Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.921
Peer-review started: December 19, 2023
First decision: January 15, 2024
Revised: January 25, 2024
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: March 27, 2024
Processing time: 94 Days and 2 Hours
Advanced pancreatic cancer is resistant to chemotherapeutic drugs, resulting in limited treatment efficacy and poor prognosis. Combined administration of the chemotherapeutic gemcitabine and erlotinib is considered a potential first-line treatment for advanced pancreatic cancer. However, their comparative benefits and potential risks remain unclear.
To assess the clinical efficacy and safety of erlotinib combined with other chemotherapy regimens for the treatment of advanced pancreatic cancer.
Literature on the clinical efficacy and safety of erlotinib combined with chemotherapy for advanced pancreatic cancer was retrieved through an online search. The retrieved literature was subjected to a methodological qualitative assessment and was analyzed using the RevMan 5.3 software. Ten randomized controlled trials involving 2444 patients with advanced pancreatic cancer were included in the meta-analysis.
Compared with chemotherapeutic treatment, erlotinib combined with chemotherapy significantly prolonged the progression-free survival time of pancreatic cancer patients [hazard ratio (HR) = 0.78, 95%CI: 0.66–0.92, P = 0.003]. Meanwhile, the overall survival (HR= 0.99, 95%CI: 0.72–1.37, and P = 0.95) and disease control rate (OR = 0.93, 95%CI: 0.45–0.91, P = 0.84) were not significantly favorable. In terms of safety, the erlotinib and chemotherapy combination was associated with a significantly higher risk of diarrhea (OR = 3.59, 95%CI: 1.63–7.90, P < 0.05) and rash (OR = 3.63, 95%CI: 1.64–8.01, P < 0.05) compared with single-agent chemotherapy. Moreover, the risk of vomiting (OR = 1.27, 95%CI: 0.62–2.59, P = 0.51), regurgitation/anorexia (OR = 1.61, 95%CI: 0.25–10.31, P = 0.62), and infection (OR = 0.72, 95%CI: 0.28-1.87, P = 0.50) were not significant in either group.
Compared with a single chemotherapeutic modality, erlotinib combined with gemcitabine can prolong progression-free survival in pancreatic cancer, but does not improve survival benefit or disease control rate, and can increase the risk of diarrhea and rash.
Core Tip: There is currently no consensus in the literature regarding which treatment (erlotinib combined with chemotherapy vs chemotherapy alone) is more beneficial among patients with pancreatic cancer. To the best of our knowledge, this is the first systematic review and meta-analysis to compare erlotinib and chemotherapy. We investigated the overall survival, disease control rate, progression-free survival, and safety of the two treatments.
- Citation: Liu XY, Pan HN, Yu Y. Clinical efficacy and safety of erlotinib combined with chemotherapy in the treatment of advanced pancreatic cancer: A meta-analysis. World J Gastrointest Surg 2024; 16(3): 921-931
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/921.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.921
Pancreatic cancer is one of the most common malignant gastrointestinal tumors worldwide, and its incidence and mortality rates are increasing each year[1]. The current mainstay of pancreatic cancer treatment is surgical resection, and early surgery has been shown to increase the likelihood of successful resection, with postoperative 5-year survival rates ranging from 70% to 85%[2]. However, pancreatic tumors tend to infiltrate surrounding tissues, and early detection and metastasis prevention pose significant challenges. Consequently, most pancreatic cancers are diagnosed at advanced stages. Only 10% of advanced pancreatic cancers can be surgically resected, resulting in a 5-year survival rate of less than 4%[3]. As such, systemic chemotherapy is the primary treatment for pancreatic cancer. However, despite the efficacy of cytotoxic drugs in inducing tumor cell apoptosis, the abnormal permeability of blood vessels surrounding the tumor tissue diminishes the influx of chemotherapeutic agents into the tumor. Furthermore, residual tumor cells can acquire essential growth-promoting substances from the surrounding blood supply, which enables continued proliferation, ultimately limiting the efficacy of chemotherapeutic drugs. This underscores the urgent need for novel and effective approaches.
The evolving fields of molecular biology and tumor immunology have paved the way for targeted drug therapy for pancreatic cancer at the molecular level. This approach allows the design of drugs that selectively act on oncogenic sites within the body, inducing tumor cell-specific death while sparing normal tissue cells surrounding the tumor[4,5]. Erlotinib is a tyrosine kinase inhibitor (EGFR antagonist) used as a molecularly targeted therapeutic drug that competitively binds to the catalytic site of the intracellular region of the tyrosine kinase receptor with adenosine triphosphate, inhibiting the phosphorylation reaction. This, in turn, blocks downstream proliferative signaling and hinders ligand-dependent HER-1/EGFR activity in tumor cells, ultimately suppressing tumor cell proliferation[6,7]. When used in conjunction with chemotherapeutic drugs for pancreatic cancer treatment, erlotinib has demonstrated favorable outcomes. Hence, this study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer, with the goal of offering valuable insights into the clinical management of this disease.
Literature searches were conducted in the PubMed, Embase, and Cochrane Library databases from inception to November 2023. The search terms used were “pancreatic cancer”, “pancreatic adenocarcinoma”, “erlotinib”, “gemcitabine”, “Erlotinib monotherapy combined with chemotherapy”, “advanced pancreatic cancer”, etc.
The inclusion criteria were: (1) Randomized controlled trials published in English; (2) studies conducted in patients with pathologically diagnosed advanced pancreatic cancer; (3) studies where patients in the control group were treated with chemotherapeutic drugs (gemcitabine and capecitabine) and those in the observation group were treated with erlotinib combined with chemotherapy; and (4) studies that assessed disease control rate (DCR), overall survival (OS), and progression-free survival (PFS) as efficacy indices. Articles that met the following criteria were excluded: (1) retrospective studies; (2) studies with incomplete data; and (3) case reports and news articles.
The literature was screened by two professionals, and relevant data were extracted. The screened information was cross-checked and disagreements were resolved by discussion with a third author. The extracted information primarily included the (1) article title, literature source, authors, and publication date; (2) literature type and relevant elements for assessing the risk of bias; (3) interventions provided to the control and study groups, patients’ age, etc.; and (4) outcome indicators found in the literature, which included efficacy (DCR, PFS, and OS) and adverse effects (diarrhea, rash, vomiting, regurgitation/anorexia, and infection).
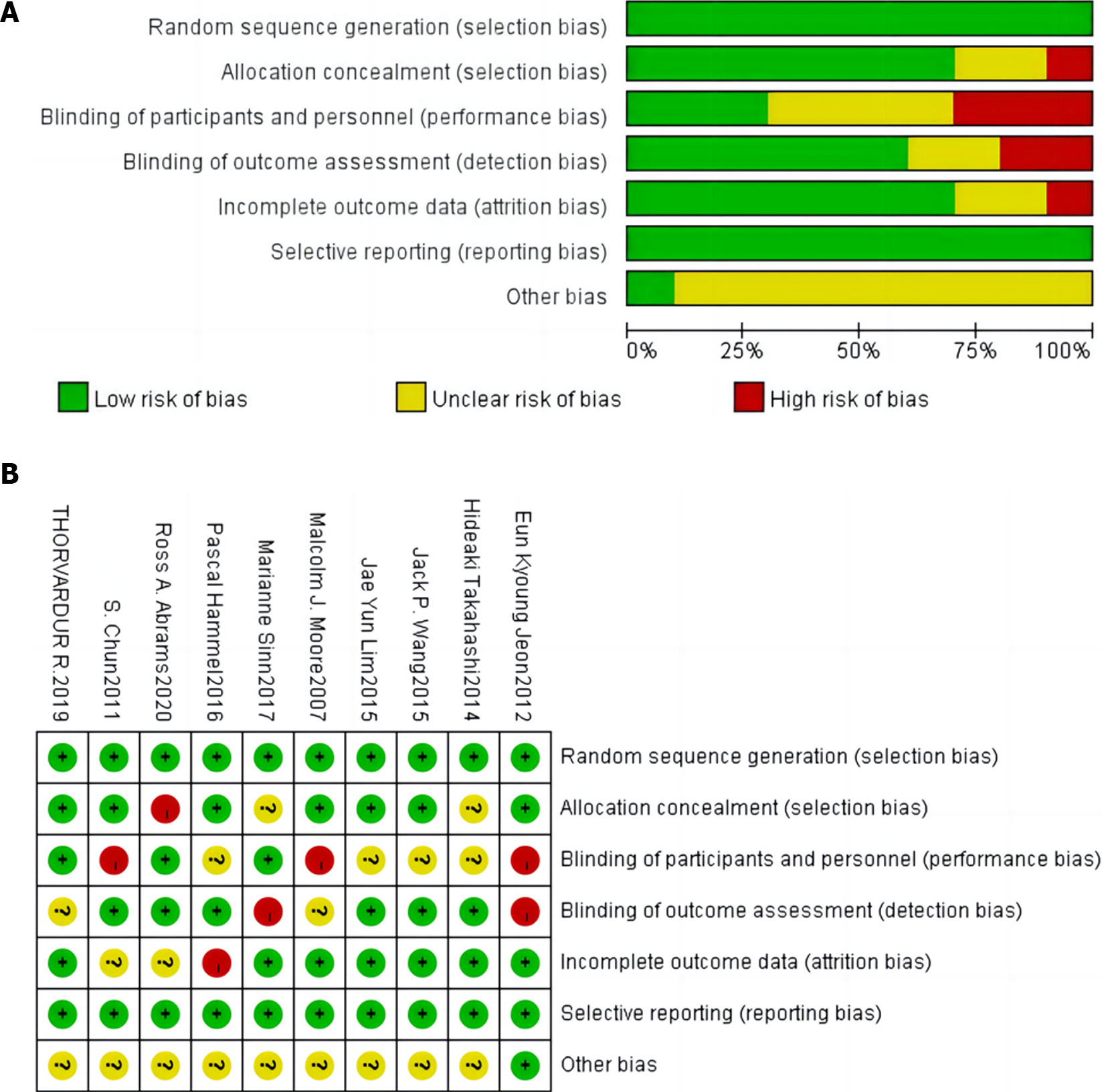
The Cochrane International Collaboration was used to evaluate the quality of the literature based on the sequence of randomization, allocation concealment, blinding implementation, presence of other biases, patient withdrawals, and loss to follow-up[8].
A meta-analysis of the efficacy and safety of erlotinib monotherapy in combination with chemotherapy for advanced pancreatic cancer was performed using RevMan 5.3 software. Dichotomous variables were evaluated as relative risks (RRs) or odds ratios (ORs) and 95%CIs for the effect analysis. For continuous variables, the mean, standard deviation, and 95%CI were used as effect statistics. Measurement data were compared using the chi-square test and combined with I2 values for the size of heterogeneity, where a P value of > 0.10 and an I2 value of < 50% indicated good statistical homogeneity. Meta-analysis was performed using a fixed-effects model, with a P value of ≤ 0.10 and an I2 value of ≥ 50% indicating the presence of statistical heterogeneity. Finally, a meta-analysis was performed using a random-effects model. If the screened literature data were not subjected to meta-analysis, a descriptive analysis was performed.
The literature search and screening processes are depicted in Figure 1. Initially, 806 papers were retrieved by searching the databases of related websites using relevant keywords. After Endnote processing, 178 duplicates were removed, leaving 628 papers for further consideration. Subsequently, the titles and abstracts of conference papers, reviews, case reports, non-randomized controlled trials, and duplicate publications were screened, resulting in the exclusion of an additional 393 articles. Finally, 235 articles were retained for further examination. After reading these articles, 225 were excluded, resulting in the inclusion of 10 articles in the final analysis.
Ten articles[9-18], all of which were randomized controlled trial studies, published between 2007 and 2020, with a total sample size of 2444 patients, were screened. The patients in the control group were treated with gemcitabine, capecitabine, or gemcitabine, whereas those in the study group were treated with gemcitabine or erlotinib. Characteristics of the included studies are listed in Table 1.
| Ref. | Vintages | Number of examples | M/F | Age/yr | Intervention | Observation indicators | ||||
| Control patients | Research group | Control patients | Research group | Control patients | Research group | Control patients | Research group | |||
| Takahashi et al[9] | 2014 | 202 | 102 | - | - | - | - | Gemcitabine (loanword) | Gemcitabine + erlotinib | 1, 2, 3 |
| Wang et al[10] | 2015 | 44 | 44 | 33/11 | 32/12 | - | - | Gemcitabine (loanword) | Gemcitabine + erlotinib | 1, 2, 3 |
| Moore et al[11] | 2007 | 284 | 285 | 162-122 | 136/149 | 64 (36.1–92.4) | 63.7 (37.9–84.4) | Gemcitabine + placebo | Gemcitabine + Erlotinib | 1, 2, 3 |
| Hammel et al[12] | 2016 | 223 | 219 | 117/106 | 111/108 | 64 (57–70) | 63 (58–71) | Gemcitabine (loanword) | Gemcitabine + erlotinib | 2, 3, 4, 6, 7 |
| Jeon et al[13] | 2012 | 19 | 34 | 14/5 | 20/14 | 58 (48–70) | 59.5 (35–73) | Gemcitabine + capecitabine | Gemcitabine + erlotinib | 1, 2, 3, 4, 5 ,6, 8 |
| Abrams et al[14] | 2020 | 163 | 167 | 88/75 | 96/63 | - | - | Gemcitabine (loanword) | Gemcitabine + erlotinib | 2, 3 |
| Sinn et al[15] | 2017 | 217 | 219 | 119/98 | 128/91 | 65 (24–82) | 63 (28–82) | Gemcitabine (loanword) | Gemcitabine + erlotinib | 2, 3 |
| Lim et al[16] | 2015 | 36 | 44 | 25/11 | 25/19 | 68 (41–84) | 63 (32–78) | Gemcitabine + capecitabine | Gemcitabine + erlotinib | 1, 2, 3, 4, 5, 6, 7, 8 |
| Halfdanarson et al[17] | 2019 | 46 | 46 | 29/17 | 31/15 | Median age: 60.5 | Median age: 62 | Gemcitabine (loanword) | Gemcitabine + erlotinib | 2, 3, 5, 6, 7 |
| Chun et al[18] | 2011 | 18 | 26 | - | - | Median age: 57.67 years | Median age: 56.58 years | Gemcitabine + capecitabine | Gemcitabine + erlotinib | 2, 3 |
The included studies exhibited a low risk of bias in terms of randomized sequence methods, blinding of literature results, and selective reporting. The overall quality of the studies was rated as B (Figure 2).
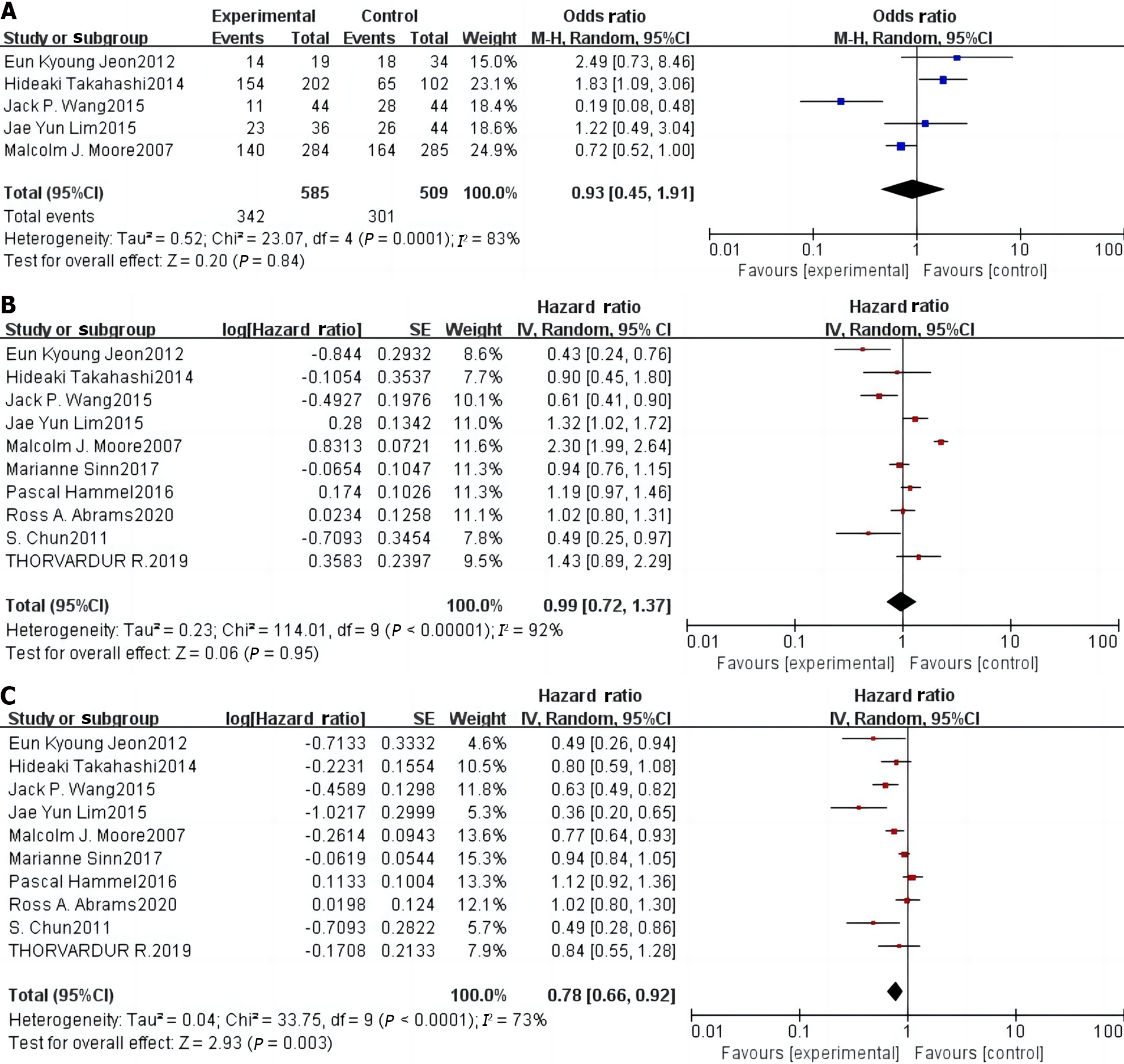
Five[9-11,13,16] of the 10 included articles reported DCR, including 509 patients in the control group and 585 patients in the study group. Statistical heterogeneity was observed among studies (P = 0.0001, I2 = 83%). Therefore, using a random-effects model, a meta-analysis showed that there was no significant difference in the DCR between the two groups of patients [OR = 0.93 (0.45–1.91), P = 0.84] (Figure 3A).
All 10 studies[9-18] reported the effect of erlotinib combination chemotherapy on OS in patients with pancreatic cancer, including 1258 patients in the control group and 1186 patients in the study group. Statistical heterogeneity was observed among the studies (P < 0.0001, I2 = 92%). Using a random-effects model, a meta-analysis showed that there was no significant difference in the comparison of OS between the two groups of patients [HR = 0.99 (0.72–1.37), P = 0.95] (Figure 3B).
All 10 studies[9-18] reported the effect of erlotinib combination chemotherapy on PFS in patients with pancreatic cancer, including 1258 patients in the control group and 1186 patients in the study group. Significant heterogeneity was observed among the studies (P < 0.0001, I2 = 73%). Using a random-effects model, the meta-analysis showed that the study group had significantly prolonged PFS compared with the control group (P < 0.001, Figure 3C).
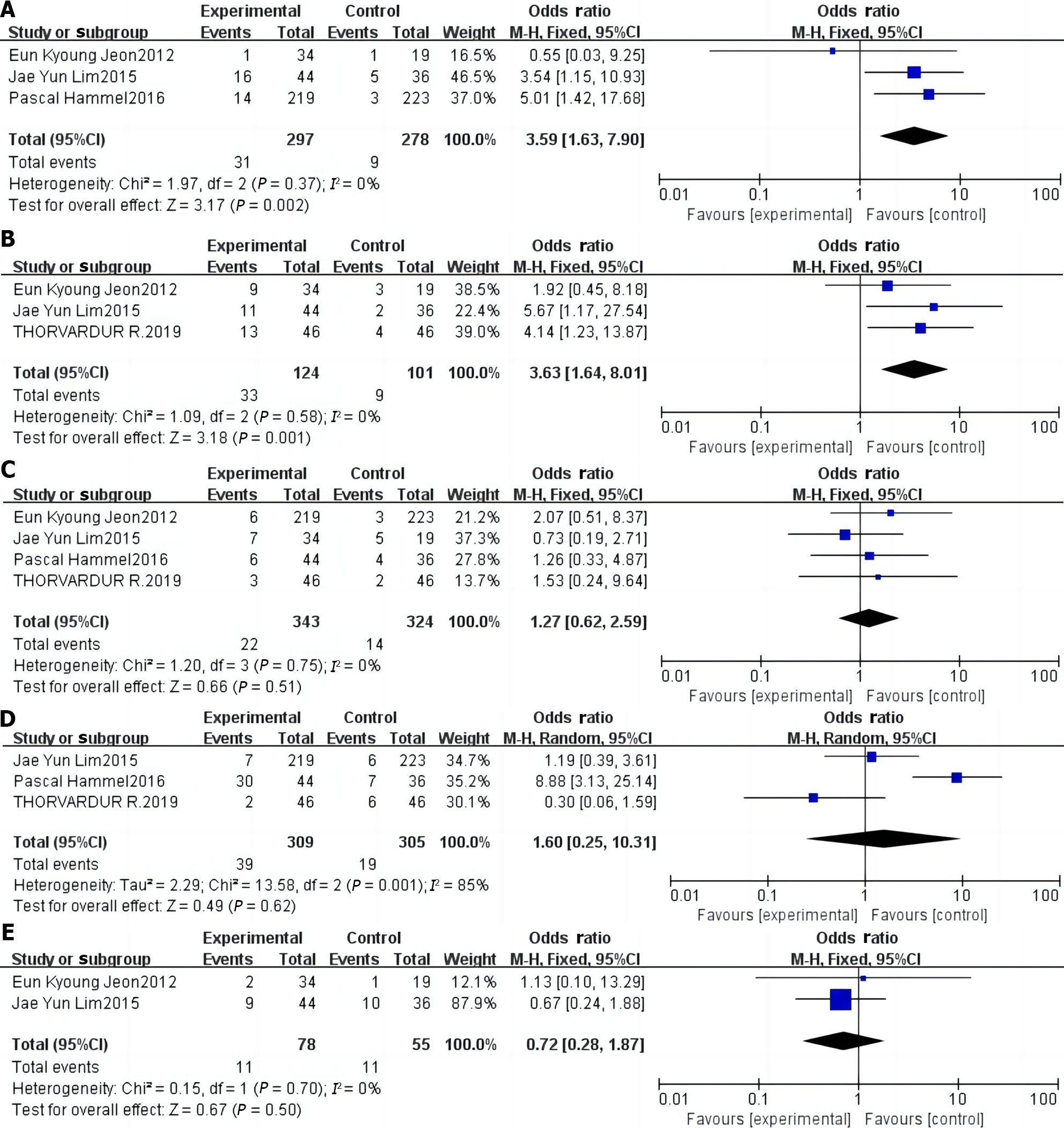
Results of the meta-analysis of diarrhea incidence: Three studies[12,13,16] reported the incidence of diarrhea, and no statistical heterogeneity was found (P > 0. 05, I2 = 0%). Hence, they were analyzed using a fixed-effects model. Results showed that the incidence of diarrhea in the study group was 3.59 times higher than that in the control group, and this difference was significant (P < 0. 001; Figure 4A).
Results of the meta-analysis of rash incidence: Three studies[13,16,17] reported the incidence of rash, but no statistical heterogeneity was found (P > 0.05, I2 = 0%). Hence, these studies were analyzed using a fixed-effects model. Results showed that the incidence of rash in the study group was 3.63 times higher than that in the control group, and this difference was significant (P < 0. 001; Figure 4B).
Results of the meta-analysis of vomiting incidence: Four studies[12,13,16,17] reported on the incidence of vomiting, among which no statistical heterogeneity was found (P > 0.05, I2 = 0%); therefore, they were analyzed using a fixed-effects model. Results showed that the incidence of vomiting in the study group was 1.27 times higher than that in the control group. However, this difference was not statistically significant (P > 0. 05; Figure 4C).
Meta-analysis results of regurgitation/anorexia: Three studies[12,16,17] reported the incidence of regurgitation/anorexia, among which a statistical heterogeneity was observed (P < 0.05, I2 = 85%); hence, these studies were analyzed using a random-effects model. Results showed that the incidence of regurgitation/anorexia was 1.60 times higher in the study group than that in the control group, but the difference was not significant (P > 0. 05, Figure 4D).
Results of the meta-analysis of infection incidence: Two studies[13,16] reported the incidence of infections, and statistical heterogeneity was observed (P > 0.05, I2 = 0%). Therefore, they were analyzed using a fixed-effects model. The results showed that the incidence of infection in the study group was 0.72 times higher than that in the control group; however, the difference was not statistically significant (P > 0. 05, Figure 4E).
Publication bias: Funnel plots were constructed based on the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for advanced pancreatic cancer, and showed good symmetry on both sides of the funnel plots with less publication bias (Figure 5).
Most patients with pancreatic cancer are diagnosed at an intermediate or advanced stage, with radical surgery considered feasible in only 15% to 20% of patients; however, the rates of recurrence and metastasis are high after surgery[19,20]. Therefore, patients with intermediate or advanced pancreatic cancer, as well as those who undergo radical surgery require further intervention. Novel pharmacological mechanisms of action for molecularly targeted drugs have garnered the attention of the medical community in recent years, leading to their rapid development. Erlotinib is a novel epidermal growth factor receptor tyrosine kinase inhibitor that can be orally administered. It effectively inhibits EGFR and blocks intracellular tyrosine kinase phosphorylation. The overexpression or mutation of EGFR in many tumors can lead to uncontrolled cell growth and malignancy. Erlotinib inhibits tumor cell proliferation, invasion, and metastasis by blocking EGFR, promoting apoptosis, enhancing sensitivity to chemotherapy, and improving the therapeutic effects. Erlotinib inhibits EGFR; hinders tumor cell proliferation, invasion, and metastasis; promotes tumor cell apoptosis; enhances sensitivity to chemotherapy; improves therapeutic effects; and prolongs the survival of patients with tumors[21,22]. However, whether erlotinib combined with chemotherapeutic drugs has significant advantages over chemotherapeutic drugs alone in the treatment of pancreatic cancer currently remains unclear. Hence, we conducted a meta-analysis of existing clinical studies to compare the efficacy and safety of erlotinib combined with chemotherapeutics and chemotherapeutic drugs alone for the treatment of pancreatic cancer.
Although molecularly targeted agents exhibit good clinical efficacy in the treatment of pancreatic cancer, the results of published clinical studies vary significantly. Moore et al[11] conducted a phase III clinical randomized controlled trial (RCT) and randomized 569 patients with locally progressive or distant metastatic pancreatic cancer into two groups in a 1:1 ratio. One group received erlotinib in combination with gemcitabine, while the other received gemcitabine alone. Results showed median OS rates of 6.24 months and 5.91 months in these groups (P = 0.038), respectively, with the combination therapy showing a better survival benefit than single-agent gemcitabine. Despite the positive results of this study, the survival benefit in the trial group was extremely limited and inconsistent with the conclusions of several subsequent prospective clinical RCTs. In a phase III clinical RCT, the LAP07 study, published in 2016, Hammel et al[12] enrolled 442 patients with locally progressive pancreatic cancer. Trial and control groups were treated with erlotinib in combination with gemcitabine or gemcitabine alone, respectively. Results showed that the median OS durations of the two groups were 11.9 and 13.6 months, respectively (P = 0.09), and the difference was not significant. Nevertheless, the results of this study showed that erlotinib combined with chemotherapy significantly prolonged the PFS of pancreatic cancer patients compared with chemotherapy alone (HR = 0.78, 95%CI: 0.66–0.92, P = 0.003). This finding indicates that erlotinib combined with chemotherapy can help patients stabilize their disease and prolong the progression-free time of pancreatic cancer. However, this combination treatment had no significant benefits on OS (HR = 0.99, 95%CI: 0.72–1.37, P = 0.95) or DCR (OR = 0.93, 95%CI: 0.45–0.91, P = 0.84). Second, in terms of safety, erlotinib combined with gemcitabine increased the risk of diarrhea (OR = 3.59, 95%CI: 1.63–7.90, P < 0.05) and rash (OR = 3.63, 95%CI: 1.64-8.01, P < 0.05) in patients with advanced pancreatic cancer compared with single-agent chemotherapy, which is not conducive to the stabilization of the disease and recovery. Although the risk of vomiting (OR = 1.27, 95%CI: 0.62-2.59, P = 0.51), regurgitation/anorexia (OR = 1.61, 95%CI: 0.25–10.31, P = 0.62), and infection (OR = 0.72, 95%CI: 0.28–1.87, P = 0.50) occurred in the two groups of patients, the difference was not significant. This observation indicates that erlotinib combination chemotherapy did not increase the risk of vomiting, regurgitation/anorexia, or infection in patients with advanced pancreatic cancer compared with single-agent chemotherapy. Moreover, the included studies exhibited good baseline comparability, and the meta-analysis was well-standardized. The symmetry on both sides of the funnel plots of DCR, OS, PFS, and adverse effects in patients after treatment indicated that publication bias had minimal impact on the study results and that the outcomes maintained a high level of confidence. Therefore, the results of this meta-analysis are reliable and stable, providing an evidence-based reference for the clinical treatment of advanced pancreatic cancer, and guiding further research.
This study had several limitations which should be mentioned. Firstly, the literature search was limited to articles published in English, only a few RCTs were included, and the sample sizes of the included studies were small. Secondly, the tumor stages of all included patients in the original studies differed or lacked relevant information. Furthermore, differences existed in the scoring criteria used to assess the physical status of included patients, with variations either between studies or due to the absence of data on the physical status of patients in the original studies. Further, the duration of follow-up between the included studies considerably varied. Finally, some of the studies do not provide relevant information; for example, only two papers mention infections in the results of adverse reactions. Given the above clinical and methodological heterogeneity and the small sample size used in the stratified analysis, the conclusions of this study need to be verified further in the clinical setting.
Systematic evaluation enables the analysis and assessment of existing clinical studies, provides guidance for clinical practice, and suggests directions for future research. Several suggestions can be made based on the limitations of this study. The process of reporting RCTs should be standardized, especially the description of methodological quality, the endpoints should be described in detail, the follow-up and recording of safety and long-term efficacy indicators should be strengthened, the economic indicators related to the report should be collected in order to make it is easier to evaluate the economic aspects of the interventions, and more high-quality, large-sample clinical studies should be conducted to validate the conclusions of this meta-analysis. Additional high-quality large-sample clinical studies are required to validate the findings of this meta-analysis. The use of erlotinib in combination with other chemotherapeutic agents to improve patient outcomes remains an important topic in clinical research.
Overall, the results of the present meta-analysis show that, in contrast to single-agent chemotherapy, the combination of erlotinib and chemotherapy prolongs PFS in patients with pancreatic cancer. Furthermore, it does not elevate the risk of vomiting, regurgitation/anorexia, or infections in patients with advanced pancreatic cancer. However, this combination does not enhance survival benefits or DCR and may increase the risk of diarrhea and rash.
Pancreatic cancer is one of the most common malignant gastrointestinal tumors worldwide, and its incidence and mortality rates are increasing each year. The current mainstay of pancreatic cancer treatment is surgical resection, and early surgery has been shown to increase the likelihood of successful resection, with postoperative 5-year survival rates ranging from 70% to 85%. However, pancreatic tumors tend to infiltrate surrounding tissues, and early detection and metastasis prevention pose significant challenges. Erlotinib is a tyrosine kinase inhibitor (EGFR antagonist) used as a molecularly targeted therapeutic drug that competitively binds to the catalytic site of the intracellular region of the tyrosine kinase receptor with adenosine triphosphate, inhibiting the phosphorylation reaction.
This study evaluated the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer, with the goal of offering valuable insights into the clinical management of this disease.
This study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer.
Literature searches were conducted in the PubMed, Embase, and Cochrane Library databases from inception to November 2023. A meta-analysis of the efficacy and safety of erlotinib monotherapy in combination with chemotherapy for advanced pancreatic cancer was performed using RevMan 5.3 software.
Compared with chemotherapeutic treatment, erlotinib combined with chemotherapy significantly prolonged the progression-free survival time of pancreatic cancer patients. Meanwhile, the overall survival and disease control rate were not significantly favorable. In terms of safety, the erlotinib and chemotherapy combination was associated with a significantly higher risk of diarrhea and rash compared with single-agent chemotherapy. Moreover, the risk of vomiting, regurgitation/anorexia, and infection were not significant in either group.
this study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer, with the goal of offering valuable insights into the clinical management of this disease.
Given the above clinical and methodological heterogeneity and the small sample size used in the stratified analysis, the conclusions of this study need to be verified further in the clinical setting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Komatsu Y, Japan S-Editor: Wang JL L-Editor: A P-Editor: Cai YX
| 1. | Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156:1951-1968.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1732] [Article Influence: 192.4] [Reference Citation Analysis (1)] |
| 3. | Roth MT, Berlin JD. Current Concepts in the Treatment of Resectable Pancreatic Cancer. Curr Oncol Rep. 2018;20:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lev S. Targeted therapy and drug resistance in triple-negative breast cancer: the EGFR axis. Biochem Soc Trans. 2020;48:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Wang K, Shen R, Meng T, Hu F, Yuan H. Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Leighl NB, Karaseva N, Nakagawa K, Cho BC, Gray JE, Hovey T, Walding A, Rydén A, Novello S. Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Greenhalgh J, Bagust A, Boland A, Dwan K, Beale S, Hockenhull J, Proudlove C, Dundar Y, Richardson M, Dickson R, Mullard A, Marshall E. Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE technology appraisals 162 and 175): a systematic review and economic evaluation. Health Technol Assess. 2015;19:1-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11036] [Article Influence: 689.8] [Reference Citation Analysis (0)] |
| 9. | Takahashi H, Kuwahara A, Okuyama H, Ohno I, Shimizu S, Mitsunaga S, Shinohara A, Kobayashi M, Okusaka T, Ikeda M. Efficacy and Possible Biomarker of Gemcitabine and Erlotinib for Advanced Pancreatic Cancer. Ann Oncol. 2014;25:v65. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Wang JP, Wu CY, Yeh YC, Shyr YM, Wu YY, Kuo CY, Hung YP, Chen MH, Lee WP, Luo JC, Chao Y, Li CP. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget. 2015;6:18162-18173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2776] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 12. | Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouché O, Shannon J, André T, Mineur L, Chibaudel B, Bonnetain F, Louvet C; LAP07 Trial Group. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 762] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 13. | Jeon EK, Won HS, Ko YH, Lee IS, Hong TH, You YK, Lee MA. Comparison of the efficacy and the toxicity between gemcitabine with capecitabine (GC) and gemcitabine with erlotinib (GE) in unresectable pancreatic cancer. J Cancer Res Clin Oncol. 2012;138:1625-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Abrams RA, Winter KA, Safran H, Goodman KA, Regine WF, Berger AC, Gillin MT, Philip PA, Lowy AM, Wu A, DiPetrillo TA, Corn BW, Seaward SA, Haddock MG, Song S, Jiang Y, Fisher BJ, Katz AW, Mehta S, Willett CG, Crane CH. Results of the NRG Oncology/RTOG 0848 Adjuvant Chemotherapy Question-Erlotinib+Gemcitabine for Resected Cancer of the Pancreatic Head: A Phase II Randomized Clinical Trial. Am J Clin Oncol. 2020;43:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 16. | Lim JY, Cho JH, Lee SJ, Lee DK, Yoon DS, Cho JY. Gemcitabine Combined with Capecitabine Compared to Gemcitabine with or without Erlotinib as First-Line Chemotherapy in Patients with Advanced Pancreatic Cancer. Cancer Res Treat. 2015;47:266-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Halfdanarson TR, Foster NR, Kim GP, Meyers JP, Smyrk TC, McCullough AE, Ames MM, Jaffe JP, Alberts SR. A Phase II Randomized Trial of Panitumumab, Erlotinib, and Gemcitabine Versus Erlotinib and Gemcitabine in Patients with Untreated, Metastatic Pancreatic Adenocarcinoma: North Central Cancer Treatment Group Trial N064B (Alliance). Oncologist. 2019;24:589-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Chun S, Lee M, Jeon E, An H, Hong T, You Y, Kim D, Lee I. Comparison of the efficacy of gemcitabine with capecitabine and gemcitabine with erlotinib combination chemotherapy in recurrent or advanced pancreatic cancer as first-line treatment: single-center experience. J Clin Oncol. 2011;29 (4_suppl):329. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 805] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 20. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 488] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 21. | Lee TG, Jeong EH, Min IJ, Kim SY, Kim HR, Kim CH. Altered expression of cellular proliferation, apoptosis and the cell cycle-related genes in lung cancer cells with acquired resistance to EGFR tyrosine kinase inhibitors. Oncol Lett. 2017;14:2191-2197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM, Harari PM. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65:3328-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |