Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1055
Peer-review started: December 19, 2023
First decision: January 9, 2024
Revised: January 18, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: April 27, 2024
Processing time: 125 Days and 9.7 Hours
Colon cancer is one of the most common malignant tumors of the digestive system. Liver metastasis after colon cancer surgery is the primary cause of death in patients with colon cancer.
To construct a novel nomogram model including various factors to predict liver metastasis after colon cancer surgery.
We retrospectively analyzed 242 patients with colon cancer who were admitted and underwent radical resection for colon cancer in Zhejiang Provincial People’s Hospital from December 2019 to December 2022. Patients were divided into liver metastasis and non-liver metastasis groups. Sex, age, and other general and clinicopathological data (preoperative blood routine and biochemical test indexes) were compared. The risk factors for liver metastasis were analyzed using single-factor and multifactorial logistic regression. A predictive model was then constructed and evaluated for efficacy.
Systemic inflammatory index (SII), C-reactive protein/albumin ratio (CAR), red blood cell distribution width (RDW), alanine aminotransferase, preoperative carcinoembryonic antigen level, and lymphatic metastasis were different between groups (P < 0.05). SII, CAR, and RDW were risk factors for liver metastasis after colon cancer surgery (P < 0.05). The area under the curve was 0.93 for the column-line diagram prediction model constructed based on these risk factors to distinguish whether liver metastasis occurred postoperatively. The actual curve of the column-line diagram predicting the risk of postoperative liver metastasis was close to the ideal curve, with good agreement. The prediction model curves in the decision curve analysis showed higher net benefits for a larger threshold range than those in extreme cases, indicating that the model is safer.
Liver metastases after colorectal cancer surgery could be well predicted by a nomogram based on the SII, CAR, and RDW.
Core Tip: Colon cancer is a highly aggressive and migratory malignant tumor for which the liver is the most common target organ for postoperative metastasis. Herein, we analyzed the general and clinicopathological data of 242 patients with colon cancer who underwent radical resection for colon cancer. The results showed that the systemic inflammatory index, C-reactive protein/albumin ratio, and red blood cell distribution width were risk factors for postoperative liver metastasis in patients with colon cancer. A columnar graph prediction model was subsequently developed based on these three factors, and its predictive efficacy was evaluated.
- Citation: Cheng DX, Xu KD, Liu HB, Liu Y. Prognostic value of a nomogram model for postoperative liver metastasis of colon cancer. World J Gastrointest Surg 2024; 16(4): 1055-1065
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1055.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1055
Colon cancer is a highly aggressive and migratory malignant tumor, which is most commonly characterized by intestinal obstruction, loss of body mass, abdominal pain, and blood in the stool[1]. The liver is the most common metastatic site in patients with colon cancer[2], with liver metastasis occurring in 25% of patients after surgery[3]. Therefore, designing a convenient and efficient predictive index for liver metastasis after colon cancer surgery would not only be beneficial for the early screening of colon cancer but also play an important role in knowing whether liver metastasis occurs after colon cancer surgery.
Several studies have shown that tumor-associated inflammatory cells can directly or indirectly act on tumors and are closely related to tumorigenesis and migration[4]. Systemic inflammation can occur within the tumor immune microenvironment and is closely related to tumor development[5,6]. The systemic inflammatory index (SII) is a test that assesses the impact of peripheral blood neutrophil, lymphocyte, and platelet counts on the biological behavior of cells. By detecting these relative values from these cell types, the SII measures whether their function is normal and subsequently assesses the patient’s status[7]. Studies have demonstrated that SII is independently associated with postoperative liver metastasis in colon cancer, and its predictive ability is superior to that of other inflammatory factors[8]. Therefore, the SII may be a marker for the prediction of liver metastasis after colon cancer surgery.
C-reactive protein (CRP) levels are influenced by tumor necrosis factor-α, interleukin (IL)-1β, and IL-6, indicating a strong association with acute inflammation[9]. Conversely, albumin levels have been associated with chronic inflammation and can indicate nutritional status[10,11]. The CRP/albumin ratio (CAR) reflects the inflammatory status and nutritional level of the patient, is less susceptible to the influence of other factors, and has better stability. Therefore, studies have confirmed that CAR can be used as a prognostic marker for colon cancer in clinical practice[12]. CAR has also been shown to be a reliable marker for predicting survival in patients with metastatic colon cancer[13]. Therefore, we hypothesized that CAR could be used as a new parameter for predicting liver metastasis after colon cancer surgery.
Owing to the many advantages of blood sample analysis, including easy accessibility and low invasiveness, as well as its ability to allow long-term monitoring and assessment of systemic status, clinical blood tests are commonplace. Among these, red cell distribution width (RDW) is a standard blood test measurement item that reflects the homogeneity of red blood cells[14]. RDW has been used as a prognostic marker for various cancers, including lung, liver, esophagogastric, and breast cancers[15,16], and has also been used as a potential prognostic marker for colon cancer. However, the predictive value of RDW for the occurrence of postoperative liver metastasis in colon cancer remains unclear. Therefore, a more accurate and comprehensive assessment of the predictive value of postoperative liver metastasis in colon cancer through the continuous exploration of new blood indices would be crucial in guiding clinical work.
In recent years, relevant studies have reported the factors influencing the occurrence of liver metastasis following colon cancer surgery; however, a more accurate prediction model is still lacking. Columnar graphs can visualize the probability of patients presenting with corresponding diseases, meaning that they are commonly used for the rapid clinical screening of high-risk groups and the development of effective interventions. Therefore, this retrospective study aimed to investigate the predictive value of the column chart model based on SII, CAR, and RDW for postoperative liver metastasis of colon cancer to aid in the clinical screening of postoperative liver metastasis of colon cancer.
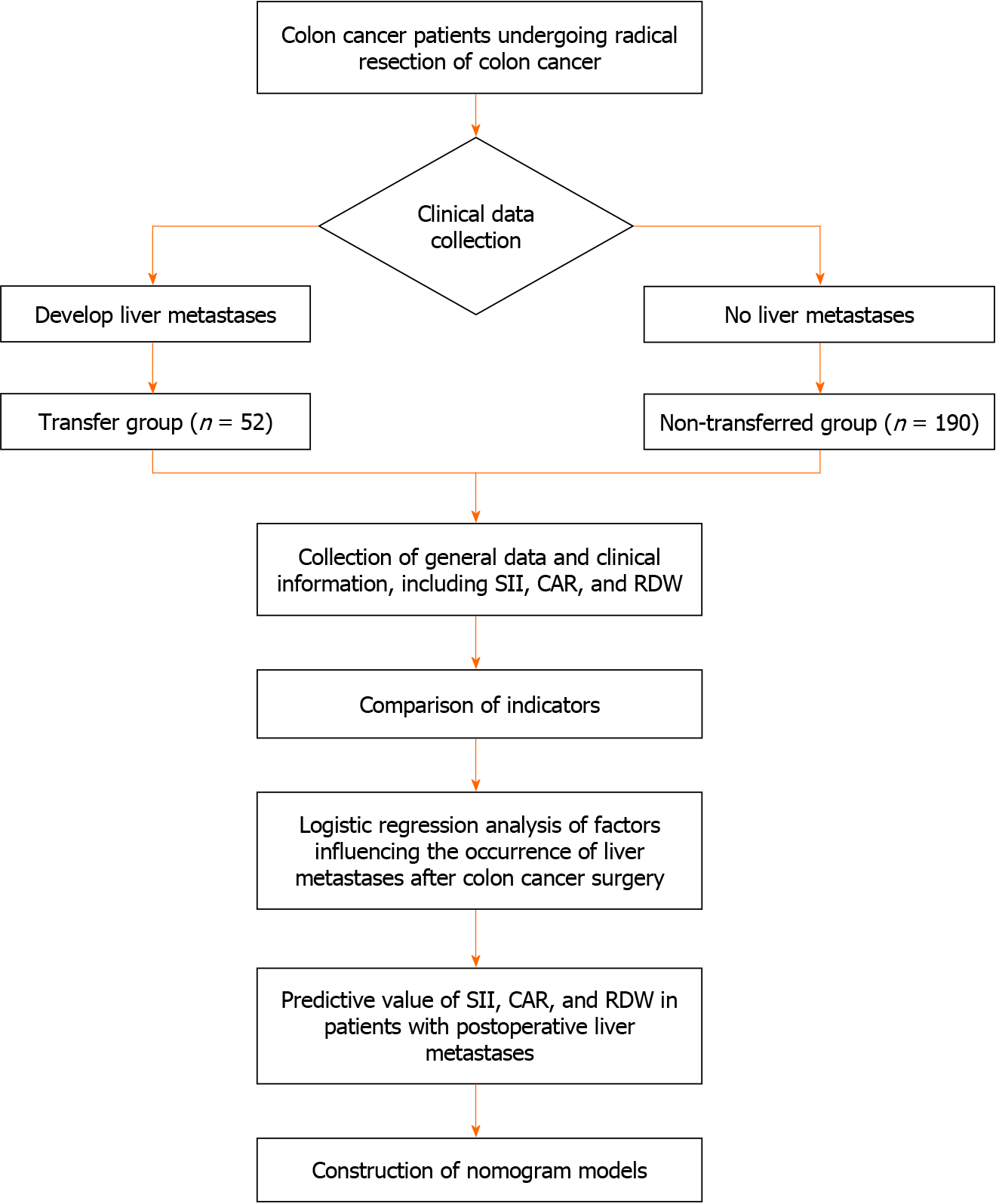
The general and clinical data of 242 patients with colon cancer admitted to the Zhejiang Provincial People’s Hospital between December 2019 and December 2022 were retrospectively analyzed. Patients were divided into the transfer (n = 52) and non-transfer (n = 190) groups based on whether they developed liver metastasis after surgery or not. The analysis process is shown in Figure 1.
The inclusion criteria were as follows: (1) Preoperative diagnosis of colon cancer by imaging and histopathology; (2) postoperative pathologic examination confirming colon cancer; (3) radical resection of colon cancer; (4) availability of complete clinicopathological data; and (5) complete follow-up data.
The exclusion criteria were as follows: (1) Preoperative colon cancer with distant metastasis; (2) colon cancer with perforation or hemorrhage; (3) previous history of malignancy; and (4) underlying severe cardiac, hepatic, renal, pulmonary, or hematological diseases.
General and clinicopathological data were collected for all patients, including age, sex, body mass index (BMI), history of alcohol consumption, smoking, and history of diseases (cardiac disease, diabetes mellitus, and hypertension). Clinicopathological data included SII, CAR, RDW, total bilirubin, albumin, platelet count, white blood cell (WBC) count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), surgical modality, vascular invasion, tumor site, type of pathology, postoperative chemotherapy, preoperative carcinoembryonic antigen (CEA) levels, and lymph node metastasis status.
The Statistical Package for Social Science (SPSS, version 26.0) was used to analyze all data. For normally distributed data points, comparisons between two groups were performed using t-test, and results are expressed as mean ± SD. Non-normally distributed data were analyzed by the Mann-Whitney U-test and expressed as M (P25-P75). Comparisons of counting data between the two groups were performed using the χ2 test, expressed as n (%). Binary logistic regression was applied to analyze the relevant influencing factors of liver metastasis after surgery for colon cancer.
A predictive model was constructed using R software based on multifactorial logistic regression analysis, and the model was internally validated using the bootstrap method with 1000 repetitive samples. The accuracy of the model was assessed by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve, the calibration curve, and the decision curve analysis (DCA).
General information such as age, BMI, sex, drinking history, smoking history, and medical history of the patients in the two groups were analyzed separately. There was no significant difference between the two groups (P > 0.05) (Table 1).
| Considerations | Transfer group (n = 52) | Non-transfer group (n = 190) | t/z/χ2 | P value |
| Age (yr) | 58.96 ± 16.59 | 61.31 ± 14.00 | 0.93 | 0.35 |
| BMI (kg/m2) | 22.16 ± 3.84 | 22.56 ± 2.92 | 0.69 | 0.50 |
| Sex | 1.01 | 0.32 | ||
| Male | 30 (57.70) | 124 (65.30) | ||
| Female | 22 (42.30) | 66 (34.70) | ||
| Drinking history | 1.41 | 0.24 | ||
| Yes | 40 (76.90) | 130 (68.40) | ||
| No | 12 (23.10) | 60 (31.60) | ||
| Smoking history | 1.13 | 0.29 | ||
| Yes | 44 (84.60) | 148 (77.90) | ||
| No | 8 (15.40) | 42 (22.10) | ||
| Heart disease | 0.01 | 0.93 | ||
| Yes | 14 (26.90) | 50 (26.30) | ||
| No | 38 (73.10) | 140 (73.70) | ||
| Diabetes | 1.58 | 0.21 | ||
| Yes | 14 (26.90) | 36 (18.90) | ||
| No | 38 (73.10) | 154 (81.10) | ||
| Hypertension | 1.84 | 0.18 | ||
| Yes | 8 (15.40) | 46 (24.20) | ||
| No | 44 (84.60) | 144 (75.80) |
To investigate the predictive value of the SII, CAR, and RDW for the development of liver metastases after surgery in patients with colon cancer, a one-way analysis of all case information collected was performed. The results revealed no significant difference between the two groups in terms of total bilirubin, albumin, platelet count, WBC count, AST level, surgical method, vascular invasion, tumor site, pathology type, or postoperative chemotherapy (P > 0.05). However, there was a significant difference between the metastatic and non-metastatic groups in the comparison of SII, CAR, RDW, ALT, preoperative CEA, and lymphatic metastasis (P < 0.05) (Table 2).
| Considerations | Transfer group (n = 52) | Non-transfer group (n = 190) | t/z/χ2 | P value |
| SII | 385.21 ± 78.63 | 264.29 ± 64.43 | -10.19 | < 0.01 |
| CAR | 1.02 ± 0.40 | 0.79 ± 0.38 | -3.88 | < 0.01 |
| RDW (FL) | 17.64 ± 1.98 | 16.28 ± 1.26 | -4.72 | < 0.01 |
| Total bilirubin (µmol/L) | 13.45 ± 2.51 | 13.75 ± 3.28 | 0.71 | 0.48 |
| Albumin (g/L) | 42.08 ± 3.81 | 42.22 ± 2.71 | 0.25 | 0.80 |
| Blood platelet count (109/L) | 167.13 ± 65.02 | 157.29 ± 40.59 | -1.04 | 0.30 |
| WBC (109/L) | 7.48 ± 2.58 | 7.52 ± 3.12 | 0.08 | 0.93 |
| ALT (U/L) | 47.88 ± 1.84 | 48.50 ± 1.59 | 2.40 | 0.017 |
| AST (U/L) | 47.29 ± 1.25 | 47.14 ± 1.76 | -0.61 | 0.54 |
| Surgical procedures | 0.01 | 0.94 | ||
| Laparoscopy | 32 (61.50) | 118 (62.10) | ||
| Open the abdomen | 20 (38.50) | 72 (37.90) | 0.01 | 0.93 |
| Vascular invasion | 3.26 | 0.07 | ||
| Yes | 24 (46.20) | 62 (32.60) | ||
| No | 28 (53.80) | 128 (67.40) | 1.58 | 0.21 |
| Tumor site | 1.01 | 0.32 | ||
| Left | 30 (57.70) | 124 (65.30) | ||
| Right | 22 (42.30) | 66 (34.70) | ||
| Pathological type | 1.05 | 0.31 | ||
| Adenocarcinoma | 48 (92.30) | 182 (95.80) | ||
| Other | 4 (7.70) | 8 (4.20) | ||
| Postoperative chemotherapy | 3.26 | 0.07 | ||
| Yes | 24 (46.20) | 62 (32.60) | ||
| No | 28 (53.80) | 128 (67.40) | ||
| Preoperative CEA (μg/L) | 9.59 | < 0.01 | ||
| < 5 | 21 (40.40) | 122 (64.20) | ||
| ≥ 5 | 31 (59.60) | 68 (35.80) | ||
| Lymphatic node transfer | 4.90 | 0.03 | ||
| Yes | 28 (53.80) | 70 (36.80) | ||
| No | 24 (46.20) | 120 (63.20) |
Binary logistic regression analysis was performed with the occurrence of liver transfer as the dependent variable and SII, CAR, RDW, ALT, preoperative CEA, and lymphatic metastasis as the independent variables. The variable assignments are shown in Table 3. Factors affecting the univariate analysis (P < 0.05) (SII, CAR, RDW, ALT, preoperative CEA, and lymphatic metastasis) in the transfer and non-transfer groups were included in the binary logistic regression analysis, which showed that SII, CAR, and RDW were independent risk factors for liver transfer in the postoperative period, and the model excluded the three factors of ALT, preoperative CEA, and lymphatic metastasis (P > 0.05) (Table 4).
| Project | Assignment description |
| Dependent variable | Transfer group '1', Non-transfer group '0' |
| SII | Continuous variable |
| CAR | Continuous variable |
| RDW | Continuous variable |
| ALT | Continuous variable |
| Preoperative CEA | ≥ 5 μg/L '1', < 5 μg/L '0' |
| Lymphatic node transfer | Yes '1', No '0' |
| Considerations | β | SE | Wald χ2 | P value | OR | 95%CI |
| SII | 0.26 | < 0.01 | 36.78 | < 0.01 | 1.03 | 1.02-1.03 |
| CAR | 1.57 | 0.56 | 7.74 | 0.01 | 4.79 | 1.59-14.44 |
| RDW | 0.57 | 0.15 | 13.77 | < 0.01 | 1.76 | 1.31-2.38 |
| ALT | -0.25 | 0.15 | 2.87 | 0.09 | 0.78 | 0.58-1.04 |
| Preoperative CEA | 0.85 | 0.48 | 3.09 | 0.08 | 2.34 | 0.91-6.04 |
| Lymphatic node transfer | 0.7 | 0.49 | 2.04 | 0.15 | 2.01 | 0.77-5.23 |
| Constant | -9.17 | 7.71 | 1.41 | 0.24 | < 0.01 | - |
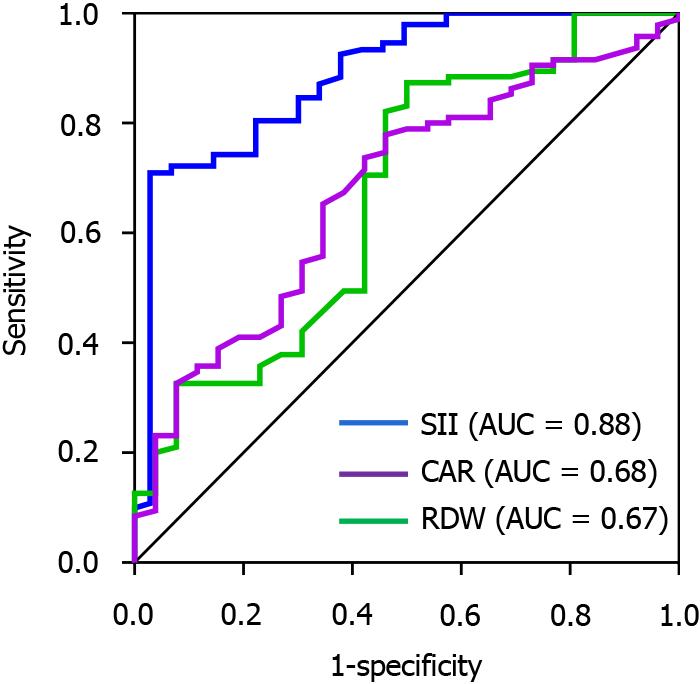
The ROC curves showed that the predicted AUCs of SII, CAR, and RDW were 0.88, 0.68, and 0.67, respectively, with sensitivities of 96.20%, 65.40%, and 50.00% and specificities of 71.60%, 67.40%, and 87.40%, respectively (Table 5 and Figure 2).
| Considerations | Truncation value | AUC | SE | P value | 95%CI | Sensitivity (%) | Idiosyncrasy (%) |
| SII | 290.52 | 0.88 | 0.03 | < 0.01 | 0.83-0.94 | 96.2 | 71.6 |
| CAR | 0.86 | 0.68 | 0.04 | < 0.01 | 0.60-0.76 | 65.4 | 67.2 |
| RDW | 16.76 | 0.67 | 0.04 | < 0.01 | 0.59-0.76 | 50 | 87.4 |
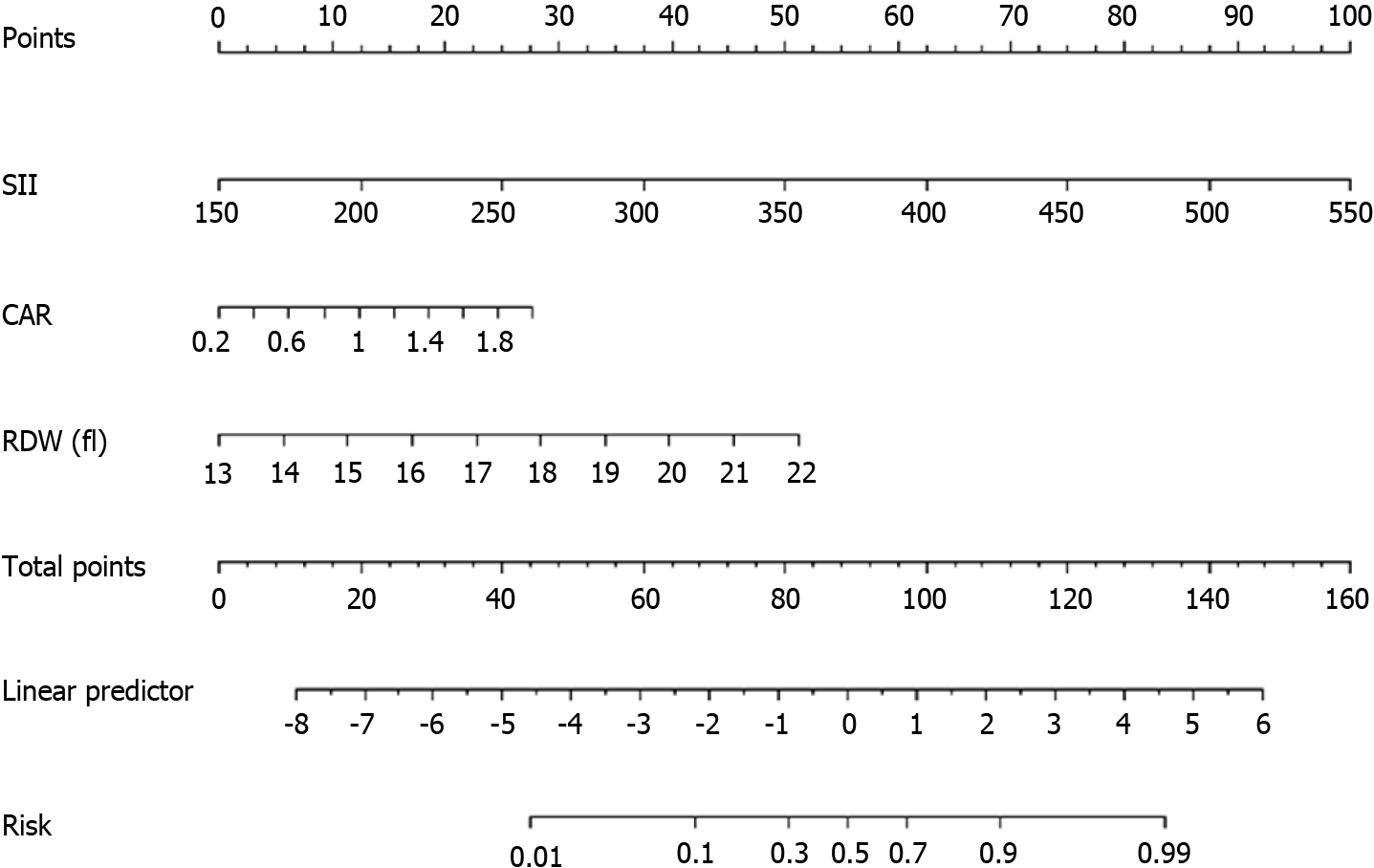
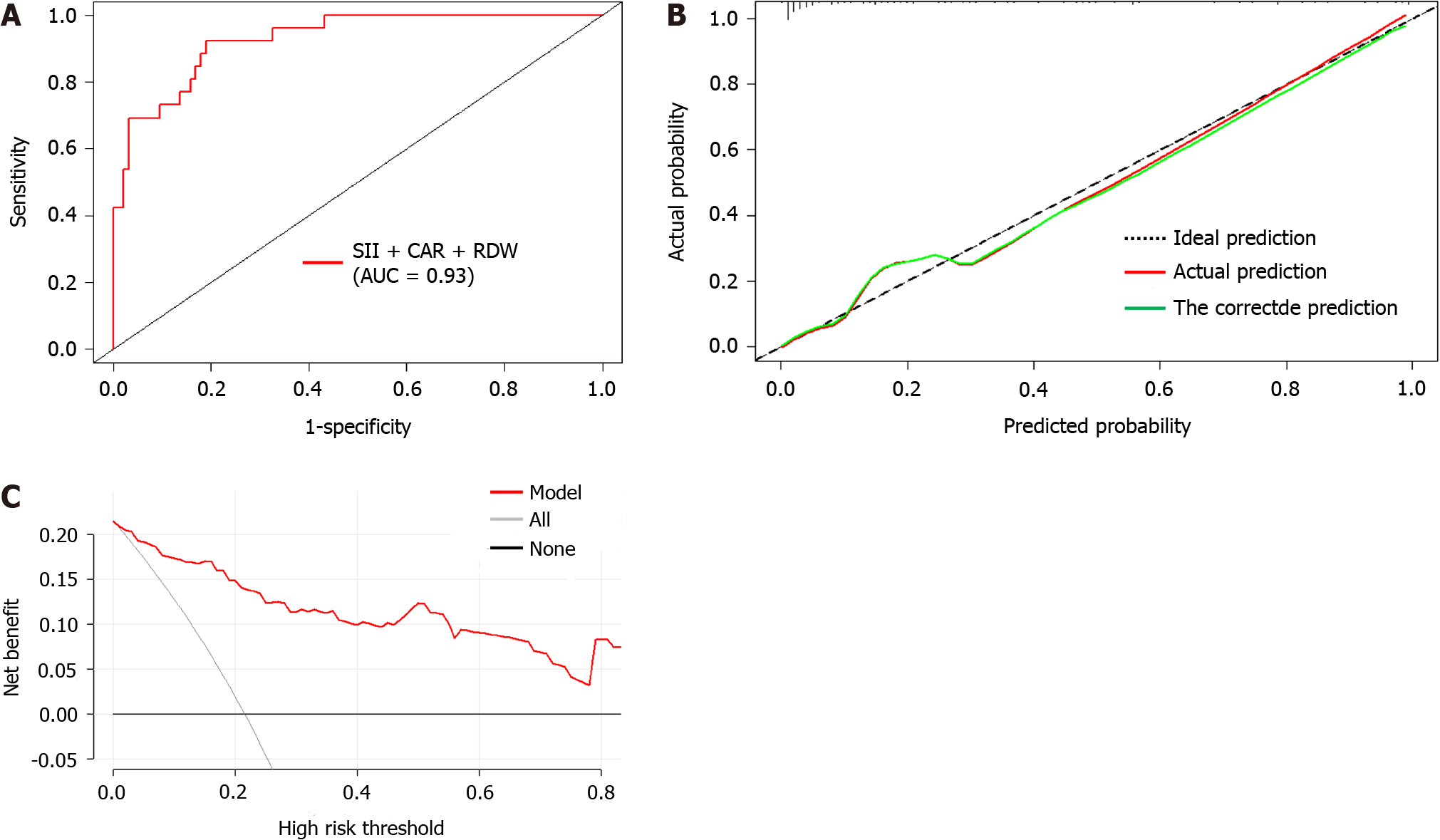
Based on the results of the multifactorial logistic regression analysis, three factors, namely SII, CAR, and RDW, were used in a columnar graphical prediction model constructed using the R software for the occurrence of liver metastasis after radical resection of colon cancer (Figure 3). The ROC curve (Figure 4A) assessed that the AUC (95%CI) of the column chart for distinguishing the occurrence of liver metastasis after radical resection of colon cancer was 0.93 (0.89-0.96), and the optimal cut-off value of the column chart was 0.16, with a sensitivity of 91.30% and a specificity of 81.10%. Asse
Colon cancer is a highly aggressive and migratory malignant tumor, and the liver is the most common organ site for postoperative metastasis[17]. Liver metastasis occurs in approximately 50% of patients with colon cancer, with metastases found at diagnosis in 25% of patients[18]. Further, metastasis is the primary cause of death in patients with colon cancer[19]. Therefore, assessing the predictive value of liver metastases after surgery in patients with colon cancer is clinically important. Overall, we found that SII, CAR, and RDW alone had some assessment value for the postoperative prognosis of patients with colon cancer[12,20,21], and the SII had a high predictive ability for the occurrence of liver metastasis after surgery in these patients[8]. Therefore, we constructed a column-line graph prediction model for the occurrence of liver metastasis after radical resection of colon cancer using SII, CAR, and RDW to help clinicians identify patients at high risk of liver metastasis. Such predictions would allow timely intervention, which could help promote optimal treatment timing.
An impaired immune function, systemic inflammatory state, and chronic inflammatory response promote the development and progression of malignant tumors and are closely associated with patient prognosis. SII is a novel inflammatory index closely related to inflammatory and immune pathways. This relationship can be expressed by the formula SII = platelets × neutrophils/Lymphocytes (× 109/L)[19]. Studies have shown that the SII has a high value in predicting the prognosis of malignant tumors[22]. In the present study, we reviewed related studies on postoperative liver transfer for colorectal cancer, which showed that the preoperative SII level of patients in the transfer group was higher than that of both the transfer and non-transfer groups, indicating that the preoperative SII level has some predictive value for liver metastasis of colorectal cancer after surgery. The results of the ROC curve analysis revealed an AUC of 0.88, which suggests that the efficacy of the SII in predicting liver metastasis of colon cancer after surgery improved, with a sensitivity of 96.20%. This result is consistent with the findings of Lu et al[8] (AUC = 0.882).
Related studies have shown that the prognoses of patients with malignant tumors such as colorectal cancer[23] and hepatocellular carcinoma[24] are closely related. Preoperative CAR (a systemic inflammatory marker) has further been found to serve as a prognostic marker for liver metastases of colorectal cancer treated with potential radical resection[25]. Meanwhile, other studies have shown that CAR reflects the inflammatory status and nutritional level of the patient, with low susceptibility and high stability[26]. In the present study, we showed that CAR levels were higher in the group with liver metastases than in those without liver metastases after colon cancer surgery; this is consistent with the findings of Liao et al[12]. These results suggest that the preoperative CAR level has some predictive value for postoperative liver metastasis of colon cancer. In our study, the ROC results showed that the efficacy of CAR in predicting postoperative hepatic metastasis of colon cancer was average (AUC = 0.68), indicating that there is a limitation to the value of CAR alone for predicting postoperative hepatic metastasis of colon cancer.
Platelet secretion, migration, and endothelial cell proliferation of vascular endothelial growth factor have an inducing effect and increase vascular permeability, while tumor cells penetrate the machinery of blood vessels to metastasize and invade. RDW reflects the heterogeneous parameters of erythrocyte size and peripheral blood volume[27]. Studies have shown that the prognosis of patients with solid tumors is closely related to RDW[16]. In the present study, we showed that the RDW level was higher in the group with liver metastasis than in the group without liver metastasis after colon cancer surgery. This result is consistent with the findings of Lu et al[28]. These results suggest that preoperative RDW has a certain predictive value for postoperative liver metastasis in colon cancer. The ROC results showed that the efficacy of RDW in predicting the postoperative incidence of liver metastasis in colon cancer was average (AUC = 0.67), suggesting that the use of RDW alone does not have a good predictive value for liver metastasis in postoperative colon cancer.
Pang et al[29] showed that column-line diagrams performed better in risk stratification of prognosis for patients undergoing radical colon cancer surgery. Therefore, in the present study, we further constructed a column-line diagram prediction model of the occurrence of liver metastasis after radical resection of colon cancer by SII, CAR, and RDW. The ROC results showed an AUC (95%CI) of 0.93 (0.89-0.96). In a prior study, Nagata et al[30] developed a prediction model for liver metastasis involving tumor differentiation, pathological staging, and preoperative CEA level. The AUC (95%CI) of this model was 0.78 (0.71-0.84), which was less effective than the model we constructed, suggesting that the models constructed by SII, CAR, and RDW can improve the prediction of liver metastasis after colon cancer surgery accuracy. In addition, we plotted calibration curves and DCA curves to further evaluate the efficacy of the model. The results of the calibration curve analysis showed that the actual curves of the column-line graph model for predicting the occurrence of postoperative liver metastasis in colon cancer largely agreed with the ideal curves, which indicated that the model had good consistency. In addition, the DCA curve suggested that the net benefit of the model was superior to the threshold value of 15%, indicating that the model was safer and had a higher clinical utility.
Overall, the results of the present study show that poor SII, CAR, and RDW results are risk factors for liver metastasis after colon cancer surgery. The prediction model established in this study based on these risk factors has good discriminatory power and a high calibrating ability for the occurrence of liver metastasis after radical resection of colon cancer. Thus, this model could help clinicians more intuitively identify high-risk patients prone to liver metastasis after surgery, take targeted screening measures, and formulate individualized medical treatment strategies. However, this study had certain limitations. First, only the data of patients who underwent radical resection for colon cancer in our hospital at a fixed time period were selected, meaning that selection bias may have been an issue. Second, as this study was a retrospective study, there is a possibility of retrospective bias. Third, the single-center design limits the generalizability of the results. Finally, only the bootstrap method was used for the internal validation of the predictive model of the risk of liver metastasis after surgery for colon cancer, and no other validation method was used. Therefore, external validation with large samples and multicenter data is needed in the future to assess the generalizability of the model and further validate and improve its prediction ability.
We identified SII, CAR, and RDW as risk factors affecting the occurrence of liver metastasis after colon cancer surgery. Further, we showed that the combination of these three factors could effectively predict the risk of liver metastasis, and our prediction model based on these three risk factors had good predictive efficacy. Therefore, in clinical practice, SII, CAR, and RDW can be beneficial for the early screening of colon cancer and predict the occurrence of liver metastasis after surgery in advance, which can help determine the best time for treatment.
Colon cancer is one of the most common malignant tumors of the digestive system. Clinical treatment primarily includes surgery, and chemo- and radiotherapy are used as auxiliary therapies to ensure comprehensive treatment. Surgical resection is an effective radical treatment for colon cancer; however, liver metastasis still occurs in 25% of patients after surgery.
In the current study, several methods to predict and assess liver metastasis after colon cancer surgery were investigated. Therefore, the identification of simple and efficient predictors of liver metastasis after colon cancer surgery is clinically important.
This study is aimed to predict the efficacy of postoperative liver metastasis in colon cancer using nomogram models constructed from systemic inflammatory index (SII), C-reactive protein/albumin ratio (CAR), and red blood cell distribution width (RDW) to provide predictive value in the clinic.
This study retrospectively analyzed the clinicopathological data of 242 patients who underwent radical resection for colon cancer; analyzed the risk factors affecting the development of liver metastases in these patients; assessed nomogram models constructed using the SII, CAR, and RDW; and evaluated the predictive efficacy of the models.
The SII, CAR, and RDW are risk factors for liver metastasis after colon cancer surgery. The area under the receiver operating characteristic curve of the column-line diagram model constructed based on these three risk factors to predict whether liver metastasis occurred after colon cancer surgery was 0.93 (95%CI: 0.89-0.96). The calibration curve of the column-line diagram predicting the risk of postoperative liver metastasis from colon cancer matched well with the actual risk of occurrence, and the net benefit of the model was better, indicating that the model was safer.
The SII, CAR, and RDW are independent risk factors for the development of liver metastases after colon cancer surgery, and the predictive efficacy of the column-line graph model constructed using the SII, CAR, and RDW was high.
To analyze the factors influencing the occurrence of liver metastases in patients with colon cancer and construct a nomogram model using the SII, CAR, and RDW to evaluate the predictive efficacy of the model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cano-Valderrama O, Spain; Tougeron D, France S-Editor: Wang JL L-Editor: A P-Editor: Yuan YY
| 1. | Ruan H, Leibowitz BJ, Zhang L, Yu J. Immunogenic cell death in colon cancer prevention and therapy. Mol Carcinog. 2020;59:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P; European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Saad AM, Abdel-Rahman O. Initial systemic chemotherapeutic and targeted therapy strategies for the treatment of colorectal cancer patients with liver metastases. Expert Opin Pharmacother. 2019;20:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Marozzi M, Parnigoni A, Negri A, Viola M, Vigetti D, Passi A, Karousou E, Rizzi F. Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Nguyen AV, Wu YY, Lin EY. STAT3 and sphingosine-1-phosphate in inflammation-associated colorectal cancer. World J Gastroenterol. 2014;20:10279-10287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Walzik D, Joisten N, Zacher J, Zimmer P. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. 2021;121:1803-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Lu Y, Xin D, Wang F. Predictive Significance Of Preoperative Systemic Immune-Inflammation Index Determination In Postoperative Liver Metastasis Of Colorectal Cancer. Onco Targets Ther. 2019;12:7791-7799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70:709-713. [PubMed] [DOI] [Full Text] |
| 10. | McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 349] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 11. | Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39:S143-S146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Liao CK, Yu YL, Lin YC, Hsu YJ, Chern YJ, Chiang JM, You JF. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J Surg Oncol. 2021;19:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Haruki K, Shiba H, Horiuchi T, Sakamoto T, Gocho T, Fujiwara Y, Furukawa K, Misawa T, Yanaga K. Impact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastases. Am J Surg. 2017;214:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Jandial A, Kumar S, Bhalla A, Sharma N, Varma N, Varma S. Elevated Red Cell Distribution Width as a Prognostic Marker in Severe Sepsis: A Prospective Observational Study. Indian J Crit Care Med. 2017;21:552-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D, Yoshimatsu H, Suzuki Y. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8:e80240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Hu L, Li M, Ding Y, Pu L, Liu J, Xie J, Cabanero M, Li J, Xiang R, Xiong S. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8:16027-16035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Polk N, Budai B, Hitre E, Patócs A, Mersich T. Corrigendum: High Neutrophil-to-Lymphocyte Ratio (NLR) and Systemic Immune-Inflammation Index (SII) are Markers of Longer Survival After Metastasectomy of Patients With Liver-Only Metastasis of Rectal Cancer. Pathol Oncol Res. 2022;28:1610658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Chandra R, Karalis JD, Liu C, Murimwa GZ, Voth Park J, Heid CA, Reznik SI, Huang E, Minna JD, Brekken RA. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, Johansson M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 21. | Bernstein CN, Benchimol EI, Bitton A, Murthy SK, Nguyen GC, Lee K, Cooke-Lauder J, Kaplan GG. The Impact of Inflammatory Bowel Disease in Canada 2018: Extra-intestinal Diseases in IBD. J Can Assoc Gastroenterol. 2019;2:S73-S80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (2)] |
| 23. | Matsuoka H, Ando K, Hu Q, Zaitsu Y, Tsuda Y, Hisamatsu Y, Nakashima Y, Kimura Y, Oki E, Mori M. Postoperative C-reactive protein/albumin ratio is a biomarker of risk of recurrence and need for adjuvant chemotherapy for stage III colorectal cancer. Int J Clin Oncol. 2020;25:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Lin N, Li J, Ke Q, Wang L, Cao Y, Liu J. Clinical Significance of C-Reactive Protein to Albumin Ratio in Patients with Hepatocellular Carcinoma: A Meta-Analysis. Dis Markers. 2020;2020:4867974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Shibutani M, Nagahara H, Fukuoka T, Iseki Y, Hirakawa K, Ohira M. Efficacy of Adjuvant Chemotherapy According to the Classification of Recurrence Risk Based on Systemic Inflammatory Markers in Patients With Liver Metastases of Colorectal Cancer. Anticancer Res. 2019;39:5039-5045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ito T, Shinkawa H, Takemura S, Tanaka S, Nishioka T, Miyazaki T, Ishihara A, Kubo S. Impact of the Preoperative C-reactive Protein to Albumin Ratio on the Long-Term Outcomes of Hepatic Resection for Intrahepatic Cholangiocarcinoma. Asian Pac J Cancer Prev. 2020;21:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Chen W, Xin S, Xu B. Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. J Healthc Eng. 2022;2022:7971415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Lu X, Huang X, Xue M, Zhong Z, Wang R, Zhang W, Wang L, Qiao Y, Ling F, Zhang Q, Zhang Y. Prognostic significance of increased preoperative red cell distribution width (RDW) and changes in RDW for colorectal cancer. Cancer Med. 2023;12:13361-13373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Pang X, Xu B, Lian J, Wang R, Wang X, Shao J, Tang S, Lu H. Real-world survival of colon cancer after radical surgery: A single-institutional retrospective analysis. Front Oncol. 2022;12:914076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 30. | Nagata H, Ishihara S, Oba K, Tanaka T, Hata K, Kawai K, Nozawa H. Development and Validation of a Prediction Model for Organ-Specific Recurrences After Curative Resection of Colon Cancer. Dis Colon Rectum. 2019;62:1043-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |