79% |
| 7 | F | 22 | 902250 | IV | 15.1 | 1203.8 | 527.3 | 30% | 4 | 177 | II | 7 | 87.9 | 533.4 | 86% | -93% |
| 8 | M | 26 | > 484000 | III | 8 | 67.1 | 347.2 | 84% | 3 | > 484000 | IV | 9.3 | 235.1 | 250.3 | 52% | 250% |
| 9 | F | 3 | 409747 | III | 10 | 459.1 | 94.4 | 17% | 6 | 260 | II | 4.2 | 25.9 | 208.9 | 89% | -94% |
| 10 | F | 32 | > 484000 | IV | 12.8 | 658.5 | 469.3 | 42% | 5 | 15470 | IV | 8.6 | 60 | 541.2 | 90% | -91% |
| 11 | M | 42 | > 484000 | III | 11 | 641.7 | 508.5 | 44% | 4 | 3144 | II | 5.6 | 79.7 | 444.4 | 85% | -88% |
| 12 | M | 53 | 269341 | IV | 13.5 | 926 | 633.8 | 41% | 4 | 89 | II | 7.3 | 91.2 | 736.6 | 89% | -90% |
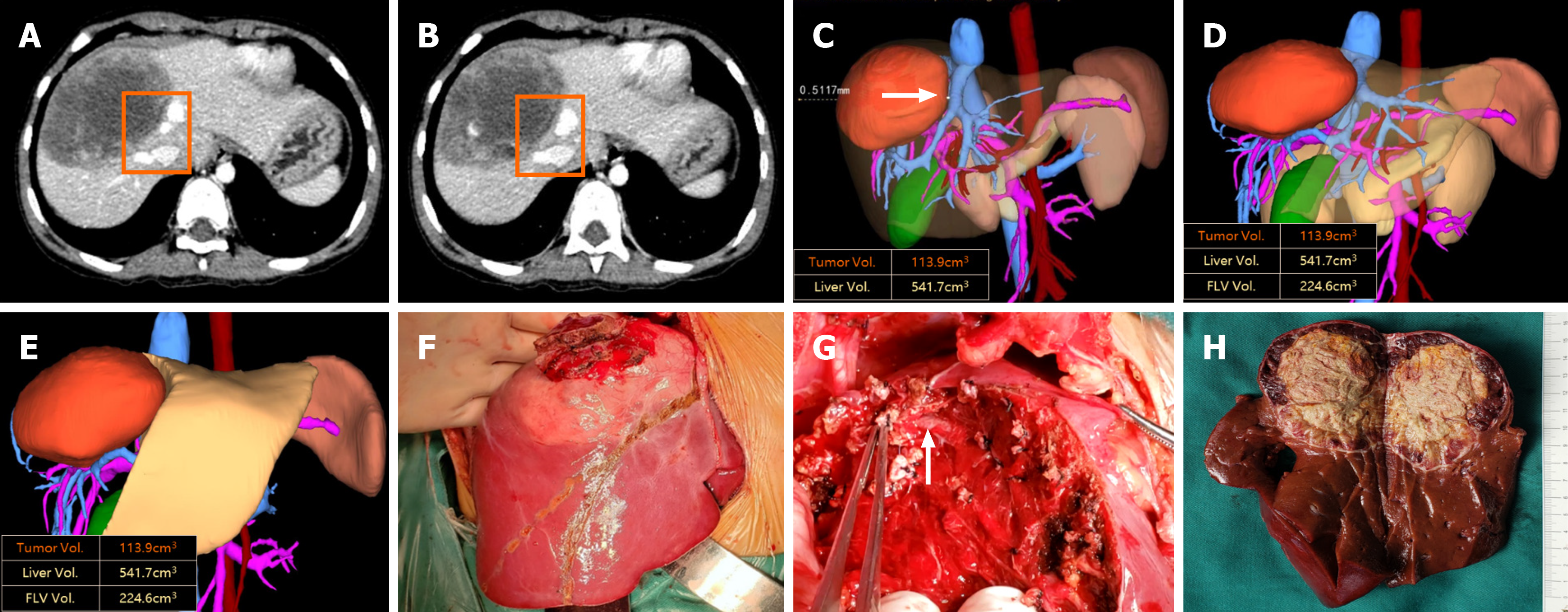
| 13 | M | 57 | 87696 | III | 14.8 | 949.2 | 599.6 | 39% | 4 | 245 | II | 7.2 | 113.9 | 541.7 | 83% | -88% |
| 14 | F | 44 | 274020 | IV | 16.5 | 1080.6 | 594.9 | 36% | 5 | 1730 | III | 6.6 | 108.2 | 503.9 | 82% | -90% |
| 15 | F | 67 | 541614 | IV | 14.5 | 1230.1 | 571.1 | 32% | 5 | 25124 | III | 5.8 | 86.4 | 727.7 | 89% | -93% |
| 16 | F | 33 | 80075 | III | 10.3 | 392.5 | 260.1 | 40% | 4 | 13033 | II | 4.5 | 54 | 336.8 | 86% | -86% |
After chemotherapy, the AFP level decreased to 2437.0 (IQR, 211.3-26512.5) ng/mL. Eight patients with PRETEXT III were downgraded to POST-TEXT II, two patients with PRETEXT IV were downgraded to POST-TEXT II, and three patients with PRETEXT IV were downgraded to POST-TEXT III. However, one patient was upgraded from PRETEXT III to POST-TEXT IV after three courses of treatment. The maximum diameter of the tumor decreased to 6.8 (IQR, 5.7-7.3) cm, with a corresponding tumor volume of 83.1 (IQR, 47.2-99.7) cm3. The percentage of tumor-free livers increased to 86.5% (IQR, 84.0%-89.0%). Except for one case of tumor progression, the tumor volume decreased in the remaining 15 patients, with a median reduction of 90%. Statistical analysis revealed significant differences in the changes in AFP level, tumor diameter and tumor volume before and after NAC (P < 0.05). However, there was no significant difference in the change in the tumor-free liver volume (P = 0.12).
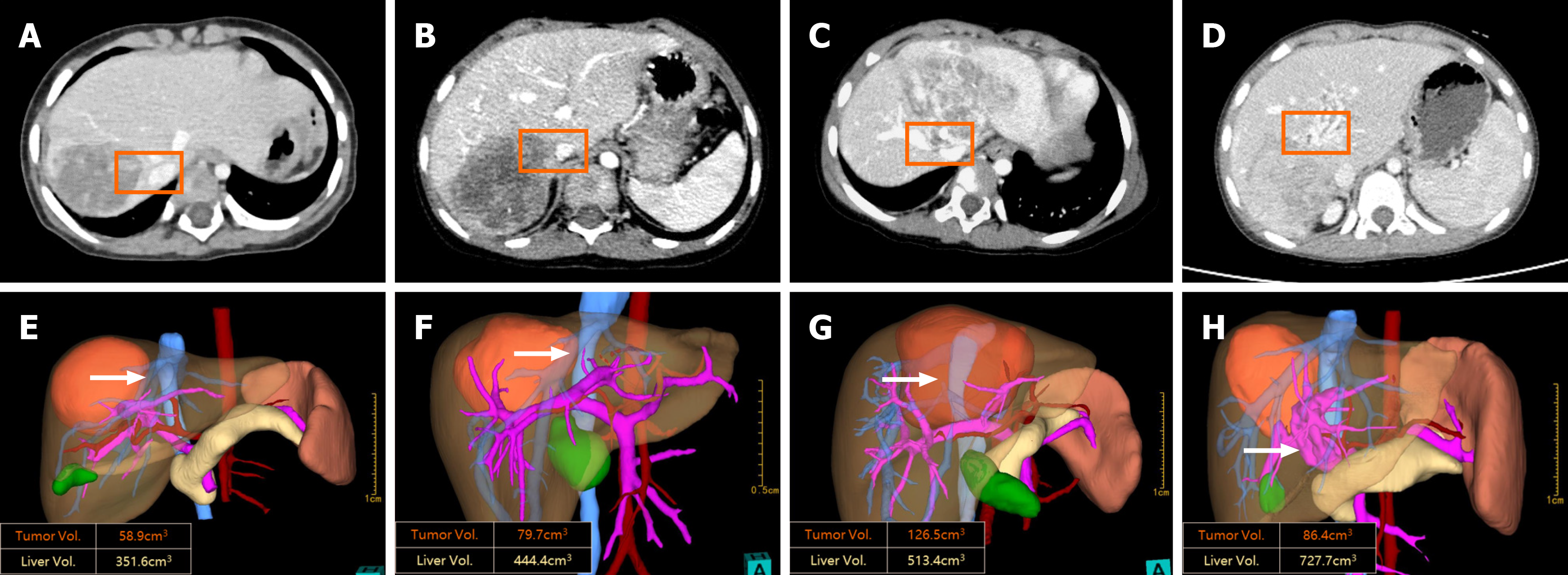
The details of the preoperative evaluation and simulated liver resection in the 16 children who still had tumors close to the major liver vasculature after a full course of NAC are presented in Table 2. Among the patients, two had tumors within 1 cm, 11 had touching, four had compression, and three had tumor thrombus. Thirteen patients had PMV with the IVC, 9 patients with MPV or BPV, and 3 patients with all HVs. Furthermore, there were 9 cases of tumors involving two lobes of the liver, including three cases of multiple tumors and 6 cases of tumors in the middle lobe of the liver. Addi
| No. | PMV | Tumor number | Tumor location | Preoperative evaluation and simulated LR | |||||
| Classification | Blood vessel | Group | Simulated mode | Surgical planning | FLV (cm3) | FLV/TLTV | |||
| 1 | Within 1 cm | IVC | V0 | 2 | Both lobes | Anatomical LR | Right hepatectomy | 373.5 | 54% |
| TT | Right HV | V3 | |||||||
| 2 | Touching | BPV | P1 | 1 | Right lobe | Anatomical LR | Right hepatectomy | 177.1 | 48% |
| 3 | Compressing | IVC | V2 | 1 | Middle lobe | Anatomical LR | Extended right hepatectomy | 109.7 | 46% |
| 4 | Touching | MPV | P1 | 1 | Middle lobe | Autonomous LR | Extended right hepatectomy; Extended left hepatectomy; Right hepatectomy | 288.2 | 74% |
| 5 | Compressing | All intrahepatic veins | V2+P2 | 1 | Middle lobe | Autonomous LR | Right hepatectomy | 343 | 54% |
| 6 | Within 1 cm Touching | IVC | V0 | 1 | Right lobe | Anatomical LR | 189.3 | 46% | |
| 7 | TT | IVC | V1 | 1 | Right lobe | Anatomical LR | Mesohepatectomy | 368 | 59% |
| Compressing | Right PV | P3 | Right hepatectomy | ||||||
| 8 | Touching | All intrahepatic veins | V2+P2 | 1 | Middle lobe | Anatomical LR | 177.5 | 37% | |
| 9 | Touching | IVC | V1 | 1 | Middle lobe | Anatomical LR | Left hepatectomy + VII | 90.6 | 39% |
| Touching | BPV | P1 | Right hepatectomy | ||||||
| 10 | Touching | BPV | P1 | 3 | Both lobes | Anatomical LR and | 348.8 | 58% | |
| 11 | Touching | IVC | V1 | 1 | Right lobe | Tumor dissection | Right hepatectomy | 287.9 | 55% |
| Touching | BPV | P1 | Anatomical LR | Right hepatectomy | |||||
| 12 | Touching | IVC | V1 | 1 | Right lobe | 355.1 | 43% | ||
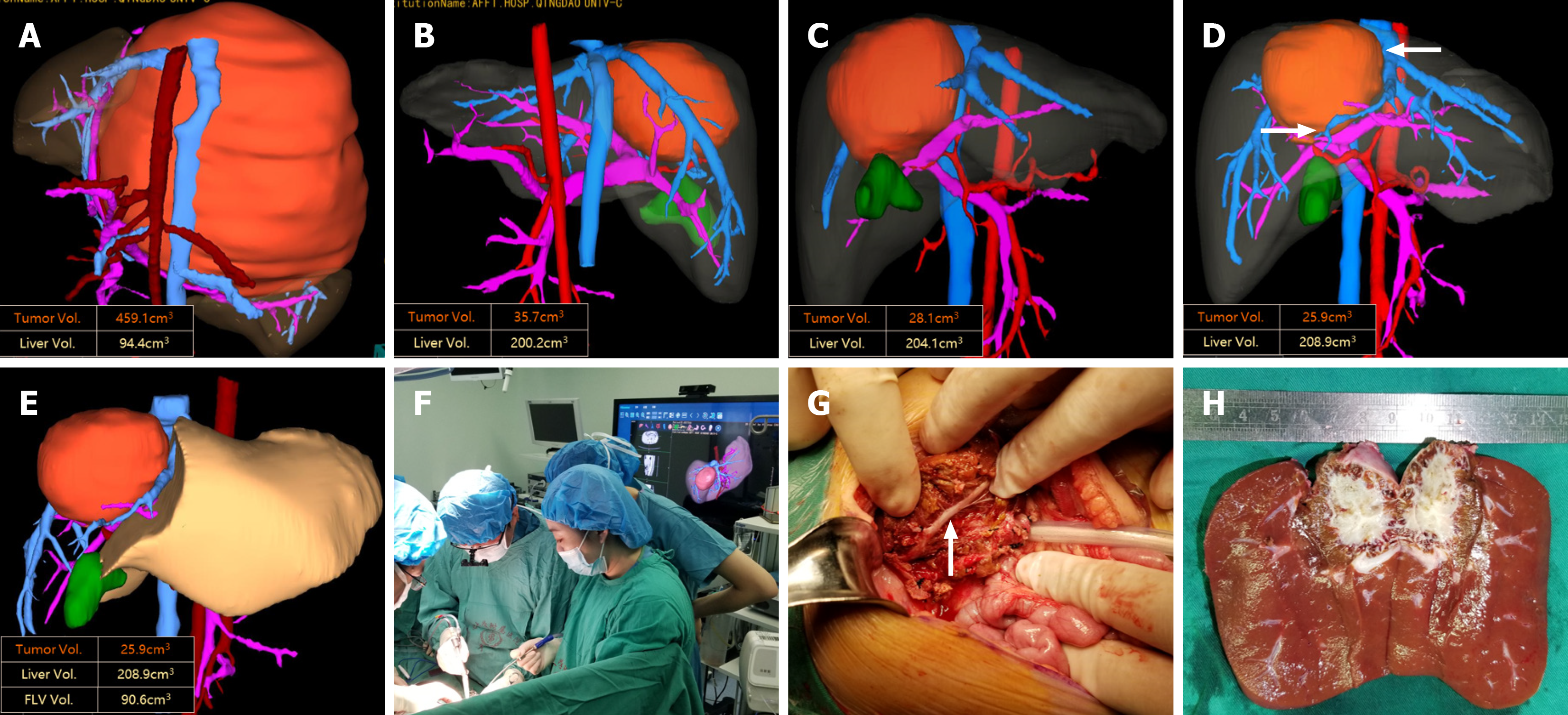
| 13 | Touching | IVC | V1 | 1 | Right lobe | Anatomical LR | Right hepatectomy + II | 224.6 | 34% |
| Touching | All HVs | V1 | Anatomical LR | Right hepatectomy | |||||
| 14 | Touching | IVC | V1 | 2 | Both lobes | 341.5 | 56% | ||
| 15 | TT | IVC | V1 | 1 | Right lobe | Anatomical LR | Right hepatectomy | 480.5 | 59% |
| 16 | Touching | PV | P3 | Anatomical LR | |||||
| Compressing | IVC | V1 | 1 | Middle lobe | 146.9 | 38% | |||
| BPV | P2 | Anatomical LR | |||||||
Extreme liver resections were performed following thorough preoperative evaluation and simulated tumor resection. During the operation, the precut lines were drawn based on the preoperative plan, and special attention was given to carefully process the blood vessels around the tumor, particularly those compressed by the tumor and any anatomical variations (Figure 3). The tumors were successfully and completely removed, and the operations were successfully com
| No. | Operation time (min) | Hepatic flow occlusion number and duration | Vascular reconstruction | Blood loss (mL) | Blood transfusion during the operation | Post-operative AFP (ng/mL) | Margin | Pathology | Discharge time (d) | Follow-up | |||
| RBC (U) | Plasma (mL) | CRYO (U) | Time (m) | Result | |||||||||
| 1 | 220 | 1, 15 min | - | 20 | 1 | 0 | 0 | 1075 | R0 | FE | 6 | 68 | Recurrence |
| 2 | 165 | 1, 15 min | - | 5 | 0 | 0 | 0 | 1.33 | R0 | MEN | 9 | 64 | |
| 3 | 190 | 1, 20 min | - | 20 | 0 | 50 | 40 | 505.9 | R0 | MEM | 14 | 61 | Recurrence |
| 4 | 185 | 1, 15 min | - | 5 | 0.5 | 0 | 0 | 1.37 | R0 | MEM | 9 | 52 | |
| 5 | 285 | 2, 20 + 10 min | IVC | 100 | 2 | 300 | 2 | 25.86 | R1 | MEM | 20 | 46 | |
| 6 | 245 | 2, 13 + 12 min | - | 50 | 1 | 100 | 2 | 0.87 | R0 | F | 21 | 42 | |
| 7 | 230 | 2, 15 + 10 min | - | 100 | 1 | 120 | 0 | 2.55 | R0 | FE | 13 | 40 | |
| 8 | 250 | 3, 13 + 12 + 8 min | IVC | 200 | 2 | 100 | 0 | 2517 | R0 | FE | 13 | 39 | Metastasis, death |
| 9 | 190 | 1, 15 min | - | 10 | 0.5 | 0 | 0 | 9.15 | R0 | FE | 10 | 33 | |
| 10 | 295 | 2, 15 + 10 min | - | 100 | 1 | 0 | 2 | 1.55 | R0 | MEM | 9 | 16 | |
| 11 | 280 | 2, 15 + 10 min | - | 100 | 2 | 0 | 0 | 3.29 | R0 | MEM | 15 | 16 | |
| 12 | 310 | 1, 15 min | - | 50 | 2 | 120 | 2 | 3.53 | R0 | F | 14 | 16 | |
| 13 | 255 | 1, 15 min | - | 50 | 1 | 0 | 0 | 1.84 | R0 | MEM | 11 | 15 | |
| 14 | 355 | 4 | IVC, MHV | 500 | 6 | 770 | 12 | 7.43 | R0 | FE | 20 | 9 | |
| 15 | 265 | 20 + 15 + 18 + 12 min | - | 100 | 1.5 | 0 | 0 | 1642 | R0 | FE | 11 | 9 | |
| 16 | 245 | 1, 20, 3, 15 + 15 + 9 min | - | 30 | 1 | 0 | 0 | 8.2 | R0 | F | 14 | 7 | Recurrence |
The study included 67 children with HB, and the median follow-up time was 62 months. The 3-year event-free survival (EFS) rate was 88%. Among the 47 children with PRE-TEXT III and IV, the median follow-up time was 58 months, and the 3-year EFS was 85%. Of the 16 children who underwent extreme liver resection, the median follow-up time was 36 months, three patients experienced recurrence, and one patient died due to multiple metastases during the follow-up period.
HB is a common malignant solid abdominal tumor in children that accounts for approximately 80% of liver tumors in childhood. The highest incidence is observed in infants and children under 5 years old, and the most common clinical manifestation is an asymptomatic abdominal mass. It has been observed that more than 50% of large HB necessitate NAC followed by delayed surgery due to the inadequate volume of the remnant liver or the involvement of major blood vessels[1,6,10]. Our retrospective analysis revealed that 58.2% of the HB patients underwent delayed resection. However, 23.9% of the HB patients received a full course of NAC; their tumor volume decreased by almost 90%, and the tumor-free liver volume accounted for approximately 85% of the total volume, still remaining close to major blood vessels. Fur
Currently, all cooperative trial group institutions recommend complex segmental resection or liver transplantation for HB evaluated for major vascular involvement after chemotherapy. Liver transplantation has traditionally been con
Due to the complex course and anatomical variation of blood vessels in the liver, as well as the limitations of two-dimensional imaging, assessing the surgical resectability of HB can be challenging. Surgeons often rely on their own anatomical knowledge and surgical experience to perform slice selection on cross-sectional CT or MRI images for eva
In addition, the guidelines for HB surgery prioritize anatomical liver resection, which involves removing more normal liver tissue (greater than 1 cm outside the tumor). Nonalcoanatomical resection or segmental resection, such as extended hepatectomy or middle lobectomy, are often considered for advanced HB[21,22]. However, these procedures make it more challenging to assess the preoperative remnant liver volume. This study utilized three dimensions to track the route of each blood vessel, determine the drainage segment of each vein, and perform the individualized liver segment ana
With the advancement of chemotherapy, 2 to 4 cycles of NAC for HB have been shown to increase the probability of surgical resection for unresectable HB at diagnosis. However, more than 4 cycles do not further increase the chance of conventional resection and may instead lead to drug resistance and worsening of chemotherapy toxicity[13]. In this study, we attempted to treat three children with 5 cycles and 1 child with 6 cycles of NAC. However, we observed that the tumor volume did not significantly decrease, and the distance from the major blood vessels increased but still in
Extreme liver resection for HB that is still in close PMV after a full course of NAC is both safe and feasible. This approach not only reduces the necessity for liver transplantation but also results in a favorable prognosis. Individualized 3D surgical planning is beneficial for accurate and complete resection of HB in children, particularly for assessing vascular involvement, remnant liver volume and anatomical variations.
Hepatoblastoma (HB) is usually a large tumor when it is detected clinically. However, there is currently no consensus on the optimal diagnosis and treatment plan for children with difficult HB who are large size and complex locations. Even after a full course of neoadjuvant chemotherapy (NAC), approximately 25% of HB patients remain in close proximity to the major liver vasculature (PMV). In recent years, aggressive extreme liver resection has become another viable option, and computer-assisted three-dimensional (3D) individualized surgical planning has also been proven to be beneficial for surgery.
Children with HB who still have PMV after a full course of NAC pose a clinical challenge in planning further treatment. After computer-assisted 3D individualized evaluation, aggressive extreme liver resection may be another viable option for reducing the need for liver transplantation.
To explore whether computer-assisted three-dimensional individualized extreme liver resection is safe and feasible for children with HB who still have PMV after a full course of NAC.
We retrospectively collected data from children with HB who underwent surgical resection at our center from June 2013 to June 2023. Then, we analyzed the clinical characteristics, PMV classification, 3D individualized assessment, preoperative planning and intraoperative and postoperative results of children with HB who still had PMV after a full course of NAC.
Sixty-seven children diagnosed with HB underwent surgical resection. After a full course of NAC, 16 patients still had close PMV (within 1 cm in two children, touching in 11 patients, compressing in four patients, and having tumor thrombus in three patients). There were 6 cases of tumors in the middle lobe of the liver, and four of those patients exhibited liver anatomy variations. These 16 children underwent extreme liver resection after comprehensive preope
Computer-assisted three-dimensional individualized extreme liver resection for HB patients who are still in close PMV after a full course of NAC is both safe and feasible.
Aggressive extreme liver resection with individualized 3D surgical planning will provide opportunities for surgical resection of difficult HB patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rasa HK, Turkey S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, Aronson DC, Malogolowkin MH, Perilongo G, von Schweinitz D, Ansari M, Lopez-Terrada D, Tanaka Y, Alaggio R, Leuschner I, Hishiki T, Schmid I, Watanabe K, Yoshimura K, Feng Y, Rinaldi E, Saraceno D, Derosa M, Czauderna P. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol. 2017;18:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Murawski M, Weeda VB, Czauderna P. Surgical management in hepatoblastoma: points to take. Pediatr Surg Int. 2023;39:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Katzenstein HM, Langham MR, Malogolowkin MH, Krailo MD, Towbin AJ, McCarville MB, Finegold MJ, Ranganathan S, Dunn S, McGahren ED, Tiao GM, O'Neill AF, Qayed M, Furman WL, Xia C, Rodriguez-Galindo C, Meyers RL. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children's Oncology Group, multicentre, phase 3 trial. Lancet Oncol. 2019;20:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Katzenstein HM, Malogolowkin MH, Krailo MD, Piao J, Towbin AJ, McCarville MB, Tiao GM, Dunn SP, Langham MR, McGahren ED, Finegold MJ, Ranganathan S, Weldon CB, Thompson PA, Trobaugh-Lotrario AD, O'Neill AF, Furman WL, Chung N, Randazzo J, Rodriguez-Galindo C, Meyers RL. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: A Children's Oncology Group study. Cancer. 2022;128:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Busweiler LA, Wijnen MH, Wilde JC, Sieders E, Terwisscha van Scheltinga SE, van Heurn LW, Ziros J, Bakx R, Heij HA. Surgical treatment of childhood hepatoblastoma in the Netherlands (1990-2013). Pediatr Surg Int. 2017;33:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Ziogas IA, Benedetti DJ, Wu WK, Matsuoka LK, Izzy M, Rauf MA, Pai AK, Bailey CE, Alexopoulos SP. Management of hepatoblastoma in the United States: Can we do better? Surgery. 2021;170:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Agarwala S, Gupta A, Bansal D, Vora T, Prasad M, Arora B, Kapoor G, Chinnaswamy G, Radhakrishnan V, Laskar S, Kaur T, Dhaliwal RS, Rath GK, Bakhshi S. Management of Hepatoblastoma: ICMR Consensus Document. Indian J Pediatr. 2017;84:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Uchida H, Sakamoto S, Komine R, Kodama T, Nakao T, Okada N, Yanagi Y, Shimizu S, Fukuda A, Shioda Y, Kiyotani C, Matsumoto K, Yoneda A, Haga C, Yoshioka T, Miyazaki O, Nosaka S, Kasahara M. Strategy for hepatoblastoma with major vascular involvement: A guide for surgical decision-making. Surgery. 2023;173:457-463. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Yang T, Whitlock RS, Vasudevan SA. Surgical Management of Hepatoblastoma and Recent Advances. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Wu X, Wang J, Duan Y, Liu Y, Chen X, Xia N, Dong Q. Surgical resection of pediatric PRETEXT III and IV hepatoblastoma: A retrospective study investigating the need for preoperative chemotherapy. Front Pediatr. 2022;10:878095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Liu J, Xiu W, Duan G, Dong Q. Application of 3D Simulation Software in Chemotherapy and Hepatoblastoma Surgery in Children. Front Surg. 2022;9:908381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Honda M, Uchida K, Irie T, Hirukawa K, Kadohisa M, Shimata K, Isono K, Shimojima N, Sugawara Y, Hibi T. Recent advances in surgical strategies and liver transplantation for hepatoblastoma. Cancer Med. 2023;12:3909-3918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 13. | Trobaugh-Lotrario AD, Meyers RL, O'Neill AF, Feusner JH. Unresectable hepatoblastoma: current perspectives. Hepat Med. 2017;9:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | El-Gendi A, Fadel S, El-Shafei M, Shawky A. Avoiding liver transplantation in post-treatment extent of disease III and IV hepatoblastoma. Pediatr Int. 2018;60:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, Hiyama E, Czauderna P, Roebuck DJ, Tiao GM. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48:536-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 16. | Aronson DC, Schnater JM, Staalman CR, Weverling GJ, Plaschkes J, Perilongo G, Brown J, Phillips A, Otte JB, Czauderna P, MacKinlay G, Vos A. Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol. 2005;23:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Xia N, Duan Y, Wu X, Zhao C, Jin C, Chen X, Gao Q, Wang Y, Wang F, Chen Y, Dong Q, Hao X. Application of computer-assisted surgery in pediatric mediastinal tumor surgery. Int J Med Robot. 2023;19:e2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Lamadé W, Glombitza G, Fischer L, Chiu P, Cárdenas CE Sr, Thorn M, Meinzer HP, Grenacher L, Bauer H, Lehnert T, Herfarth C. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000;135:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Zhang G, Zhou XJ, Zhu CZ, Dong Q, Su L. Usefulness of three-dimensional(3D) simulation software in hepatectomy for pediatric hepatoblastoma. Surg Oncol. 2016;25:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Warmann SW, Schenk A, Schaefer JF, Ebinger M, Blumenstock G, Tsiflikas I, Fuchs J. Computer-assisted surgery planning in children with complex liver tumors identifies variability of the classical Couinaud classification. J Pediatr Surg. 2016;51:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Lake CM, Tiao GM, Bondoc AJ. Surgical management of locally-advanced and metastatic hepatoblastoma. Semin Pediatr Surg. 2019;28:150856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Fuchs J, Cavdar S, Blumenstock G, Ebinger M, Schäfer JF, Sipos B, Warmann SW. POST-TEXT III and IV Hepatoblastoma: Extended Hepatic Resection Avoids Liver Transplantation in Selected Cases. Ann Surg. 2017;266:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Ren X, Li H, Diao M, Chen L, Xu H, Li L. Results of surgical resections with positive margins for children with hepatoblastoma: Case series from a single Asian center. Pediatr Blood Cancer. 2019;66:e27479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Aronson DC, Weeda VB, Maibach R, Czauderna P, Dall'Igna P, de Ville de Goyet J, Branchereau S, Perilongo G, Brock P, Zsiros J, Semeraro M, Chardot C, Wildhaber B, Morland B, Brugières L; Childhood Liver Tumour Strategy Group (SIOPEL). Microscopically positive resection margin after hepatoblastoma resection: what is the impact on prognosis? A Childhood Liver Tumours Strategy Group (SIOPEL) report. Eur J Cancer. 2019;106:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Shen G, Wu L, Zhao J, Wei B, Zhou X, Zhuo X, Dong Q. Imaging and Pathology Study of the Chemotherapy Regression Area of Hepatoblastoma - A Prospective Single-Center Study. Fetal Pediatr Pathol. 2020;39:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Liu J, Wu X, Xu C, Ma M, Zhao J, Li M, Yu Q, Hao X, Wang G, Wei B, Xia N, Dong Q. A Novel Method for Observing Tumor Margin in Hepatoblastoma Based on Microstructure 3D Reconstruction. Fetal Pediatr Pathol. 2022;41:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Ke M, Zhou Y, Yang CZ, Li L, Diao M. Analysis of risk factors for angiolymphatic invasion and establishment of a predictive nomogram for hepatoblastomas. J Pediatr Surg. 2022;57:430-437. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |