Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.907
Peer-review started: October 7, 2023
First decision: December 8, 2023
Revised: January 7, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 27, 2024
Processing time: 166 Days and 19.2 Hours
Endoscopic ultrasound-guided biliary drainage using electrocautery-enhanced (ECE) delivery of lumen-apposing metal stent (LAMS) is gradually being re
To update the efficacy and safety of ECE-LAMS for treatment of biliary ob
We searched PubMed, EMBASE, and Scopus databases from the inception of the ECE technique to May 13, 2022. Primary outcome measure was pooled technical success rate, and secondary outcomes were pooled rates of clinical success, re
Fourteen eligible studies involving 620 participants were ultimately included. The pooled rate of technical success was 96.7%, and clinical success was 91.0%. Adverse events were reported in 17.5% of patients. Overall reintervention rate was 7.3%. Subgroup analyses showed results were generally consistent.
ECE-LAMS has favorable success with acceptable adverse events in relieving biliary obstruction when ERCP is impossible. The consistency of results across most subgroups suggested that this is a generalizable approach.
Core Tip: Electrocautery-enhanced lumen-apposing metal stent (ECE-LAMS) has been developed to perform biliary drainage. Because small, heterogeneous observational studies might be underpowered to identify the efficacy and safety of endoscopic ultrasound (EUS)-guided biliary drainage with ECE-LAMS, a comprehensive study is required. Therefore, we did a meta-analysis and systematic review to evaluate the efficacy and safety of EUS-guided biliary drainage with ECE-LAMS.
- Citation: Peng ZX, Chen FF, Tang W, Zeng X, Du HJ, Pi RX, Liu HM, Lu XX. Endoscopic-ultrasound-guided biliary drainage with placement of electrocautery-enhanced lumen-apposing metal stent for palliation of malignant biliary obstruction: Updated meta-analysis. World J Gastrointest Surg 2024; 16(3): 907-920
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/907.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.907
Endoscopic retrograde cholangiopancreatography (ERCP)-guided biliary drainage is the first-line therapeutic technique for management of patients with distal malignant biliary obstruction[1]. However, even though it is performed by ex
Previously, surgical bypass or percutaneous transhepatic biliary drainage (PTBD) was considered as an alternative for palliative treatment of malignant biliary obstruction when ERCP failed[6]. Although surgical biliary bypass has a high rate of technical success, this approach showed a high rate of major complications and mortality[7,8]. PTBD has had a high rate of complication and leads to poor health-related quality of life, and its adverse event rate is up to 60%[9-11].
Endoscopic ultrasound (EUS)-BD has emerged over the past decade as a safer and more effective treatment in patients who failed ERCP. When compared with PTBD, EUS-BD showed a higher rate of clinical success, lower rate of adverse events, and lower reintervention rate[12,13]. However, EUS-BD using conventional stent involves multiple procedural steps, which considerably increases the risk of adverse events[14].
A revolutionary stent delivery system, electrocautery-enhanced (ECE) technology, was launched in 2013 for the placement of lumen-apposing metal stents (LAMSs), which simplified the procedure and allowed one-step stent deployment[15]. EUS-BD with dedicated ECE-LAMS is gradually being recognized as a viable technique when ERCP is impossible. Nevertheless, ambiguous evidence for its efficacy and safety still hampers adoption of this approach in clinical practice. In an effort to help overcome such ambiguity, we conducted a descriptive meta-analysis in 2021 focusing on studies published before April 2020, which investigated the EUS-guided choledochoduodenostomy using ECE-LAMS for biliary drainage. However, only six studies involving 270 patients were considered eligible for analysis[16]. In
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline[17]. We searched PubMed, EMBASE, and Scopus databases from January 1, 2012 to May 13, 2022, since the introduction of ECE-LAMS in 2013[18]. The following keywords were used: “endoscopic ultrasound”, “EUS”, “lumen-apposing metal stent”, “LAMS”, “electrocautery-enhanced”, “electrocautery-enabled”, “biliary drainage”, “transmural drainage”, “biliary obstruction”, “bile duct obstruction”, and “obstructive jaundice”. Searches were re
Two authors (Peng ZX and Chen FF) independently filtered titles and abstracts of potentially eligible articles following duplicate removal. Case reports, reviews, meta-analyses, letters to the editor, conference abstracts, comments, study protocols, and articles focusing on pancreatic fluid collection drainage using ECE-LAMS were excluded. For overlapping studies, only the most recent publication was included. The inclusion criteria were: (1) EUS-BD involving ECE-LAMS; and (2) Technical success rate reported. We manually retrieved full text for further evaluation. Any discrepancy was discussed with a senior author (Lu XX) in consultation.
Two authors (Peng ZX and Wen T) independently extracted data from each study using a predetermined data extraction sheet, with differences resolved through discussion. Extracted data included: Study characteristics (i.e., first author, country, publication year, study design, and period of recruitment); study population (i.e., total analyzed number of pa
The primary outcome was pooled technical success rate. The secondary outcomes were pooled rates of clinical success, reintervention, and adverse events. In most of the included studies, technical success was defined as accurate deployment of ECE-LAMS between the common bile duct and duodenal wall. However, the definitions of clinical success were variable among the included studies (Supplementary Table 1). Unfortunately, we could not redefine clinical success using a uniform definition in the present study. We thus stipulated the definitions of the clinical success based on the individual studies. Based on the American Society for Gastrointestinal Endoscopy lexicon, the adverse events were classified into three categories: Intraprocedural, postprocedural (up to 14 d), and late (any time after 14 d)[19].
Two authors (Chen FF and Wen T) independently evaluated the quality of the included studies using the Methodological Index for Non-randomized Studies (MINORS)[20]. Any differences were resolved by discussion. Eight items for noncomparative studies were evaluated: Clearly study aim; inclusion of consecutive patients; prospective data collection; ap
We calculated the pooled rate of technical success, clinical success, reintervention, adverse events and its 95% confidence intervals (CIs). Considering that zero-event studies were also included in the present meta-analysis, we transformed the data using the Freeman-Tukey double-arcsine transformation to stabilize variance[21]. The random-effects model was used to calculate the pooled rate since the heterogeneity existed in each outcome[22]. The heterogeneity between studies was assessed using τ2 and I2 statistics[23]. The I2 values < 50%, 50%-75%, and > 75% were suggestive of low, moderate, and high heterogeneity, respectively[24]. We ran subgroup analysis comparing studies of different study quality (low vs high), region of origin (Europe vs others), year of publication (before 2021 vs 2021 onwards), cohort size (> 50 vs < 50), and study scale (single-center vs multicenter). Funnel plot asymmetry and Egger’s test were used to assess publication bias. Statistical analyses were performed using R software (version 4.1.3) and P < 0.05 was considered significant.
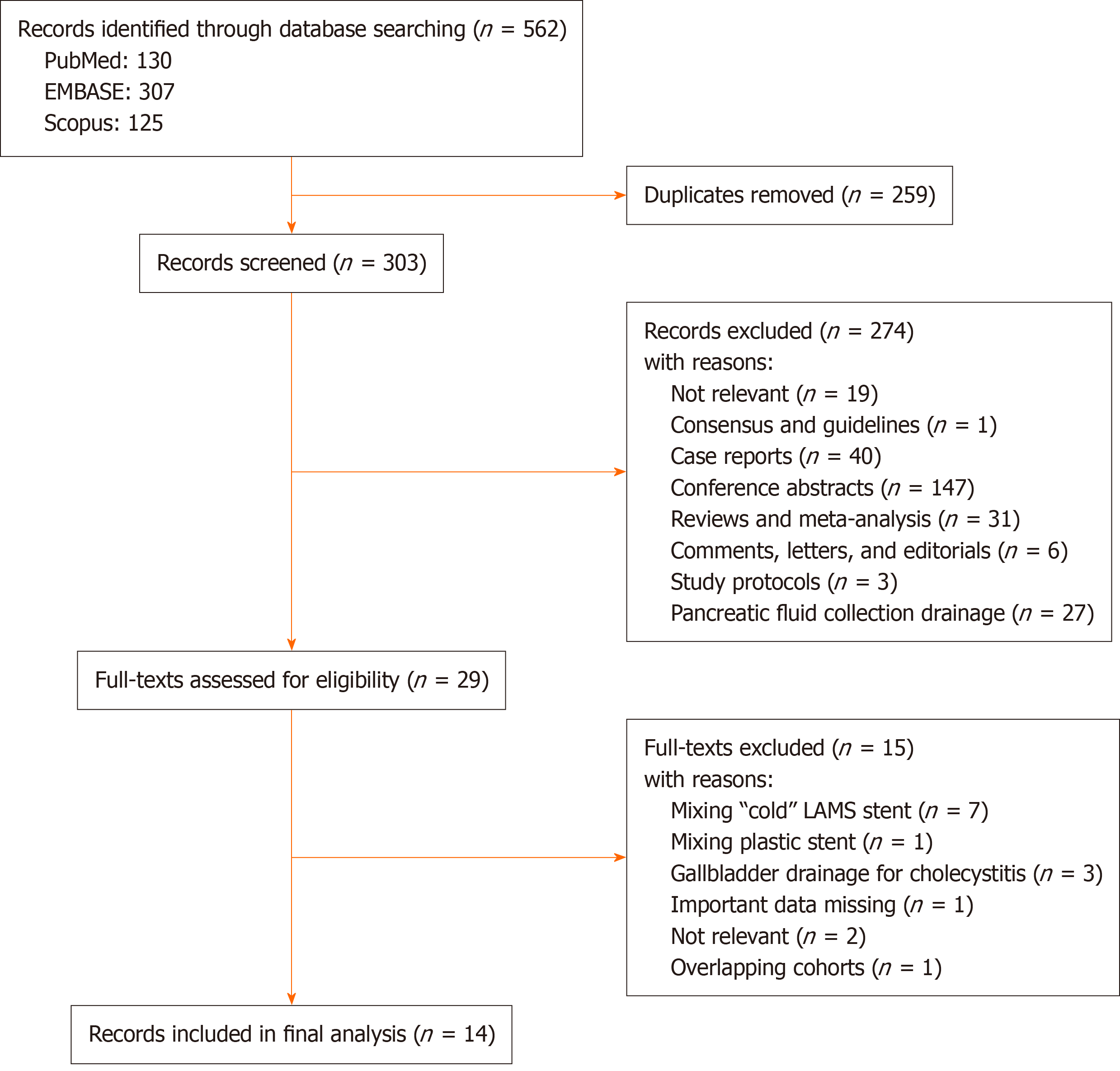
The initial search from PubMed, Scopus and EMBASE yielded 130, 125 and 307 articles, respectively. After removal of duplicates and initial screening of titles and abstracts, 29 studies were selected for full-text assessment. Finally, a total of 14 studies were included in the meta-analysis[25-38]. The flow diagram of the selection process is shown in Figure 1.
The present meta-analysis comprised 620 participants (319 male, 51.5%). Table 1 summarizes the characteristics of the 14 included studies from tertiary centers in Italy (n = 3)[27,30,35], France (n = 3)[28,31,37], United Kingdom (n = 2)[32,34], Belgium (n = 1)[38], Japan and China (n = 1)[33], South Korea (n = 1)[29], New Zealand (n = 1)[26], Spain (n = 1)[36] and United States (n = 1)[25]. Seven studies were multicenter, and seven were single center[25,28,31-34,36]. Two studies were prospective and 12 were retrospective cohort studies[33,34]. All studies were published from 2018 onwards.
| Ref. | Country | Setting, center involved (n) | Patient (n) | Age (yr) | Etiology (n) | Reason for failed ERCP (n) | Stent diameter | Follow-up (d) |
| Tsuchiya et al[33], 2018 | Japan, China | Prospective, multicenter (5) | 19 | mean 70.6 (SD, 13.9) | Pancreatic cancer (10), cholangiocarcinoma (2), metastatic renal cancer (2), ampullary cancer (1), metastatic colon cancer (1), sarcoma (1), duodenal cancer (1), metastatic gastric cancer (1) | Duodenal stenosis (4), cannulation failure (15) | 6 mm (10), 8 mm (9) | mean 205.0 (SD, 187.9) |
| Anderloni et al[30], 2019 | Italy | Retrospective, single-center | 46 | mean 73.1 (SD, 12.6) | Pancreatic cancer (40), duodenal cancer (3), ampullary cancer (2), cholangiocarcinoma (1) | Tumoral involvement of the papilla (19), duodenal stenosis (9), cannulation failure (12), ampulla obscured by prior stents (6) | 6 mm (21), 8 mm (19), 10 mm (6) | mean 114.4 (95%CI: 73.2-155.4) |
| El Chafic et al[25], 20191 | United States | Retrospective, multicenter (6) | 67 | mean 68.8 (SD, 11.8) | Peri-ampullary cancer (56), metastatic cancer (11) | Duodenal stenosis (29), tumoral involvement of the papilla (29), cannulation failure (9) | 10 mm (67) | median 119.0 (range, 28-567) |
| Jacques et al[28], 2019 | France | Retrospective, multicenter (10) | 52 | mean 78 (range, 61-92) | Pancreatic cancer (43), cholangiocarcinoma (2), degenerated IPMN (2), duodenal lymphoma (1), peritoneal carcinomatosis (1), stones (1), duodenal cancer (2) | Tumoral involvement of the papilla (10), duodenal stenosis (18), cannulation failure (14), ampulla obscured by prior stents (9), other (1) | 6 mm (43), 8 mm (7), 10 mm (2) | mean 157 |
| Chin et al[26], 20202 | New Zealand | Retrospective, single-center | 60 | median 76 (range, 52-90) | Pancreatic cancer (47), cholangiocarcinoma (3), metastatic cancer (6), duodenal cancer (2), lymphoma (2) | All patients underwent biliary drainage after ERCP failed, but details were not reported | 8 mm (43), 10 mm (17) | mean 237 |
| Jacques et al[31], 2020 | France | Retrospective, multicenter (8) | 70 | mean 75 (range, 61-92) | Pancreatic cancer (54), cholangiocarcinoma (3), ampullary cancer (4), duodenal cancer (2), other (7) | Tumoral involvement of the papilla (15), duodenal stenosis (31), cannulation failure (17), other (7) | 6 mm (60), 8 mm (9), 10 mm (1) | mean 153 |
| Di Mitri et al[35], 2022 | Italy | Retrospective, single-center | 36 | median 75 (IQR, 61-82.5) | Pancreatic cancer (30), duodenal cancer (1), ampullary cancer (2), cholangiocarcinoma (3) | Tumoral involvement of the papilla (9), duodenal stenosis (17), cannulation failure (5), ampulla obscured by prior stents (5) | 6 mm (1), 8 mm (24), 10 mm (9), 15 mm (2) | median 160.0 (IQR, 102-205) |
| Tarantino et al[27], 2021 | Italy | Retrospective, single-center | 21 | NA | Pancreatic cancer (14), duodenal cancer (1), ampullary cancer (3), cholangiocarcinoma (3) | Tumoral involvement of the papilla (12), duodenal stenosis (2), cannulation failure (5), other (2) | 6 mm (1), 8 mm (16), 10 mm (3), 15 mm (1) | mean 188.0 (range, 8-554) |
| Venkatachalapathy et al[34], 2021 | United Kingdom | Prospective, multicenter (3) | 20 | median 76 (range, 65-81) | Pancreatic cancer (12), metastatic cancer (8) | All patients underwent biliary drainage after ERCP failed, but details were not reported | 6 mm (3), 8 mm (17) | NA |
| Garcia-Sumalla et al[36], 20213 | Spain | Retrospective, multicenter (3) | 39 | mean 72.3 (SD, 12.5) | Pancreatic cancer (31), pancreatic neuroendocrine tumor (2), ampullary cancer (3), gastric cardia adenocarcinoma (2), metastatic cancer (1) | Duodenal stenosis (13), cannulation failure (26) | 6 mm (19), 8 mm (20) | mean 81.8 (SD, 82.8) |
| Hindryckx et al[38], 2021 | Belgium | Retrospective, single-center | 13 | NA | NA | NA | NA | NA |
| Yoo et al[29], 2021 | Korea | Retrospective, single-center | 7 | NA | Malignant distal biliary stricture | Tumoral involvement of the papilla (5), ampulla obscured by prior stents (2) | 8 mm (6), 10 mm (1) | NA |
| Ginestet et al[37], 2022 | France | Retrospective, single-center | 50 | mean 76.5 | Pancreatic cancer (43), cholangiocarcinoma (2), ampullary cancer (3), other (2) | Tumoral involvement of the papilla (10), duodenal stenosis (15), cannulation failure (13), other (12) | 6 mm (50) | NA |
| On et al[32], 20224 | United Kingdom, Ireland | Retrospective, multicenter (8) | 120 | median 73.0 (IQR, 17.0) | Pancreatic cancer (77), cholangiocarcinoma (8), ampullary cancer (9), duodenal cancer (14), metastatic cancer (12) | Tumoral involvement of the papilla (32), duodenal stenosis (53), cannulation failure (35) | 6 mm (46), 8 mm (68), 10 mm (5) | median 70.0 (range, 3-869) |
Pancreatic cancer was the most common disease, and the others included cholangiocarcinoma, metastatic cancer and ampullary cancer. One study did not report whether the failed ERCP attempt was prior to EUS-BD[38], and two studies did not report the reason for failed ERCP[26,34]. Duodenal stenosis was the most common cause for ERCP failure.
In most of the studies, the choice of the stent diameter was at the discretion of the operator. Thirteen studies including 607 cases reported the diameter of the stent in the EUS-BD procedure[25-37]: 6-, 8-, 10- and 15-mm stents were used in 254 (41.9%), 238 (39.3%), 111 (18.3%) and three (0.5%) cases, respectively. ECE-LAMS was manufactured by Boston Scientific in 13 studies[25-28,30-38] and Taewoong Medical Corporation in the other study[29]. Four studies with 109 cases received routine double stent placement with a pigtail plastic stent[25,26,32,36].
The results for study quality are shown in Supplementary Table 2. None of the studies reported the cohort size cal
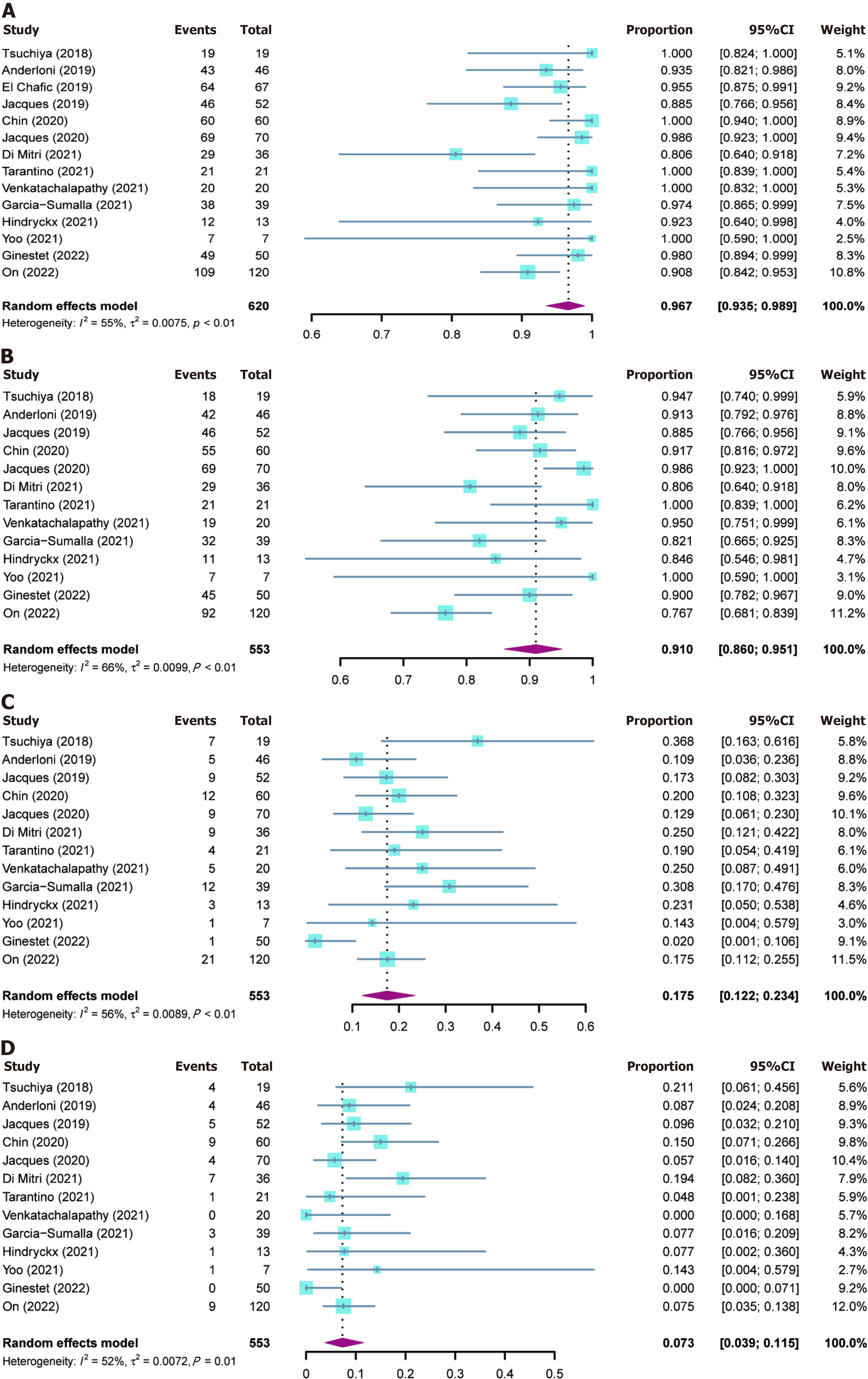
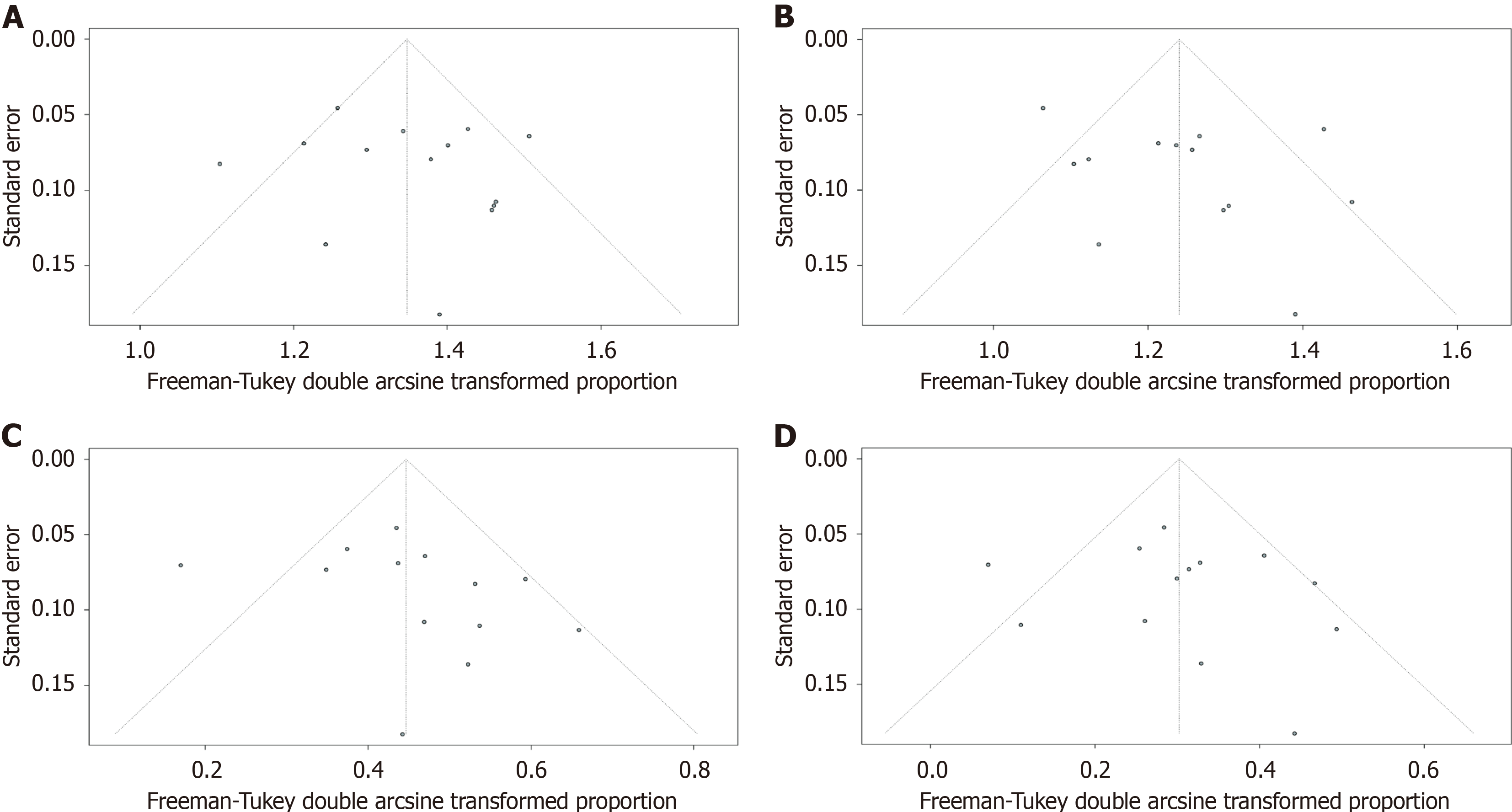
The outcomes of included studies are summarized in Table 2. The pooled rate of technical success was 96.7% (95%CI: 93.5%-98.9%; I2 = 55%; τ2 < 0.01, P < 0.01), which included 620 patients from 14 studies (Figure 2A). Moderate heterogeneity in this outcome was observed. We carried out subgroup analyses focusing on the region of origin, size of study, year of publication, and study scale (single center or multicenter) and found that results were generally consistent across subgroups (Table 3; Supplementary Figure 1). The funnel plot showed no asymmetry. No significant publication bias assessed by Egger’s test (P = 0.55) was observed (Figure 3A).
| Ref. | Total patient (n) | Technical success | Clinical success | Adverse events | Reintervention (n) | Intra-procedural adverse events (n) | Post-procedural adverse events (n) | Late adverse events (n) |
| Tsuchiya et al[33], 2018 | 19 | 19 | 18 | 7 | 4 | 0 | Cholangitis (2), fever (1) | Stent occlusion (1), stent kinking (1), tumor obstruction (1), stent migration (1) |
| Anderloni et al[30], 2019 | 46 | 43 | 42 | 5 | 4 | 0 | 0 | Bleeding (1), stent occlusion (3), stent migration (1) |
| El Chafic et al[25], 2019 | 67 | 64 | - | - | - | - | - | - |
| Jacques et al[28], 2019 | 52 | 46 | 46 | 9 | 5 | 0 | Cholangitis (1), bleeding (1) | Sump syndrome (2), tumor obstruction (4), stent migration (1) |
| Chin et al[26], 2020 | 60 | 60 | 55 | 12 | 9 | NA | NA | NA |
| Jacques et al[31], 2020 | 70 | 69 | 69 | 9 | 4 | 0 | Bleeding (1), primary dysfunction of stent (1), cholangitis (1) | Tumor obstruction (4), stent migration (1), bacteremia (1) |
| Di Mitri et al[35], 2022 | 36 | 29 | 29 | 9 | 7 | First flange malemployment (2), bleeding (5) | 0 | Tumor obstruction (2) |
| Tarantino et al[27], 2021 | 21 | 21 | 21 | 4 | 1 | 0 | 0 | Stent occlusion (2), tumor obstruction (1), stent migration (1) |
| Venkatachalapathy et al[34], 2021 | 20 | 20 | 19 | 5 | 0 | 0 | Pain (3), cholangitis (1), stent migration (1), | |
| Garcia-Sumalla et al[36], 2021 | 39 | 38 | 32 | 12 | 3 | 0 | Duodenal perforation (1), cholangitis (2), stent migration (1), bleeding (1) | Stent occlusion (7) |
| Hindryckx et al[38], 2021 | 13 | 12 | 11 | 3 | 1 | Duodenal perforation (1) | Cholangitis (1), cholecystitis (1) | - |
| Yoo et al[29], 2021 | 7 | 7 | 7 | 1 | 1 | 0 | Stent occlusion (1) | - |
| Ginestet et al[37], 2022 | 50 | 49 | 45 | 1 | 0 | Bleeding (1) | 0 | 0 |
| On et al[32], 2022 | 120 | 109 | 92 | 21 | 9 | Pneumoperitoneum (2), retroperitoneal air (1), bile leak (2), duodenal perforation (2) | Cholangitis (3), stent occlusion (4) | Cholangitis (2), stent occlusion (3), Stent migration (2) |
| Subgroup | No. of studies | No. of patients | Pooled rates as % (95 %CI) | I2 as % | τ2 |
| Technical success rate | |||||
| Low-quality | 5 | 173 | 94.7 (86.7-99.5) | 53.0 | 0.01 |
| High-quality | 9 | 447 | 97.3 (93.8-99.5) | 59.0 | < 0.01 |
| European | 10 | 467 | 95.1 (91.1-98.1) | 55.0 | < 0.01 |
| Outside the European | 4 | 153 | 99.7 (96.1-100.0) | 16.0 | < 0.01 |
| Cohort size (≥ 50 patients) | 6 | 419 | 96.1 (91.8-99.0) | 69.0 | < 0.01 |
| Cohort size (< 50 patients) | 8 | 201 | 96.8 (91.5-99.9) | 45.0 | < 0.01 |
| Single-center | 7 | 233 | 97.0 (90.7-100.0) | 66.0 | 0.01 |
| Multi-center | 7 | 387 | 96.0 (92.3-98.7) | 46.0 | < 0.01 |
| Published before 2021 | 6 | 324 | 97.0 (92.8-99.6) | 60.0 | < 0.01 |
| Published from 2021 onwards | 8 | 306 | 96.1 (90.9-99.4) | 51.0 | < 0.01 |
| Clinical success rate | |||||
| Low-quality | 4 | 106 | 88.4 (80.8-94.6) | 0 | < 0.01 |
| High-quality | 9 | 447 | 91.6 (85.6-96.3) | 75.0 | 0.01 |
| European | 10 | 467 | 89.9 (83.7-94.9) | 73.0 | 0.01 |
| Outside the European | 3 | 86 | 94.6 (87.7-99.1) | 0 | 0 |
| Cohort size (≥ 50 patients) | 5 | 352 | 90.0 (81.4-96.2) | 84.0 | 0.01 |
| Cohort size (< 50 patients) | 8 | 201 | 91.5 (85.1-96.5) | 36.0 | < 0.01 |
| Single-center | 7 | 233 | 91.9 (86.9-95.9) | 29.0 | < 0.01 |
| Multi-center | 6 | 320 | 90.0 (81.2-96.4) | 81.0 | 0.02 |
| Published before 2021 | 5 | 247 | 93.7 (88.6-97.5) | 41.0 | < 0.01 |
| Published from 2021 onwards | 8 | 306 | 88.7 (81.0-94.7) | 60.0 | 0.01 |
| Adverse events rate | |||||
| Low-quality | 4 | 106 | 13.1 (1.6-30.6) | 77.0 | 0.03 |
| High-quality | 9 | 447 | 18.5 (14.4-22.9) | 28.0 | < 0.01 |
| European | 10 | 467 | 16.4 (10.7-22.9) | 60.0 | < 0.01 |
| Outside the European | 3 | 86 | 22.9 (11.9-35.8) | 11.0 | < 0.01 |
| Cohort size (≥ 50 patients) | 5 | 352 | 13.3 (7.1-21.0) | 69.0 | < 0.01 |
| Cohort size (< 50 patients) | 8 | 201 | 22.3 (15.4-30.0) | 13.0 | < 0.01 |
| Single-center | 7 | 233 | 14.1 (6.7-23.3) | 63.0 | 0.01 |
| Multi-center | 6 | 320 | 20.4 (14.2-27.3) | 43.0 | < 0.01 |
| Published before 2021 | 5 | 247 | 16.7 (11.6-22.4) | 39.0 | < 0.01 |
| Published from 2021 onwards | 8 | 306 | 17.8 (9.8-27.3) | 66.0 | 0.01 |
| Reintervention rate | |||||
| Low-quality | 4 | 106 | 6.8 (0-22.6) | 80.0 | 0.03 |
| High-quality | 9 | 447 | 7.9 (5.4-10.8) | 17.0 | < 0.01 |
| European | 10 | 467 | 6.0 (2.8-10.0) | 48.0 | < 0.01 |
| Outside the European | 3 | 86 | 15.1 (7.5-24.2) | 0 | 0 |
| Cohort size (≥ 50 patients) | 5 | 352 | 6.5 (2.1-12.7) | 70.0 | 0.01 |
| Cohort size (< 50 patients) | 8 | 201 | 8.8 (4.0-14.7) | 28.0 | < 0.01 |
| Single-center | 7 | 233 | 7.8 (2.1-15.8) | 67.0 | 0.01 |
| Multi-center | 6 | 320 | 6.9 (4.2-10.2) | 24.0 | < 0.01 |
| Published before 2021 | 5 | 247 | 10.1 (6.1-14.9) | 22.0 | < 0.01 |
| Published from 2021 onwards | 8 | 306 | 5.2 (1.0-11.3) | 59.0 | 0.01 |
Thirteen studies reported clinical success rates[26-38]. The remaining study was excluded due to a high rate of loss to follow-up (35.8%)[25]. The pooled clinical success rate was 91.0% (95%CI: 86.0%-95.1%; I2 = 66%; τ2 < 0.01, P < 0.01) (Figure 2B). Similarly, we did encounter moderate heterogeneity in this outcome and we were unable to explain the heterogeneity by subgroup analysis (Table 3; Supplementary Figure 2). Furthermore, no significant publication bias was observed (Figure 3B).
As mentioned above, one study was excluded from the analysis of adverse event rates[25]. The pooled rate of adverse events was 17.5% (95%CI: 12.2%-23.4%; I2 = 56%; τ2 < 0.01, P < 0.01; Egger’s test for bias, P = 0.27) (Figures 2C and 3C). The most common intraprocedural, postprocedural, and late adverse events were bleeding, cholangitis, and stent oc
The pooled reintervention rate was 7.3% (95%CI: 3.9%-11.5%) (Figure 2D), which included 553 patients from 13 studies (I2 = 52%; τ2 < 0.01, P < 0.01; Egger’s test for bias, P = 0.59) (Figure 3D). Results of subgroup analyses on the rates of adverse events and reintervention are given in Table 3 and Supplementary Figures 3 and 4.
This meta-analysis pooled data from 14 eligible studies involving 620 patients with biliary obstruction, and we analyzed the pooled rates of technical success, clinical success, adverse events, and reintervention for ECE-LAMS for palliation of biliary obstruction. This updated meta-analysis had more than twice as many patients as previous analyses.
EUS-BD with dedicated ECE-LAMS is a viable approach after ERCP failure and has potential for reproducible and generalized clinical application. For instance, a recent meta-analysis of five studies with 201 patients revealed a 93.8% technical and 88.8% clinical success rate for EUS-BD using ECE-LAMS for biliary drainage[39]. Our former meta-analysis (6 studies, 270 participants) also published in 2020 demonstrated comparable rates[16]. Furthermore, this updated meta-analysis consistently indicated similar pooled rates of technical success (96.7%) and clinical efficacy (91.0%). Although ECE-LAMS is being used in increasingly more medical centers worldwide, its reproducibility seemed not to be com
ECE-LAMS is also a safe technique. The low incidence of adverse events makes the ECE delivery system an ideal device for LAMS, with a pooled rate of only 17.5% in this meta-analysis, which is comparable to results reported in other studies (13.6%-17.1%)[16,40,41]. To reduce the adverse event rate of EUS-BD, accumulation of endoscopic experience is usually advocated since the procedures are highly operator dependent[42-44]. However, the adverse event rate of the ECE-LAMS procedures performed by nonexperts was similar to that of experts[32]. The similar satisfactory success rate for expert and nonexpert operators[28] suggests that the ECE-LAMS technique is reproducible and generalizable.
Many attempts have been made to reduce the incidence of adverse events in ECE-LAMS placement. Anchoring a coaxial double-pigtail plastic stent (DPS) through ECE-LAMS may prevent stent migration. Several recent studies have found that placement of a DPS within the ECE-LAMS is superior to ECE-LAMS alone in reducing the reintervention rate[25,26,32]. In this meta-analysis, the pooled reintervention rate was 7.3% (95%CI: 3.9%-11.5%). However, a retrospective study of 41 participants from three Spanish tertiary centers demonstrated no significant difference in reintervention rates between DPS within ECE-LAMS and ECE-LAMS alone (9.1% vs 5.8%, P = 0.99)[36]. Thus, the potential of positioning a DPS through ECE-LAMS for reducing the need for reintervention should be evaluated further in large-scale prospective studies, such as the ongoing BAMPI trial initiated in November 2020[45].
ECE-LAMS sizes ranging from 6 mm to 8 mm in diameter were used in about 80% of cases in the present meta-analysis. It should be noted that small stent diameter is more likely to be associated with increased rates of specific adverse events (e.g., stent lumen occlusion because of sludge accumulation and food waste)[32,33]. These adverse events relating to stent diameter could be prevented by the use of a larger-bore stent but may at the expense of technical success[32]. Hence, choosing an optimal stent size necessitates considering the balance between the reduction in risk of adverse events and superior technical success.
EUS-BD using ECE-LAMS has been previously considered as a palliative treatment, yet now this is being recognized as a bridge to surgery approach. Several recent studies have provided evidence that ECE-LAMS as a bridge to elective surgery (e.g., pancreatoduodenectomy, PD) in malignant biliary obstruction is feasible and safe[32,46]. Considering influences of preoperative BD on the eventual surgical removal of the obstruction, we suggest that: (1) ECE-LAMS deployment from the duodenal bulb to the common bile duct is preferable to stent placement between the stomach and left intrahepatic bile duct, given the extent of PD; (2) Insofar as possible postprocedural cholangitis should be avoided, because it is an independent risk factor for mortality following PD[47]; and (3) Treatment strategy involving multidisciplinary consultation is recommended.
The present study had several limitations. The lack of a uniform definition for clinical success is a potential contributor to study heterogeneity although the heterogeneity between the included studies was moderate in this meta-analysis. We were not able to identify sources of heterogeneity by conducting subgroup analyses because of the original study design
The ECE-LAMS is an effective and safe approach for patients who bear biliary obstruction when ERCP is impossible. This approach could be generalizable and extensible to the practitioners in this field. Therefore, this should be acknowledged as a standard core component of management of malignant biliary obstruction. Large and prospective observational studies are needed to further investigate and confirm these findings. Further investigations regarding the use of ECE-LAMS as the first-line intervention for biliary drainage or the “bridge to surgery” approach should be performed to invigorate more practical applications with hopefully favorable outcomes.
Endoscopic-ultrasound-guided biliary drainage (EUS-BD) with electrocautery-enhanced lumen-apposing metal stent (ECE-LAMS) has recently been reported as an alternative treatment approach for malignant biliary obstruction after endoscopic retrograde cholangiopancreatography (ERCP) failure.
In 2021, we conducted a meta-analysis focusing on the EUS-guided choledochoduodenostomy with ECE-LAMS for BD. However, only six studies involving 270 patients were included in the analyses. Intriguingly, studies regarding EUS-BD using ECE-LAMS have been widely performed in the last 2 years. Hence, an updated meta-analysis was warranted to determine further the feasibility and safety of ECE-LAMS for palliation of biliary obstruction when ERCP is impossible.
To evaluate the efficacy and safety of EUS-BD with ECE-LAMS for treatment of biliary obstruction after ERCP failure.
We searched PubMed, EMBASE and Scopus databases from January 1, 2012 to May 13, 2022. The following search keywords were used: “endoscopic ultrasound”, “EUS”, “lumen-apposing metal stent”, “LAMS”, “electrocautery-enhanced”, “electrocautery-enabled”, “biliary drainage”, “transmural drainage”, “biliary obstruction”, “bile duct obstruction”, and “obstructive jaundice”. The primary outcome of our study was pooled technical success rate. The secondary outcomes were pooled rates of clinical success, reintervention, and adverse events. We ran subgroup analysis comparing studies of different study quality (low vs high), region of origin (Europe vs others), year of publication (before 2021 vs 2021 onwards), cohort size (> 50 vs < 50), and study scale (single center vs multicenter). Funnel plot asymmetry and Egger’s test were used to assess the publication bias. Meta-analysis was performed using a random-effects model following Freeman-Tukey double-arcsine transformation in R software.
Fourteen studies involving 620 participants were included in the analysis. The pooled rate of technical success was 96.7%, and clinical success was 91.0%. Adverse events were reported in 17.5% of patients. Overall reintervention rate was 7.3%. Subgroup analyses showed results were generally consistent.
EUS-BD using ECE-LAMS is an effective and safe approach for patients with biliary obstruction when ERCP is impossible. This approach could be generalizable to practitioners in this field.
The present meta-analysis adopted strict inclusion and exclusion criteria to ensure appropriate methodological quality to evaluate the efficacy and safety of EUS-BD using ECE-LAMS. Large and prospective observational studies are needed to further investigate and confirm our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moyana T, Canada S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, Costamagna G; European Society of Gastrointestinal Endoscopy. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Ekkelenkamp VE, de Man RA, Ter Borg F, Borg PC, Bruno MJ, Groenen MJ, Hansen BE, van Tilburg AJ, Rauws EA, Koch AD. Prospective evaluation of ERCP performance: results of a nationwide quality registry. Endoscopy. 2015;47:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Salerno R, Davies SEC, Mezzina N, Ardizzone S. Comprehensive review on EUS-guided biliary drainage. World J Gastrointest Endosc. 2019;11:354-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, Takasawa O, Koshita S, Kanno Y. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009;21:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Stark A, Hines OJ. Endoscopic and operative palliation strategies for pancreatic ductal adenocarcinoma. Semin Oncol. 2015;42:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Artifon EL, Sakai P, Cunha JE, Dupont A, Filho FM, Hondo FY, Ishioka S, Raju GS. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol. 2006;101:2031-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Bliss LA, Eskander MF, Kent TS, Watkins AA, de Geus SW, Storino A, Ng SC, Callery MP, Moser AJ, Tseng JF. Early surgical bypass versus endoscopic stent placement in pancreatic cancer. HPB (Oxford). 2016;18:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Rees J, Mytton J, Evison F, Mangat KS, Patel P, Trudgill N. The outcomes of biliary drainage by percutaneous transhepatic cholangiography for the palliation of malignant biliary obstruction in England between 2001 and 2014: a retrospective cohort study. BMJ Open. 2020;10:e033576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Nam K, Kim DU, Lee TH, Iwashita T, Nakai Y, Bolkhir A, Castro LA, Vazquez-Sequeiros E, de la Serna C, Perez-Miranda M, Lee JG, Lee SS, Seo DW, Lee SK, Kim MH, Park DH. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol. 2015;49:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (2)] |
| 13. | Baniya R, Upadhaya S, Madala S, Subedi SC, Shaik Mohammed T, Bachuwa G. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography: a meta-analysis. Clin Exp Gastroenterol. 2017;10:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Anderloni A, Troncone E, Fugazza A, Cappello A, Del Vecchio Blanco G, Monteleone G, Repici A. Lumen-apposing metal stents for malignant biliary obstruction: Is this the ultimate horizon of our experience? World J Gastroenterol. 2019;25:3857-3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 15. | ASGE technology committee; Law RJ, Chandrasekhara V, Bhatt A, Bucobo JC, Copland AP, Krishnan K, Kumta NA, Pannala R, Parsi MA, Rahimi EF, Saumoy M, Trikudanathan G, Trindade AJ, Yang J, Lichtenstein DR. Lumen-apposing metal stents (with videos). Gastrointest Endosc. 2021;94:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 16. | Peng ZX, Li S, Tang YL, Wei WJ, Pi RX, Liang XC, Wan YF, Liu HM. Efficacy and Safety of EUS-Guided Choledochoduodenostomy Using Electrocautery-Enhanced Lumen-Apposing Metal Stents (ECE-LAMS) in the Treatment of Biliary Obstruction: A Systematic Review and Meta-Analysis. Can J Gastroenterol. 2021;2021. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7645] [Article Influence: 477.8] [Reference Citation Analysis (1)] |
| 18. | Kunda R, Pérez-Miranda M, Will U, Ullrich S, Brenke D, Dollhopf M, Meier M, Larghi A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1851] [Article Influence: 123.4] [Reference Citation Analysis (1)] |
| 20. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5641] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 21. | Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1500] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 22. | DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1927] [Article Influence: 192.7] [Reference Citation Analysis (0)] |
| 23. | Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 1780] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 24. | Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 302] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 25. | El Chafic AH, Shah JN, Hamerski C, Binmoeller KF, Irani S, James TW, Baron TH, Nieto J, Romero RV, Evans JA, Kahaleh M. EUS-Guided Choledochoduodenostomy for Distal Malignant Biliary Obstruction Using Electrocautery-Enhanced Lumen-Apposing Metal Stents: First US, Multicenter Experience. Dig Dis Sci. 2019;64:3321-3327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Chin JY, Seleq S, Weilert F. Safety and outcomes of endoscopic ultrasound-guided drainage for malignant biliary obstruction using cautery-enabled lumen-apposing metal stent. Endosc Int Open. 2020;8:E1633-E1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 27. | Tarantino I, Peralta M, Ligresti D, Amata M, Barresi L, Cipolletta F, Antonio G, Traina M. Endoscopic ultrasound-guided biliary drainage of malignant stenosis, not treatable with endoscopic retrograde cholangiopancreatography: a single-center, prospective observational study. Endosc Int Open. 2021;9:E110-E115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Jacques J, Privat J, Pinard F, Fumex F, Valats JC, Chaoui A, Cholet F, Godard B, Grandval P, Legros R, Kerever S, Napoleon B. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: a retrospective analysis. Endoscopy. 2019;51:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Yoo HW, Moon JH, Jo SJ, Lee YN, Park JK, Lee TH, Cha SW, Cho YD, Park SH, Park SI, Jeong S, Lee DH. A novel electrocautery-enhanced delivery system for one-step endoscopic ultrasound-guided drainage of the gallbladder and bile duct using a lumen-apposing metal stent: a feasibility study. Endoscopy. 2021;53:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Anderloni A, Fugazza A, Troncone E, Auriemma F, Carrara S, Semeraro R, Maselli R, Di Leo M, D'Amico F, Sethi A, Repici A. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Jacques J, Privat J, Pinard F, Fumex F, Chaput U, Valats JC, Cholet F, Jezequel J, Grandval P, Legros R, Lepetit H, Albouys J, Napoleon B. EUS-guided choledochoduodenostomy by use of electrocautery-enhanced lumen-apposing metal stents: a French multicenter study after implementation of the technique (with video). Gastrointest Endosc. 2020;92:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | On W, Paranandi B, Smith AM, Venkatachalapathy SV, James MW, Aithal GP, Varbobitis I, Cheriyan D, McDonald C, Leeds JS, Nayar MK, Oppong KW, Geraghty J, Devlin J, Ahmed W, Scott R, Wong T, Huggett MT. EUS-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing metal stents in patients with malignant distal biliary obstruction: multicenter collaboration from the United Kingdom and Ireland. Gastrointest Endosc. 2022;95:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 33. | Tsuchiya T, Teoh AYB, Itoi T, Yamao K, Hara K, Nakai Y, Isayama H, Kitano M. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: a prospective multicenter study. Gastrointest Endosc. 2018;87:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 34. | Venkatachalapathy SV, James MW, Huggett MT, Paranandi B, Pereira SP, Johnson G, Aravinthan AD, Aithal GP. Utility of palliative EUS-guided biliary drainage using lumen-apposing metal stents: a prospective multicenter feasibility study (with video). Gastrointest Endosc. 2021;94:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Di Mitri R, Amata M, Mocciaro F, Conte E, Bonaccorso A, Scrivo B, Scimeca D. EUS-guided biliary drainage with LAMS for distal malignant biliary obstruction when ERCP fails: single-center retrospective study and maldeployment management. Surg Endosc. 2022;36:4553-4569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Garcia-Sumalla A, Loras C, Guarner-Argente C, Velasquez-Rodriguez JG, Andujar X, Salord S, Busquets J, Tebe C, Laquente B, Gornals JB. Is a coaxial plastic stent within a lumen-apposing metal stent useful for the management of distal malignant biliary obstruction? Surg Endosc. 2021;35:4873-4881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Ginestet C, Sanglier F, Hummel V, Rouchaud A, Legros R, Lepetit H, Dahan M, Carrier P, Loustaud-Ratti V, Sautereau D, Albouys J, Jacques J, Geyl S. EUS-guided biliary drainage with electrocautery-enhanced lumen-apposing metal stent placement should replace PTBD after ERCP failure in patients with distal tumoral biliary obstruction: a large real-life study. Surg Endosc. 2022;36:3365-3373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Hindryckx P, Degroote H. Lumen-apposing metal stents for approved and off-label indications: a single-centre experience. Surg Endosc. 2021;35:6013-6020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Krishnamoorthi R, Dasari CS, Thoguluva Chandrasekar V, Priyan H, Jayaraj M, Law J, Larsen M, Kozarek R, Ross A, Irani S. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS): a systematic review and meta-analysis. Surg Endosc. 2020;34:2866-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Amato A, Sinagra E, Celsa C, Enea M, Buda A, Vieceli F, Scaramella L, Belletrutti P, Fugazza A, Cammà C, Radaelli F, Repici A, Anderloni A. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy: a systematic review and meta-analysis. Endoscopy. 2021;53:1037-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Mohan BP, Shakhatreh M, Garg R, Ponnada S, Navaneethan U, Adler DG. Efficacy and Safety of Endoscopic Ultrasound-guided Choledochoduodenostomy: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2019;53:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Tyberg A, Mishra A, Cheung M, Kedia P, Gaidhane M, Craig C, Tarnasky PR, Ardengh JC, Kahaleh M. Learning curve for EUS-guided biliary drainage: What have we learned? Endosc Ultrasound. 2020;9:392-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, Abadia MA, Pérez-Millán A, González-Huix F, Gornals J, Iglesias-Garcia J, De la Serna C, Aparicio JR, Subtil JC, Alvarez A, de la Morena F, García-Cano J, Casi MA, Lancho A, Barturen A, Rodríguez-Gómez SJ, Repiso A, Juzgado D, Igea F, Fernandez-Urien I, González-Martin JA, Armengol-Miró JR. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Poincloux L, Rouquette O, Buc E, Privat J, Pezet D, Dapoigny M, Bommelaer G, Abergel A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 45. | Garcia-Sumalla A, Loras C, Sanchiz V, Sanz RP, Vazquez-Sequeiros E, Aparicio JR, de la Serna-Higuera C, Luna-Rodriguez D, Andujar X, Capilla M, Barberá T, Foruny-Olcina JR, Martínez B, Dura M, Salord S, Laquente B, Tebe C, Videla S, Perez-Miranda M, Gornals JB; Spanish Working Group on Endoscopic Ultrasound Guided Biliary Drainage. Multicenter study of lumen-apposing metal stents with or without pigtail in endoscopic ultrasound-guided biliary drainage for malignant obstruction-BAMPI TRIAL: an open-label, randomized controlled trial protocol. Trials. 2022;23:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Gaujoux S, Jacques J, Bourdariat R, Sulpice L, Lesurtel M, Truant S, Robin F, Prat F, Palazzo M, Schwarz L, Buc E, Sauvanet A, Taibi A, Napoleon B. Pancreaticoduodenectomy following endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents an ACHBT - SFED study. HPB (Oxford). 2021;23:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 47. | Darnell EP, Wang TJ, Lumish MA, Hernandez-Barco YG, Weniger M, Casey BW, Qadan M, Lillemoe KD, Ferrone CR, Fernandez-Del Castillo C, Krishnan K. Preoperative cholangitis is an independent risk factor for mortality in patients after pancreatoduodenectomy for pancreatic cancer. Am J Surg. 2021;221:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |