Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.932
Peer-review started: November 6, 2023
First decision: December 6, 2023
Revised: December 29, 2023
Accepted: February 23, 2024
Article in press: February 23, 2024
Published online: March 27, 2024
Processing time: 137 Days and 7.7 Hours
Genetic factors of chronic intestinal ulcers are increasingly garnering attention. We present a case of chronic intestinal ulcers and bleeding associated with mu
A 20-year-old man was admitted to our center with a 6-year history of recurrent abdominal pain, diarrhea, and dark stools. At the onset 6 years ago, the patient had received treatment at a local hospital for abdominal pain persisting for 7 d, under the diagnosis of diffuse peritonitis, acute gangrenous appendicitis with perforation, adhesive intestinal obstruction, and pelvic abscess. The surgical treat
Mutations in the ACVRL1 and PLA2G4A genes may be one of the causes of chronic intestinal ulcers and bleeding in IBD. Orally administered Kangfuxin liquid may have therapeutic potential.
Core Tip: We present a case of chronic intestinal ulcers and bleeding associated with mutations of the activin A receptor type II-like 1 (ACVRL1) and phospholipase A2 group IVA (PLA2G4A) genes. The patient’s symptoms started at an early age, with the first manifestation being acute gangrenous appendicitis with perforation. However, the patient experienced recurrent abdominal pain and bloody stools one year after surgery. After colonoscopy and intestinal mucosal pathological examination, the diagnosis was established as inflammatory bowel disease without the characteristic features of Crohn's disease or ulcerative colitis. Aminosalicylic acid and immunotherapy were not sufficiently effective. Further investigations revealed mutations in the ACVRL1 and PLA2G4A genes. From these observations, we inferred that these mutations may be linked to chronic intestinal ulcers and bleeding. Kangfuxin liquid appears to be promising in terms of therapeutic potential in this type of chronic intestinal ulcers.
- Citation: Tang YJ, Zhang J, Wang J, Tian RD, Zhong WW, Yao BS, Hou BY, Chen YH, He W, He YH. Link between mutations in ACVRL1 and PLA2G4A genes and chronic intestinal ulcers: A case report and review of literature. World J Gastrointest Surg 2024; 16(3): 932-943
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/932.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.932
Chronic intestinal ulcers are caused by multiple factors and have a complex etiopathogenesis. On the basis of causation, chronic gastrointestinal ulcers are classified as those due to infectious causes such as bacteria, fungi, viruses, and in
We present a case of chronic intestinal ulcer and bleeding in IBD, which were probably associated with mutations in the active A receptor type II-like 1 (ACVRL1) and phospholipase A2 group IVA (PLA2G4A) genes. In addition, we review the relevant literature and summarize the diagnosis and treatment in such cases.
A 20-year-old male patient was admitted to our hospital on July 9, 2021, for evaluation of recurring abdominal pain, diarrhea, and black stools, which had been persisting for 6 years.
The patient received treatment at a local hospital 6 years back for seven days of unexplained abdominal pain. On exa
On July 9, 2021, the patient presented with persistence and worsening of the abovementioned symptoms. The fre
The patient's medical history was the same as before, with no other surgical or traumatic history. He also denied having any history of long-term use of NSAIDs or glucocorticoids. Due to gastrointestinal bleeding, the patient received red blood cell infusion twice, without any negative reactions during the process. There was no evidence of infectious diseases, such as typhoid fever and tuberculosis, or any sexually transmitted diseases.
The individual reported a history of alcohol consumption for three years, although the amount consumed was unknown. He denied having any history of smoking or exposure to toxins; he also denied any family history of genetic diseases.
On examination, the patient had stable vital signs, a clear mind, an anemic face, and pallor of the palpebral conjunctiva, lips, and nail beds. Superficial lymph nodes were not palpable, and cardiopulmonary examination revealed no apparent positive signs; only an old longitudinal surgical scar measuring approximately 20 cm in length was noted on the abdo
Routine blood tests revealed moderate anemia and a decrease in the average red blood cell volume (Table 1). Other tests conducted after admission showed no abnormalities in coagulation function and blood biochemistry. No abnormal find
| Date | WBCs, × 109/L | RBCs, × 1012/L | HB, g/L | PLTs, × 109/L | MCV, fl |
| Ref: 3.5-9.5 | Ref: 4.3-5.8 | Ref: 130-175 | Ref: 125-350 | Ref: 80-100 | |
| 2021-07-09 | 5.13 | 3.7↓ | 75↓ | 312 | 68.3↓ |
| 2021-07-11 | 4.83 | 3.3↓ | 66↓ | 249 | 68.7↓ |
| 2021-07-18 | 6.25 | 3.8↓ | 76↓ | 429↑ | 67.4↓ |
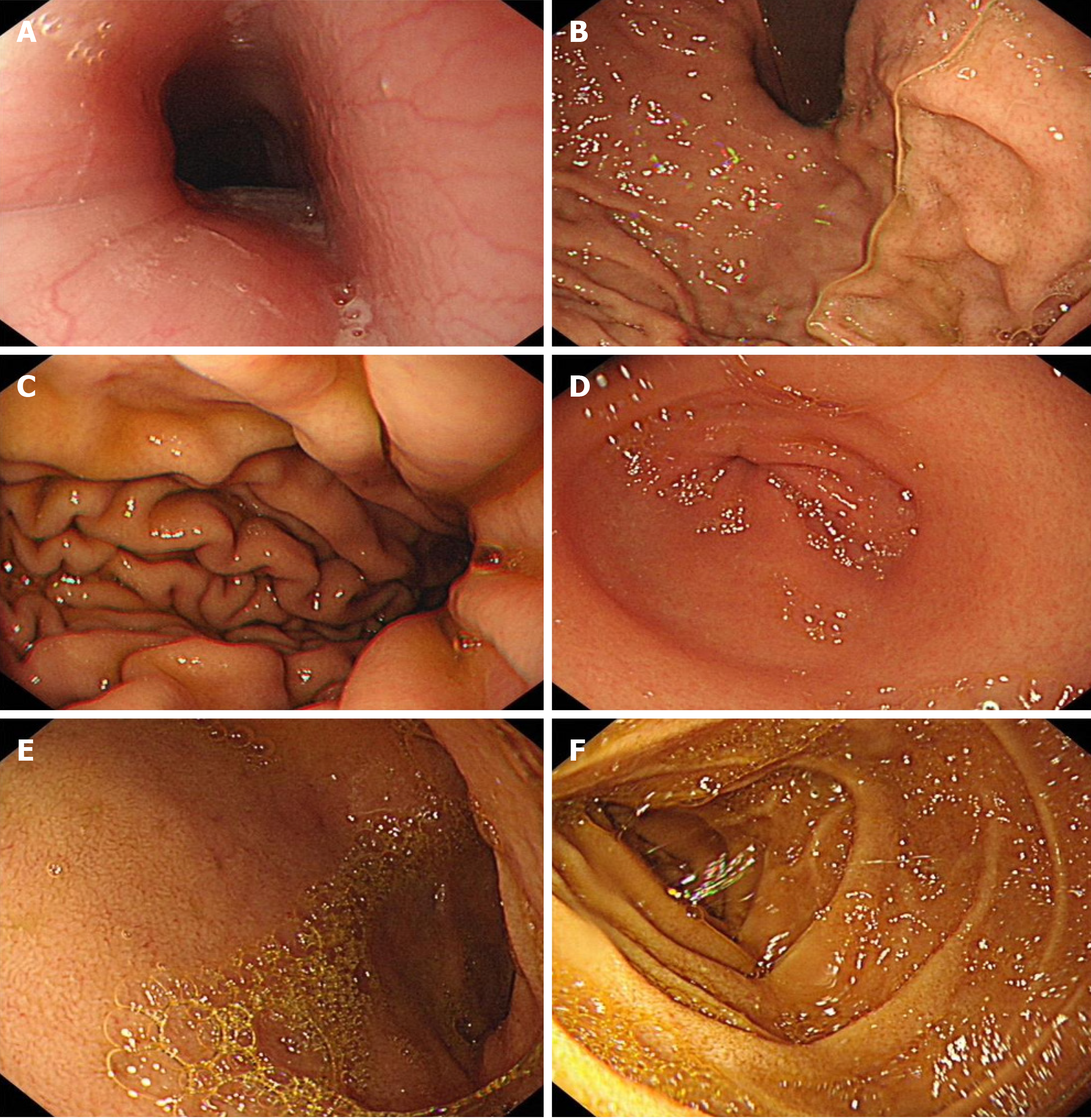
On gastroscopy performed on July 12, 2021, the esophagus appeared to have normal morphology and color, with no evident abnormalities (Figure 2). The distance from the cardia to the incisor was approximately 40 cm, and the dentate line was clearly visible. No abnormalities were visible in the mucosa and structure of the gastric fundus and gastric body. The gastric fundus showed a moderate amount of mucus and yellow turbidity, with a smoothly curved gastric angle. In addition, congestion and edema of the gastric antrum mucosa were noted, with no signs of ulcers or masses. The pylorus appeared to be circular and functioning properly, with smooth opening and closure. Similarly, no abnormalities were detected in the duodenal bulb and mucosa of the descending duodenum. The above findings led to the conclusion of chronic non-atrophic gastritis with bile reflux.
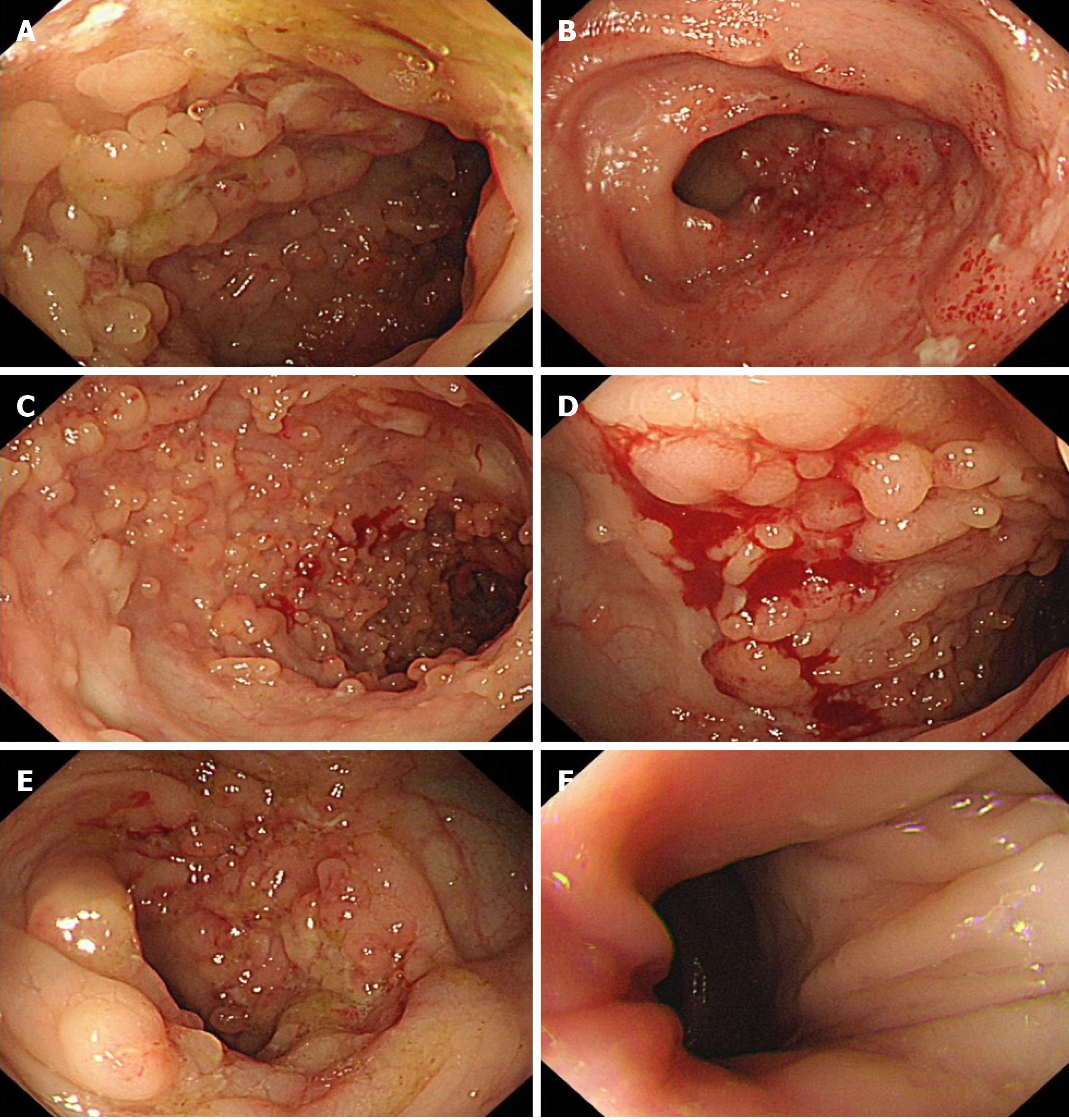
Colonoscopy performed on July 16, 2021 revealed that the surgical repair site between the ascending colon and the ileum was visible 55 cm from the anus (usually 60-70 cm away from the anus); the ileocecal valve and cecum were in
Pathological examination of the appendix removed on July 2, 2015, showed acute gangrenous appendicitis, peritonitis, appendix perforation, and fecal stone incarceration within the cavity.
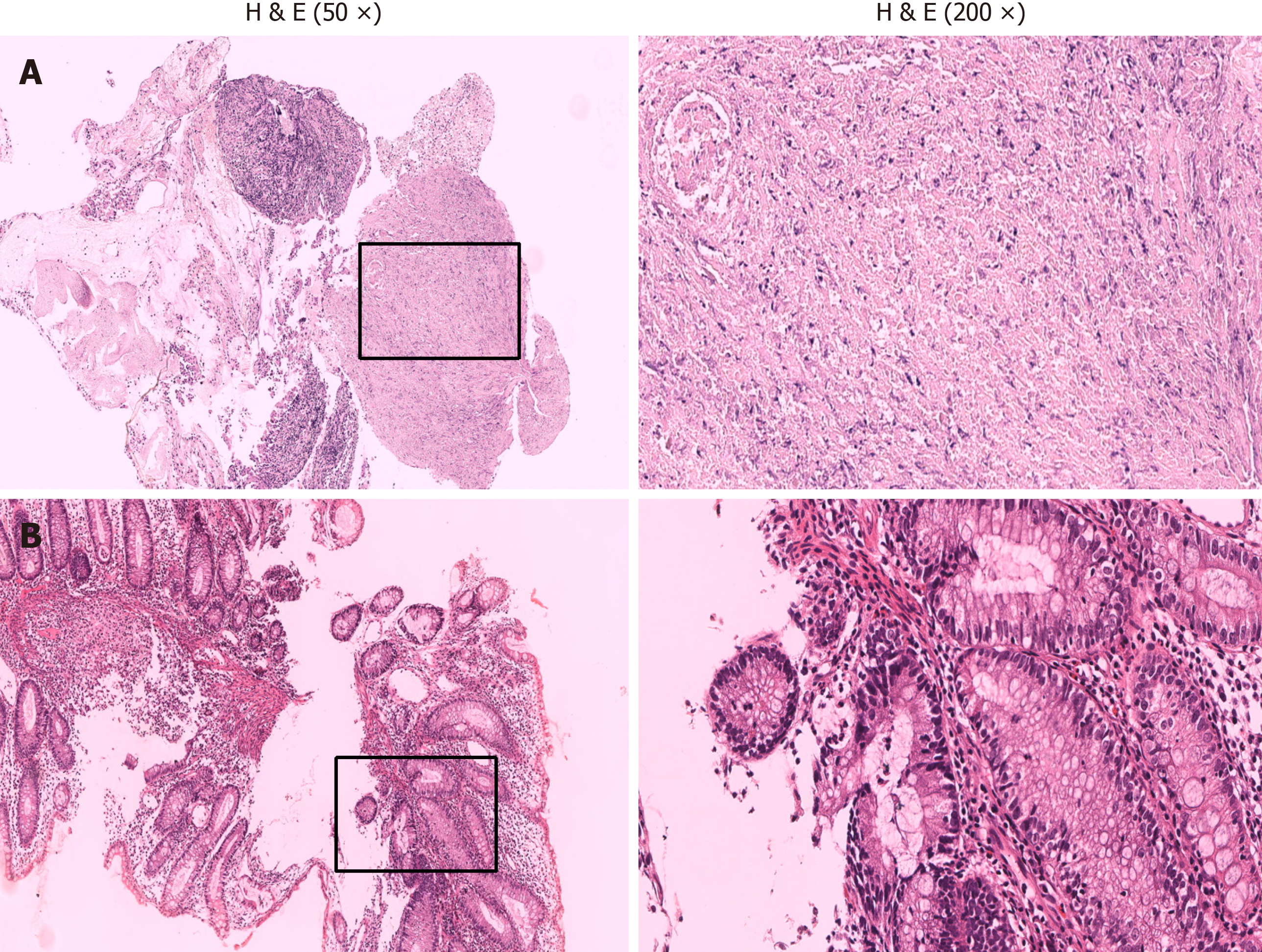
Histopathologic examination of a sample of intestinal mucosa obtained on July 19, 2021 revealed superficial mucosa ulceration in the ileum with abundant inflammatory exudates, formation of granulomas, infiltration of lymphocytes and plasma cells, and no caseous necrosis (Figure 4). Additionally, the superficial mucosa of the colon showed signs of acute and chronic inflammation.
Multiple routine investigations during hospitalization revealed a decrease in the levels of red blood cells, hemoglobin, and average red blood cell volume; slightly higher fibrinogen levels; and a positive fecal occult blood. Given the patient’s history of appendiceal perforation, special care was taken to remain vigilant for signs of gastrointestinal ulcers, and a careful gastroenteroscopy and pathological examination was performed, which validated the atypical intestinal lesions of CD or UC. Additionally, the patient did not exhibit symptoms such as low fever or night sweats, and did not have ab
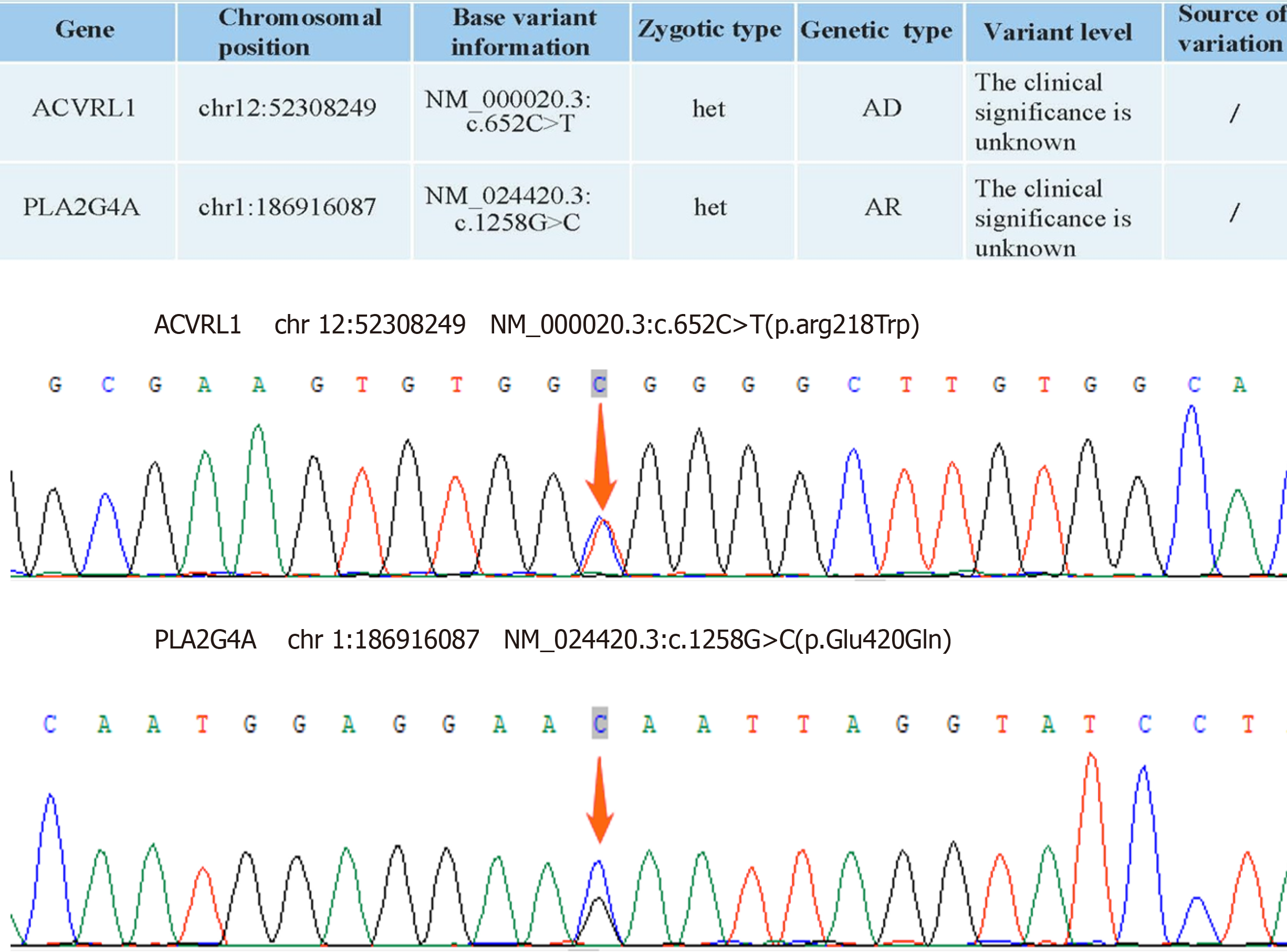
Subsequently, whole-exome sequencing for genetic diseases was performed, which revealed mutations in the ACVRL1 and PLA2G4A genes. Mutations of both these genes are known to cause a decrease in the ability of the intestinal mucosa to resist injury and sustain repair. Kangfuxin liquid is known to have an effect of accelerating the repair of pathological tissue, shedding of necrotic tissue, and healing of ulcers and wounds. Accordingly, we modified the treatment plan and administered oral Kangfuxin liquid of 10 mL three times daily to promote the repair of the intestinal repair. The patient was discharged once his symptoms improved on initiating this treatment, and treatment was continued for 4 wk after discharge. If the patient occasionally experiences symptoms such as abdominal pain, diarrhea, and black stool, oral administration of Kangfuxin liquid can alleviate the symptoms.
Taking into consideration the patient's medical history and the results of investigations, the diagnosis was determined to be IBD, and the patient’s chronic intestinal ulcer and bleeding may be related to mutations in the ACVRL1 and PLA2G4A genes.
The treatment plan comprised the following: Yunnan Baiyao for hemostasis, blood transfusion to correct anemia, and omeprazole to inhibit gastric acid secretion. However, this treatment plan was not sufficiently effective. The patient re
Modification of the treatment plan to include Kangfuxin liquid was found to have a satisfactory therapeutic effect. The patient’s symptoms of bloody stools and abdominal pain improved. However, without treatment, there still remains the possibility of these symptoms resurfacing. The patient opted to continue receiving Kangfuxin liquid for symptom relief.
In this paper, we present the case of a 20-year-old man with IBD, whose chronic intestinal ulcer and bleeding may be associated with mutations in the ACVRL1 and PLA2G4A genes. At the age of 14 years, the patient had developed acute gangrenous appendicitis with perforation for which he received surgical treatment. Subsequently, the patient had re
Our patient first developed acute gangrenous appendicitis with perforation at the age of 14 years, and one year after surgical treatment, experienced recurrent abdominal pain and bloody stools. During follow-up, colonoscopy revealed scattered nodular protrusions, lamellar vesicles, and shallow ulcers at the site of surgical repair and in the ileum. Addi
IBD is a chronic inflammatory disease of the gastrointestinal mucosa that occurs due to various genetic and environmental factors. IBD encompasses two chronic conditions: CD and UC[8,9]. The clinical symptoms of IBD primarily include abdominal pain, bloody stools, and diarrhea.
In IBD, aminosalicylic acid and immunomodulators are frequently employed to control the imbalance between pro
CD is more prevalent among adolescents and causes transmural inflammation that may affect any section of the gastrointestinal tract, although it most commonly affects the end of the ileum or adjacent portion of the colon sporadically[11]. The clinical symptoms of CD include abdominal pain, diarrhea, weight loss, anemia, fever, and bloody stools. The histological features of CD are submucosal thickening, transmural inflammation, fissure ulcers, and non-caseating gra
Similar to CD, UC is more prevalent among young individuals and often presents with diarrhea, abdominal pain, mucopurulent and bloody stools, anemia, and fever[12]. UC is characterized by inflammatory changes of the mucosal lining, which begin in the rectum and continuously spread proximally. It primarily affects the mucosal layer and sub
In the present case, the histopathologic examination revealed ulceration of the superficial mucosa of the ileum, with substantial inflammatory exudate and granuloma formation. Significant infiltration of lymphocytes and plasma cells was noted. The superficial mucosa of the colon exhibited signs of both acute and chronic inflammation. The colonoscopy revealed that the surgical repair site between the ascending colon and the ileum was visible 55 cm from the anus, with an indistinguishable ileocecal valve and cecum (usually 60-70 cm away from the anus), which suggests that the length of the colon may have been shortened and the shape of the ileocecal region may have changed. There were ulcers in the ileum and transverse colon. The ileal mucosa exhibited scattered nodular protrusions; flaky erosions; and shallow ulcers, with nodular protrusions on the mucosa; as well as substantial inflammatory exudate and granuloma formation. The histo
The underlying pathogenesis of IBD is the disruption of proinflammatory and anti-inflammatory responses, following injury to the intestinal mucosa. Intestinal inflammation in IBD is maintained by lymphocyte subpopulations, especially Th12 Lymphocytes, which are derived from immature Th23 Lymphocytes. The activity of Th12 Lymphocytes is mediated by interleukin-17 (IL-17) and IL-10. IL-23, IL-12, IL-25, and other molecules produced by activated antigen-presenting cells (APCs) bind to IL-12Rb1, thus initiating the intracellular activation pathway of the effector cells. Subsequent cytokine-mediated signaling through the JAK-STAT pathway leads to the activation of Th17 cells, which, in turn, produce several proinflammatory cytokines, such as IL-17A, IL-17F, IL-22, IL-26, and the chemokine CCL20. These cytokines are expressed at high levels during the course of IBD[14]. IL-10 is regulated by the level of phosphorylation achieved through the action of JAK1. This occurs by the binding of IL-10 to the IL-10R1 receptor on white blood cells and is mediated by the STAT 3 signaling pathway. IL-10 can inhibit APC by decreasing the expression of the major histocompatibility complex (MHC). Therefore, increased MHC expression is advantageous in delaying the progression of IBD. Additionally, IL-10 is known to facilitate the proper functioning of the intestinal epithelial barrier by stimulating the regeneration of intestinal stem cells and regulating gut microbiota during fucosylation. Studies have shown that the lack of IL-10 contributes to the onset of IBD[15]. Therefore, the patient was previously administered aminosalicylic acid and immunomodulatory the
Considering the patient’s early age of onset, recurring condition, lack of typical CD and UC colonoscopy features, and poor response to aminosalicylic acid and immunomodulatory therapy, we sought to investigate the potential invol
The ACVRL1 gene is responsible for encoding the protein activin receptor-like kinase 1 (ALK1)—a type I receptor of the transforming growth factor-β (TGF-β) family[18] primarily expressed in vascular endothelial cells and other tissues with rich vasculature. Additionally, ALK1 is a homodimeric transmembrane glycoprotein, which implies that it is inte
Our patient had a mutation in the ACVRL1 gene inherited in an autosomal dominant manner. Specifically, the mu
Phospholipase A2 (PLA2) refers to an important group of enzymes that can break down the Sn-2 ester bond structure in phospholipids. Cytoplasmic PLA2 (cPLA2) catalyzes the hydrolysis of membrane phospholipids, resulting in the release of arachidonic acid (AA) and lysophospholipids. cPLA2 also plays crucial roles in phospholipid reconstruction, pulmonary surfactant metabolism, cell signaling, host response, the promotion of blood coagulation, etc.[28,29]. PL
Our patient had the PLA2G4A gene mutation acquired via autosomal recessive inheritance. A heterozygous transition from guanine to uracil (c.1258G>C) occurs in this mutation, resulting in a missense mutation and consequent partial loss of PLA2G4A function and expression. Increased levels of PLA2G4A contribute to the development of an immunosuppressive microenvironment[7]. Additionally, recessive mutations in the PLA2G4A gene can result in the formation of multiple small intestinal ulcers, which aligns with the findings in our case. There are no reports of mutations at this site, but briefly, we speculate that mutations in the PLA2G4A (c.1258G>C) gene result in a partial loss of function, decreased expression, and hindered conversion of AA to mediators such as PGs and TXA2. As a result, there is a dilatation of blood vessels, weakened endothelial cell growth, increased inflammatory response and weakened platelet aggregation ability. This could at least partly explain why the patient encountered recurrent episodes of severe intestinal ulcers, and bleeding during adolescence.
Intestinal injury triggers an inflammatory response as part of the host’s defense mechanism. This response is responsible for clearing damaged cells and pathogens, as well as for the restoration of tissue structure and physiological function. However, if the inflammatory response is sustained for a long period and is intense, it can cause harm to cells of healthy tissue during the response against pathogens, resulting in the occurrence of inflammatory diseases and negative effects on human health[40].
Therefore, the body also has an anti-inflammatory system to counterbalance excessive inflammatory responses. Typically, these systems remain in a state of dynamic equilibrium. The intestinal epithelial cells continuously undergo rapid proliferation and differentiation to repair the intestine in case of damage, under the influence of genes like ACVRL1, thus maintaining the integrity of the mucosal lining and blood vessels in the intestines. Mutations of this gene can disrupt this process, leading to damage in the intestinal mucosa and blood vessels and consequently triggering the inflammatory response and anti-inflammatory system. This is further compounded if mutations of the PLA2G4A gene are concurrently present, it can cause a partial loss of function and reduced expression of the gene, which hinders the conversion of AA into mediators like PGs and TXA2, attenuating the vasodilatory effect and exacerbating vascular ischemic necrosis; Reduced platelet production function leads to coagulation disorders; in addition, the ability to upregulate vascular en
Our patient was found to have mutations of the ACVRL1 and PLA2G4A genes. Currently, no specific treatment is available for patients presenting with these mutations. Therefore, we decided to try Kangfuxin liquid as a treatment option. Kangfuxin liquid is a patented traditional Chinese medicine prepared using the extract from Periplaneta ame
However, this case report has some limitations. First, he link between mutations in the ACVRL1 and PLA2G4A genes and chronic intestinal ulcers needs to be further verified in pathological studies and in vivo animal experiments. Second, the patient’s parents did not undergo whole-exome sequencing and colonoscopy examinations. Finally, the therapeutic effect of Kangfuxin liquid in chronic intensive ulcers and bleeding needs further observation or more case support.
Mutations in the ACVRL1 and PLA2G4A genes may be one of the underlying causes of chronic intestinal ulcers and bleeding in IBD. These mutations may compromise the ability of the intestinal mucosa to resist injury and to repair. Oral administration of Kangfuxin liquid appears to be promising in terms of therapeutic potential in chronic gastrointestinal ulcers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Park AM, Khadka S, Sato F, Omura S, Fujita M, Hsu DK, Liu FT, Tsunoda I. Galectin-3 as a Therapeutic Target for NSAID-Induced Intestinal Ulcers. Front Immunol. 2020;11:550366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Narayanan M, Reddy KM, Marsicano E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo Med. 2018;115:219-224. [PubMed] |
| 3. | Šterbenc A, Jarc E, Poljak M, Homan M. Helicobacter pylori virulence genes. World J Gastroenterol. 2019;25:4870-4884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 4. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4097] [Article Influence: 512.1] [Reference Citation Analysis (110)] |
| 5. | Kedia S, Das P, Madhusudhan KS, Dattagupta S, Sharma R, Sahni P, Makharia G, Ahuja V. Differentiating Crohn's disease from intestinal tuberculosis. World J Gastroenterol. 2019;25:418-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (6)] |
| 6. | Kulikauskas MR, Oatley M, Yu T, Liu Z, Matsumura L, Kidder E, Ruter D, Bautch VL. Endothelial Cell SMAD6 Balances ACVRL1/Alk1 Function to Regulate Adherens Junctions and Hepatic Vascular Development. bioRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Zhan Y, Zheng L, Liu J, Hu D, Wang J, Liu K, Guo J, Zhang T, Kong D. PLA2G4A promotes right-sided colorectal cancer progression by inducing CD39+γδ Treg polarization. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Astorga J, Gasaly N, Dubois-Camacho K, De la Fuente M, Landskron G, Faber KN, Urra FA, Hermoso MA. The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD. Front Immunol. 2022;13:1028953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, Peyrin-Biroulet L, Danese S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2023;8:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 10. | Balderramo D. Role of the combination of biologics and/or small molecules in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. 2022;28:6743-6751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 11. | Zhang Y, Si X, Yang L, Wang H, Sun Y, Liu N. Association between intestinal microbiota and inflammatory bowel disease. Animal Model Exp Med. 2022;5:311-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 12. | Haneishi Y, Furuya Y, Hasegawa M, Picarelli A, Rossi M, Miyamoto J. Inflammatory Bowel Diseases and Gut Microbiota. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 98] [Reference Citation Analysis (0)] |
| 13. | Karmiris K, Avgerinos A, Tavernaraki A, Zeglinas C, Karatzas P, Koukouratos T, Oikonomou KA, Kostas A, Zampeli E, Papadopoulos V, Theodoropoulou A, Viazis N, Polymeros D, Michopoulos S, Bamias G, Kapsoritakis A, Karamanolis DG, Mantzaris GJ, Tzathas C, Koutroubakis IE. Prevalence and Characteristics of Extra-intestinal Manifestations in a Large Cohort of Greek Patients with Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 14. | Kofla-Dłubacz A, Pytrus T, Akutko K, Sputa-Grzegrzółka P, Piotrowska A, Dzięgiel P. Etiology of IBD-Is It Still a Mystery? Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol. 2019;44:101344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 16. | Turpin W, Goethel A, Bedrani L, Croitoru Mdcm K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm Bowel Dis. 2018;24:1133-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Borowitz SM. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front Pediatr. 2022;10:1103713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 18. | Zhao D, Yang F, Wang Y, Li S, Li Y, Hou F, Yang W, Liu D, Tao Y, Li Q, Wang J, He F, Tang L. ALK1 signaling is required for the homeostasis of Kupffer cells and prevention of bacterial infection. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Guo X, Niu Y, Han W, Han X, Chen Q, Tian S, Zhu Y, Bai D, Li K. The ALK1-Smad1/5-ID1 pathway participates in tumour angiogenesis induced by low-dose photodynamic therapy. Int J Oncol. 2023;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Bofarid S, Hosman AE, Mager JJ, Snijder RJ, Post MC. Pulmonary Vascular Complications in Hereditary Hemorrhagic Telangiectasia and the Underlying Pathophysiology. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 21. | Salibe-Filho W, Oliveira FR, Terra-Filho M. Update on pulmonary arteriovenous malformations. J Bras Pneumol. 2023;49:e20220359. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Wang G, Wen B, Deng Z, Zhang Y, Kolesnichenko OA, Ustiyan V, Pradhan A, Kalin TV, Kalinichenko VV. Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling. Nat Commun. 2022;13:2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Shovlin CL, Simeoni I, Downes K, Frazer ZC, Megy K, Bernabeu-Herrero ME, Shurr A, Brimley J, Patel D, Kell L, Stephens J, Turbin IG, Aldred MA, Penkett CJ, Ouwehand WH, Jovine L, Turro E. Mutational and phenotypic characterization of hereditary hemorrhagic telangiectasia. Blood. 2020;136:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Fan J, Xia X, Fan Z. Hsa_circ_0129047 regulates the miR-375/ACVRL1 axis to attenuate the progression of lung adenocarcinoma. J Clin Lab Anal. 2022;36:e24591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Robert F, Desroches-Castan A, Bailly S, Dupuis-Girod S, Feige JJ. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis. 2020;15:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Errasti Díaz S, Peñalva M, Recio-Poveda L, Vilches S, Casado-Vela J, Pérez Pérez J, Botella LM, Albiñana V, Cuesta AM. A Novel Splicing Mutation in the ACVRL1/ALK1 Gene as a Cause of HHT2. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Schmid CD, Olsavszky V, Reinhart M, Weyer V, Trogisch FA, Sticht C, Winkler M, Kürschner SW, Hoffmann J, Ola R, Staniczek T, Heineke J, Straub BK, Mittler J, Schledzewski K, Ten Dijke P, Richter K, Dooley S, Géraud C, Goerdt S, Koch PS. ALK1 controls hepatic vessel formation, angiodiversity, and angiocrine functions in hereditary hemorrhagic telangiectasia of the liver. Hepatology. 2023;77:1211-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Wang S, Li B, Solomon V, Fonteh A, Rapoport SI, Bennett DA, Arvanitakis Z, Chui HC, Sullivan PM, Yassine HN. Calcium-dependent cytosolic phospholipase A(2) activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol Neurodegener. 2022;17:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Zhao R, Lv Y, Feng T, Zhang R, Ge L, Pan J, Han B, Song G, Wang L. ATF6α promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate. 2022;82:617-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | Hassan JJ, Lieske A, Dörpmund N, Klatt D, Hoffmann D, Kleppa MJ, Kustikova OS, Stahlhut M, Schwarzer A, Schambach A, Maetzig T. A Multiplex CRISPR-Screen Identifies PLA2G4A as Prognostic Marker and Druggable Target for HOXA9 and MEIS1 Dependent AML. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Sun R, Liu Z, Qiu B, Chen T, Li Z, Zhang X, Xu Y, Zhang Z. Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine. 2019;47:142-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 33. | Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol. 2019;176:337-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 34. | Adler DH, Cogan JD, Phillips JA 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Sarkar C, Jones JW, Hegdekar N, Thayer JA, Kumar A, Faden AI, Kane MA, Lipinski MM. PLA2G4A/cPLA2-mediated lysosomal membrane damage leads to inhibition of autophagy and neurodegeneration after brain trauma. Autophagy. 2020;16:466-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 36. | Hudson CJ, Zhu JXG, Durocher AM. Re-analysis of genetic polymorphism data supports a relationship between schizophrenia and microsatellite variability in PLA2G4A. Psychiatr Genet. 2021;31:102-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Bazhan D, Khaniani MS. Supplementation with omega fatty acids increases the mRNA expression level of PLA2G4A in patients with gastric cancer. J Gastrointest Oncol. 2018;9:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | McGraw KL, Cheng CH, Chen YA, Hou HA, Nilsson B, Genovese G, Cluzeau T, Pellagatti A, Przychodzen BP, Mallo M, Arenillas L, Mohamedali A, Adès L, Sallman DA, Padron E, Sokol L, Moreilhon C, Raynaud S, Tien HF, Boultwood J, Ebert BL, Sole F, Fenaux P, Mufti GJ, Maciejewski JP, Kanetsky PA, List AF. Non-del(5q) myelodysplastic syndromes-associated loci detected by SNP-array genome-wide association meta-analysis. Blood Adv. 2019;3:3579-3589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | González LM, Robles NR, Mota-Zamorano S, Arévalo-Lorido JC, Valdivielso JM, López-Gómez J, Gervasini G. Tag-SNPs in Phospholipase-Related Genes Modify the Susceptibility to Nephrosclerosis and its Associated Cardiovascular Risk. Front Pharmacol. 2022;13:817020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 40. | Liu R, Qin S, Li W. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed Pharmacother. 2022;153:113362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 41. | Zou JB, Zhang XF, Shi YJ, Tai J, Wang Y, Liang YL, Wang F, Cheng JX, Wang J, Guo DY. Therapeutic Efficacy of Kangfuxin Liquid Combined with PPIs in Gastric Ulcer. Evid Based Complement Alternat Med. 2019;2019:1324969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Wang J, Zhong L, Bo Y, Luo N, Hao P. Pharmaceutical preparations of Periplaneta americana (KangFuXin liquid) in the treatment of pressure ulcer: A meta-analysis. Int Wound J. 2023;20:2855-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 43. | Wang T, Lu H, Li F, Zhang Q. Effect of Kangfuxin Liquid enema combined with mesalazine on gestational outcomes and quality of life in child-bearing female with active ulcerative colitis: A protocol for randomized, double-blind, controlled trial. Medicine (Baltimore). 2021;100:e23915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Lin M, Zhang S, Zhang M, Shi J, Zhang C, Luo R, You J, Sun J, Zhang J, Gao F. Therapeutic efficacy and safety of Kangfuxin in combination with rabeprazole in the treatment of peptic ulcer: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 45. | Tian M, Dong J, Wang Z, Lu S, Geng F. The effects and mechanism of Kangfuxin on improving healing quality and preventing recurrence of gastric ulcer. Biomed Pharmacother. 2021;138:111513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Wan X, Gen F, Sheng Y, Ou M, Wang F, Peng T, Guo J. Meta-Analysis of the Effect of Kangfuxin Liquid on Diabetic Patients with Skin Ulcers. Evid Based Complement Alternat Med. 2021;2021:1334255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |