Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.893
Peer-review started: October 18, 2023
First decision: December 12, 2023
Revised: December 28, 2023
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 27, 2024
Processing time: 155 Days and 15.6 Hours
Colorectal cancer is a major global health challenge that predominantly affects older people. Surgical management, despite advancements, requires careful consideration of preoperative patient status for optimal outcomes.
To summarize existing evidence on the association of frailty with short-term postoperative outcomes in patients undergoing colorectal cancer surgery.
A literature search was conducted using PubMed, EMBASE and Scopus databases for observational studies in adult patients aged ≥ 18 years undergoing planned or elective colorectal surgery for primary carcinoma and/or secondary metastasis. Only studies that conducted frailty assessment using recognized frailty assess
A total of 24 studies were included. Compared with nonfrail patients, frailty was associated with an increased risk of mortality at 30 d (RR: 1.99, 95%CI: 1.47–2.69), at 90 d (RR: 4.76, 95%CI: 1.56–14.6) and at 1 year (RR: 5.73, 95%CI: 2.74–12.0) of follow up. Frail patients had an increased risk of any complications (RR: 1.81, 95%CI: 1.57–2.10) as well as major complications (Clavien–Dindo classification grade ≥ III) (RR: 2.87, 95%CI: 1.65–4.99) compared with the control group. The risk of reoperation (RR: 1.18, 95%CI: 1.07–1.31), readmission (RR: 1.70, 95%CI: 1.36–2.12), need for blood transfusion (RR: 1.67, 95%CI: 1.52–1.85), wound complications (RR: 1.49, 95%CI: 1.11–1.99), delirium (RR: 4.60, 95%CI: 2.31–9.16), risk of prolonged hospitalization (RR: 2.09, 95%CI: 1.22–3.60) and discharge to a skilled nursing facility or rehabilitation center (RR: 3.19, 95%CI: 2.0–5.08) was all higher in frail patients.
Frailty in colorectal cancer surgery patients was associated with more complications, longer hospital stays, higher reoperation risk, and increased mortality. Integrating frailty assessment appears crucial for tailored surgical management.
Core Tip: This meta-analysis focused on understanding the impact of frailty on short-term outcomes in individuals undergoing colorectal cancer surgery. We analyzed 24 studies involving adult patients aged ≥ 18 years who underwent planned colorectal surgery. Relevant literature search, until August 2023, was conducted using PubMed, EMBASE and Scopus. Observational studies of prospective and retrospective cohort design, as well as case–control studies were included. Pooled findings indicated that frailty was associated with a significant increase in perioperative complications, longer hospital stays, higher risk of reoperation, and increased mortality rate.
- Citation: Zhou Y, Zhang XL, Ni HX, Shao TJ, Wang P. Impact of frailty on short-term postoperative outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis. World J Gastrointest Surg 2024; 16(3): 893-906
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/893.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.893
Colorectal cancer presents a significant healthcare challenge. It currently ranks as the fourth most prevalent cancer worldwide and predominantly affects the older population[1]. The management of colorectal cancer is centered on surgical procedures, with or without neoadjuvant therapy[2]. A surgical approach is a challenging procedure, often involving complex resections and substantial postoperative recovery and is associated with numerous short-term complications. While advancements in surgical techniques and perioperative care have improved outcomes over the years, a growing body of evidence suggests that the preoperative status of patients plays a crucial role in determining postoperative results[3,4]. Therefore, it is crucial to identify the risk factors, associated with postoperative complications in colorectal cancer patients, to enhance the care provided and to reduce potential complications. Frailty is one of the factors that has gained increasing attention in recent years. It is a complex multidimensional syndrome known to involve a range of characteristics, such as reduced muscle mass, lower levels of physical activity, cognitive challenges, and nutritional deficiencies[5-7]. Frailty can affect individuals of various ages, and may potentially affect short-term postoperative outcomes in many cancers[8-10]. Research on the impact of frailty on postoperative outcomes in patients undergoing colorectal cancer surgery has profound implications for the entire medical and surgical team. They should have the necessary skills to assess and recognize frailty in these patients. This recognition is crucial for tailoring preo
Previous reviews have shown that frailty in patients undergoing colorectal cancer surgery is associated with higher mortality, prolonged hospital stay, serious complications, increased risk of readmission, and requirement for more support outside the home[11,12]. With new studies being published on this issue, our study aims to update the existing evidence and to use a subgroup analysis to provide a more in-depth understanding of the impact of frailty on short-term postoperative outcomes, primarily, mortality and risk of complications in this cohort of patients.
The review was performed following the PRISMA guidelines[13], and the protocol was registered with PROSPERO (registration number CRD42023461812). We conducted a thorough search on PubMed, EMBASE and Scopus to identify relevant studies published until August 31, 2023. Our search strategy involved a combination of specific terms, including (Frailty OR muscle weakness OR sarcopenia OR impaired muscle function) AND (colorectal cancer surgery OR colorectal resection OR curative colorectal resection) AND (clinical outcomes OR post-operative outcomes OR mortality OR survi
We included observational studies of prospective and retrospective cohort design, as well as case–control studies, which involved adult participants aged ≥ 18 years undergoing planned or elective colorectal surgery for primary carcinoma and/or secondary metastasis. Only studies that performed frailty assessments using established frailty assessment tools and defined and categorized frailty based on recognized criteria were included. The comparator group of the included studies should have consisted of nonfrail participants undergoing elective colorectal surgery for malignancy. Studies with either laparoscopic/robotic (minimally invasive) or open surgical approaches were eligible for inclusion. Additionally, the included studies should have reported at least one short-term postoperative outcome of interest and provided sufficient data for effect size calculation. There were no restrictions on the publication date.
Case reports, case series, reviews, conference abstracts, editorials, studies involving pediatric populations (participants aged < 18 years), or patients undergoing emergency colorectal surgery were excluded. Additionally, studies with patients undergoing colorectal surgery for non-carcinomatous indications were excluded. To prevent duplication, in cases where multiple publications originated from the same study, only the most comprehensive and recent publication was considered.
After the initial search across the databases and the removal of duplicates, two researchers from our team conducted a meticulous review of the remaining studies. During the initial screening phase, titles and abstracts were searched. Full texts of the studies that met the predefined criteria were, subsequently, examined for eligibility. Any disagreements were resolved through discussion between the two authors. If necessary, we sought the perspective of a third author to reach a consensus.
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the studies[14]. Data extraction was conducted systematically by two independent reviewers using a standardized data extraction spreadsheet and included study identifiers such as author's name, publication year, study location, subject characteristics, sample size, the definition of frailty used, and the outcomes of interest. Any discrepancies during the data extraction process were resolved by discussion or by consulting a third senior reviewer.
All the analysis was done using STATA version 15.0. We reported the effect size as the relative risk (RR) for categorical outcomes and as the weighted mean difference (WMD) for continuous outcomes. Effect sizes were reported along with 95% confidence intervals (CIs). A random-effects model was used for all our analyses to account for variations in baseline characteristics among the included studies. Publication bias was assessed using both Egger’s test and visual inspection of the funnel plot[15]. We conducted subgroup analysis based on study design (retrospective and prospective cohort), type of surgery (minimally invasive and open), and tumor stage. P < 0.05 indicated statistical significance.
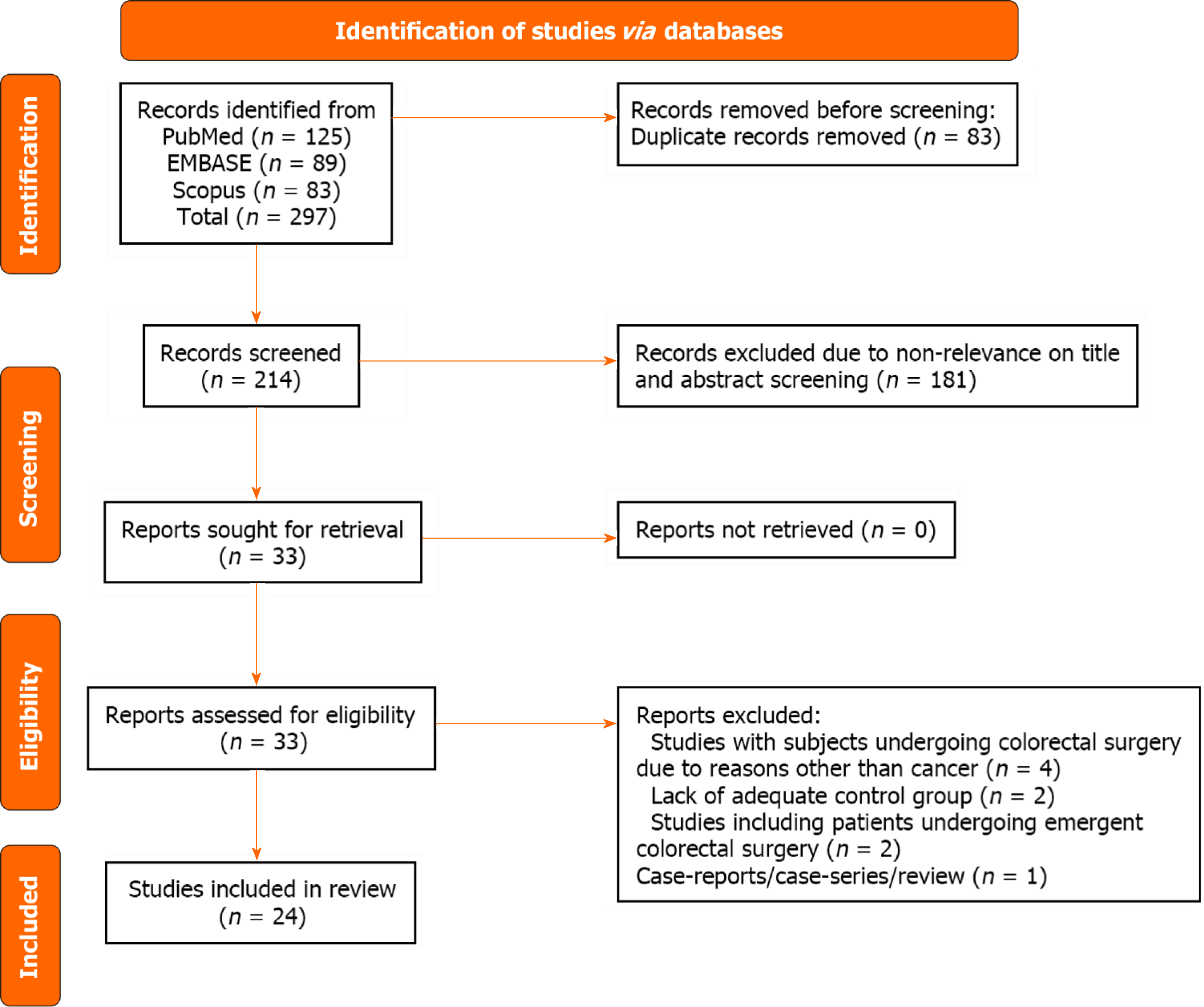
Our search strategy initially identified 297 studies. After eliminating 83 duplicates, 214 unique studies remained. After the review of titles and abstracts, 181 studies were excluded. A thorough review of the complete texts of the remaining 33 studies was done, and eventually, 24 studies were eligible for inclusion in our meta-analysis[16-39] (Figure 1).
Of 24 studies, 14 were retrospective cohort studies and the remaining 10 studies had a prospective design. The majority of the studies were conducted in the United States (n = 5), Japan (n = 4) and Netherlands (n = 4). Two studies each were conducted in Norway and Spain. One study each was conducted in the United Kingdom, China, Italy, New Zealand, Mexico and Finland. One study was multicentric (Singapore and Japan). The assessment criteria for frailty varied significantly among the studies. Even when studies used the same assessment tool, the cutoff values of frailty differed. Modified Frailty Index (n = 5) and the Clinical Frailty Scale (n = 5) were the most used assessment criteria. The studies included a total of 277993 patients (77091 with frailty and 200902 without). The mean quality assessment score of the included studies was 7.3, indicating that the studies were of acceptable quality. There were eleven studies with an NOS score of 7 (out of the maximum attainable score of 9), eight studies with a score of 8, four studies with a score of 6, and one study scoring 9 (Table 1).
| Refs | Study design | Country | Subject characteristics | Definitions used for frailty | Sample size | Newcastle– Ottawa score |
| McGovern et al[16], 2023 | Retrospective cohort | United Kingdom | Age ≥ 65 yr (66%); males (55%). TNM Stage 1 and 2 (65%) | 5-item mFI; mFI ≥ 2 indicated frailty | Frailty: 221. No frailty: 781 | 7 |
| Sibia et al[17], 2023 | Retrospective cohort | United States | Mean age of subjects around 62 yr; males (> 50%); frail patients older, had higher BMI, and with more comorbidities than nonfrail patients. Data on TNM stage not available | 5-item mFI; mFI ≥ 0.4 indicated frailty | Frailty: 3606. No frailty: 13855 | 8 |
| Abdelfatah et al[18], 2023 | Retrospective Cohort | United States | Mean age of around 75 yr and males (51%); compared with the nonfrail patients, the frail group had an older mean age. Cancer histology-adenocarcinoma (80%). Majority underwent minimally invasive procedure (laparoscopic and robotic; 78%). Stage 0-2 (63%) | Frailty scores calculated using the revised RAI-A; score of 38 or higher considered as frail | Frailty: 123. No frailty: 288 | 8 |
| Aguilar-Frasco et al[19], 2023 | Retrospective cohort | Mexico | Mean age of 72 yr and males (56%); mean BMI approximately 2 kg/m2 | Memorial Sloan Kettering-FI score ≥ 3 denoted frailty | Frailty: 56. No frailty: 160 | 7 |
| Garcia-Perez et al[20], 2023 | Retrospective cohort | Spain | Mean age of 76 yr and males (58%); majority with ASA class 2 or 3; all subjects with laparoscopic surgery | mFI developed based on the CSHA-FI, consisting of 11 variables, mFI score of ≥ 2 considered as frailty | Frailty: 46. No frailty: 126 | 7 |
| Argillander et al[21], 2022 | Retrospective cohort | Netherlands | Median age of 76 yr; females (55%); with ASA class 1 or 2 (78%); stage 1 or 2 (72%); laparoscopic surgery (64%); those who were frail were older, had higher ASA (3 or 4) and increased comorbidities | Groningen frailty indicator; score of ≥ 4 indicative of frailty | Frailty: 44. No frailty: 187 | 8 |
| Nakao et al[22], 2022 | Retrospective cohort | Japan | Median age of 70 yr; male (65%); patients with stage 2 or 3 cancer; ASA score higher in the frail group | Assessed using CFS score of ≥ 4 points- frail | Frailty: 11. No frailty: 97 | 7 |
| Niemeläinen et al[23], 2021 | Prospective cohort | Finland | Median age of 84 yr; females (60%); with ASA score of 3 (67%); stage 1 or 2 (72%); majority with either open surgery or conversion to open surgery (approximately 65%) | Assessed using CFS score of 5 to 9 points-frail | Frailty: 43. No frailty: 117 | 7 |
| Artiles-Armas et al[24], 2021 | Prospective cohort | Spain | Median age of 72 yr; males (65%); frail patients older and with more comorbidities, than nonfrail patients. Majority with TNM Stage 1 and 2 (66%); majority with open surgery (61%) | CSHA-CFS score ≥ 4 indicated frailty | Frailty: 59. No frailty: 90 | 7 |
| Pata et al[25], 2021 | Prospective cohort | Italy | Median age of 81 yr; males (53%); higher proportion of females, those with ASA 3 or 4 and co-morbidities in those who were frail; laparoscopic surgery (53%) | The multidimensional prognostic index; MPI score > 0.33 considered as frailty | Frailty: 34. No frailty: 70 | 8 |
| Tamura et al[26], 2021 | Prospective cohort | Japan | Mean age of 76 yr; male (58%); stage 0–2 (56%); laparoscopic surgery (90%) | The KCL; score of ≥ 8 was considered as frailty | Frailty: 164. No frailty: 336 | 7 |
| Richards et al[27], 2021 | Prospective cohort | New Zealand | Median age of 76 yr; male (50%); patients with ASA 3 or 4 (48%); Compared with the nonfrail patients, the frail group had an older median age, high ASA score and higher proportion underwent open surgery; stage 1 or 2 tumor (72%); laparoscopic surgery (57%) | Edmonton frail scale; those scoring ≥ 8 classified as frail | Frailty: 12. No frailty: 74 | 8 |
| Bessems et al[28], 2021 | Retrospective cohort | Netherlands | Patients older than 75 yr; females (51%); with ASA class 1 or 2 (67%); stage 1 or 2 (64%); laparoscopic surgery (75%); those who were frail were older, had higher ASA (3 or 4) and increased comorbidities | G8 and 4MGST (G8 ≤ 14 and/or 4MGST < 1 m/s) indicated frailty | Frailty: 53. No frailty: 79 | 8 |
| Mima et al[29], 2020 | Retrospective cohort | Japan | Majority aged < 75 yr (54%); male (53%); stage 1 or 2 (66%) | Assessed using CFS score of ≥ 4 points-frail | Frailty: 253. No frailty: 476 | 6 |
| Gong and Qi[30], 2020 | Retrospective cohort | China | Mean age of 68 yr; males (54%); with ASA 1 or 2 score (80%); stage 1 or 2 (63%); minimal invasive surgery (75%) | mFI developed based on the CSHA-FI, consisting of 11 variables. mFI score of ≥ 4 considered as high frailty | Frailty: 19. No frailty: 141 | 6 |
| Al-Khamis et al[31], 2019 | Retrospective cohort | United States | Patients aged > 50 yr and females (51%); compared with the nonfrail patients, the frail group had an older mean age, higher BMI, comorbidities (COPD, diabetes and hypertension) and high ASA score; Majority underwent open surgery (53%) | 5-item mFI; mFI ≥ 2 indicated frailty | Frailty: 53230. No frailty: 135356 | 9 |
| Okabe et al[32], 2019 | Retrospective cohort | Japan | Median age of subjects higher in those who were frail (80 yr vs 68 yr); males (62%); laparoscopic surgery (63%); stage 1 or 2 (70%) | Assessed using CFS score of ≥ 4 points-frail | Frailty: 78. No frailty: 191 | 7 |
| Pandit et al[33], 2018 | Retrospective cohort | United States | Mean age of 69 yr; males (62%); majority with open surgery; those who were frail had increased comorbidities (diabetes, cardiovascular disease) | CSHA-FI; score of > 0.27 was defined as frail | Frailty: 18241. No frailty: 35411 | 8 |
| Souwer et al[34], 2018 | Prospective cohort | Netherlands | Median age of 77 yr; females (45%); stage 1 or 2 (65%); majority with laparoscopic surgery (76%) | G8 and ISARHP scales. Score of ≤ 14 on G8: frail. Score of ≥ 2 on ISARHP: frail | Frailty: 20. No frailty: 117 | 8 |
| Reisinger et al[35], 2015 | Prospective cohort | Netherlands | Mean age of 69 yr; male (50%); majority with open surgery (90%; stage 3 or 4 (> 50%) | Groningen frailty indicator; score of 5 or more labeled as frail | Frailty: 41. No frailty: 269 | 7 |
| Ommundsen et al[36], 2014 | Prospective cohort | Norway | Majority aged 7 to 89 yr (94%); female (57%); stage 0 to 2 (60%); open surgery (66%) | GA | Frailty: 76. No frailty: 102 | 6 |
| Neuman et al[37], 2013 | Retrospective cohort | United States | Mean age of 84 yr; stage 1 or 2 (72%); male (39%) | JHACG frailty-defining diagnosis indicator was used. It uses 11 categories of ICD-10 codes to predict a patient’s frailty status | Frailty: 566. No frailty: 12413 | 7 |
| Tan et al[38], 2012 | Prospective cohort | Multicenter (Singapore and Japan) | Mean age of around 81 yr; ASA 3 or more (31%); majority undergoing laparoscopic surgery | Based on weight loss, physical exhaustion, physical activity level, grip strength, and walking speed | Frailty: 19. No frailty: 64 | 6 |
| Kristjansson et al[39], 2010 | Prospective cohort | Norway | Mean age of 80 yr; females (57%); stage 0 to 2 (62%); majority with open surgery (66%) | Based on CGA | Frailty: 76. No frailty: 102 | 7 |
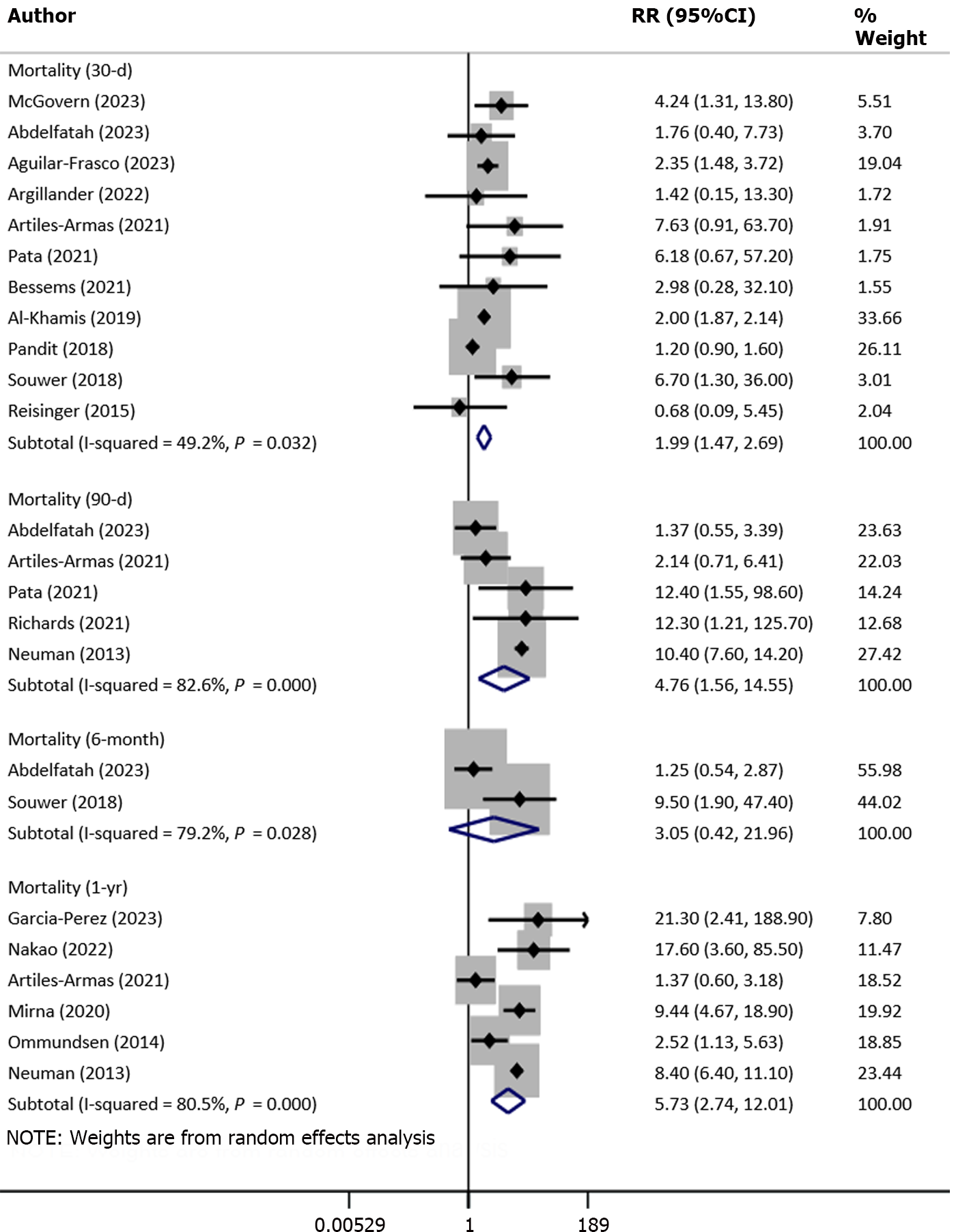
Compared with nonfrail patients, patients with frailty had increased risk of mortality at 30 d (RR: 1.99, 95%CI: 1.47-2.69; n = 11, I2 = 49.2%), at 90 d (RR: 4.76, 95%CI: 1.56–14.6; n = 5, I2 = 82.6%) and at 1 year (RR: 5.73, 95%CI: 2.74–12.0; n = 6, I2 = 80.5%) of follow-up (Figure 2). The observed pooled effect size of the risk of mortality at 6 mo of follow-up was not significant (RR: 3.05, 95%CI: 0.42–21.9; n = 2, I2 = 79.2%), possibly due to a limited number of studies (n = 2) reporting on the mortality outcome at this time point. We did not find the presence of publication bias either on Egger’s test or on the visual inspection of the funnel plot. Egger’s P value for mortality at 30-d, 90-d and 1-year of follow-up was 0.19, 0.44 and 0.83 respectively. The funnel plots for mortality outcomes at different time points of follow-up have been presented in Supplementary Figures 1–3.
Subgroup analysis based on the study design showed that the risk of mortality for frail patients was higher and statistically significant in both retrospective and prospective cohort studies (Table 2). Similarly, there was an increased risk of mortality in frail patients irrespective of the mode of surgical management; that is, open surgery or minimally invasive surgery. Subgroup analysis of patients with stage 0 to 2 tumor showed that frailty was associated with the increased risk of mortality at 30 d, 90 d and 12 months of follow-up (Table 2).
| Mortality (30-d) | Mortality (90-d) | Mortality (1-yr) | Any complications | Major complications | |
| RR (95%CI) (n; I2) | |||||
| Study design | |||||
| Retrospective | 1.85 (1.37, 2.50) (7; 57.1%)1 | 3.96 (0.54, 28.8) (2; 94.1%) | 8.80 (6.84, 11.3) (4; 0.0%)1 | 2.02 (1.64, 2.49) (9; 89.9%)1 | 2.38 (0.93, 6.07) (4; 94.6%) |
| Prospective | 3.94 (1.30, 12.0) (4; 19.7%)1 | 5.03 (1.38, 18.3) (3; 38.9%)1 | 1.88 (1.03, 3.41) (2; 6.1%)1 | 1.54 (1.33, 1.78) (6; 17.5%)1 | 3.23 (2.11, 4.95) (5; 32.7%)1 |
| Tumor stage | |||||
| Stage 0 to 22 | 3.54 (1.79, 7.02) (6; 0.0%)1 | 4.07 (1.14, 14.6) (4; 86.8%)1 | 4.29 (1.83, 10.1) (4; 87.0%)1 | 1.67 (1.42, 1.97) (9; 42.4%)1 | 2.57 (1.41, 4.66) (5; 64.7%)1 |
| Type of surgery3 | |||||
| Minimal invasive | 3.10 (1.33, 7.22) (5; 0.0%)1 | 4.61 (0.85, 25.1) (3; 65.3%) | 18.8 (5.22, 67.7) (2; 0.0%)1 | 2.02 (1.59, 2.57) (8; 58.0%)1 | 3.12 (1.71, 5.68) (5; 60.2%)1 |
| Open | 1.63 (1.01, 2.63) (4; 78.7%)1 | 2.14 (0.71, 6.43) (1; -) | 1.88 (1.03, 3.41) (2; 6.1%)1 | 1.51 (1.27, 1.81) (4; 60.8%)1 | 1.80 (1.09, 2.98) (3; 68.5%)1 |
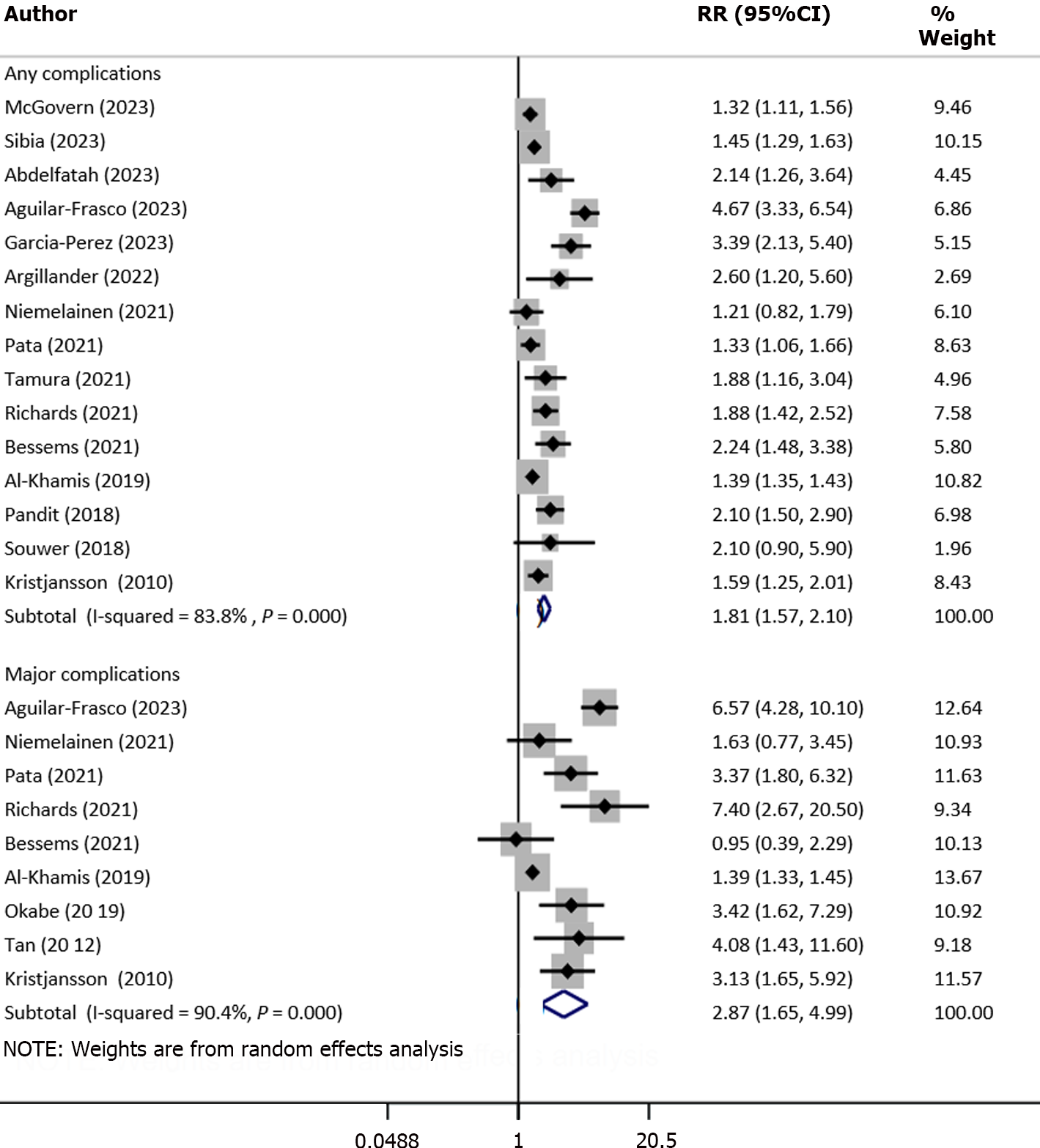
Frail patients had an increased risk of any complications (RR: 1.81, 95%CI: 1.57–2.10; n = 15, I2 = 83.8%) as well as major complications (Clavien–Dindo classification grade ≥ III) (RR: 2.87, 95%CI: 1.65–4.99; n = 9, I2 = 90.4%) (Figure 3). There was evidence for the presence of significant publication bias for any complications (P = 0.02) or major complications (P = 0.04) on Egger’s test. The funnel plots for both these outcomes are presented in Supplementary Figures 4 and 5. In the subgroup analysis, the increased risk of complications was observed among frail patients, irrespective of the study design (retrospective and prospective cohort) and mode of surgical management (open or minimally invasive surgery) (Table 2). Upon pooling of studies with patients with stage 0 to 2 tumor, we showed that frailty was associated with the increased risk of complications (Table 2).
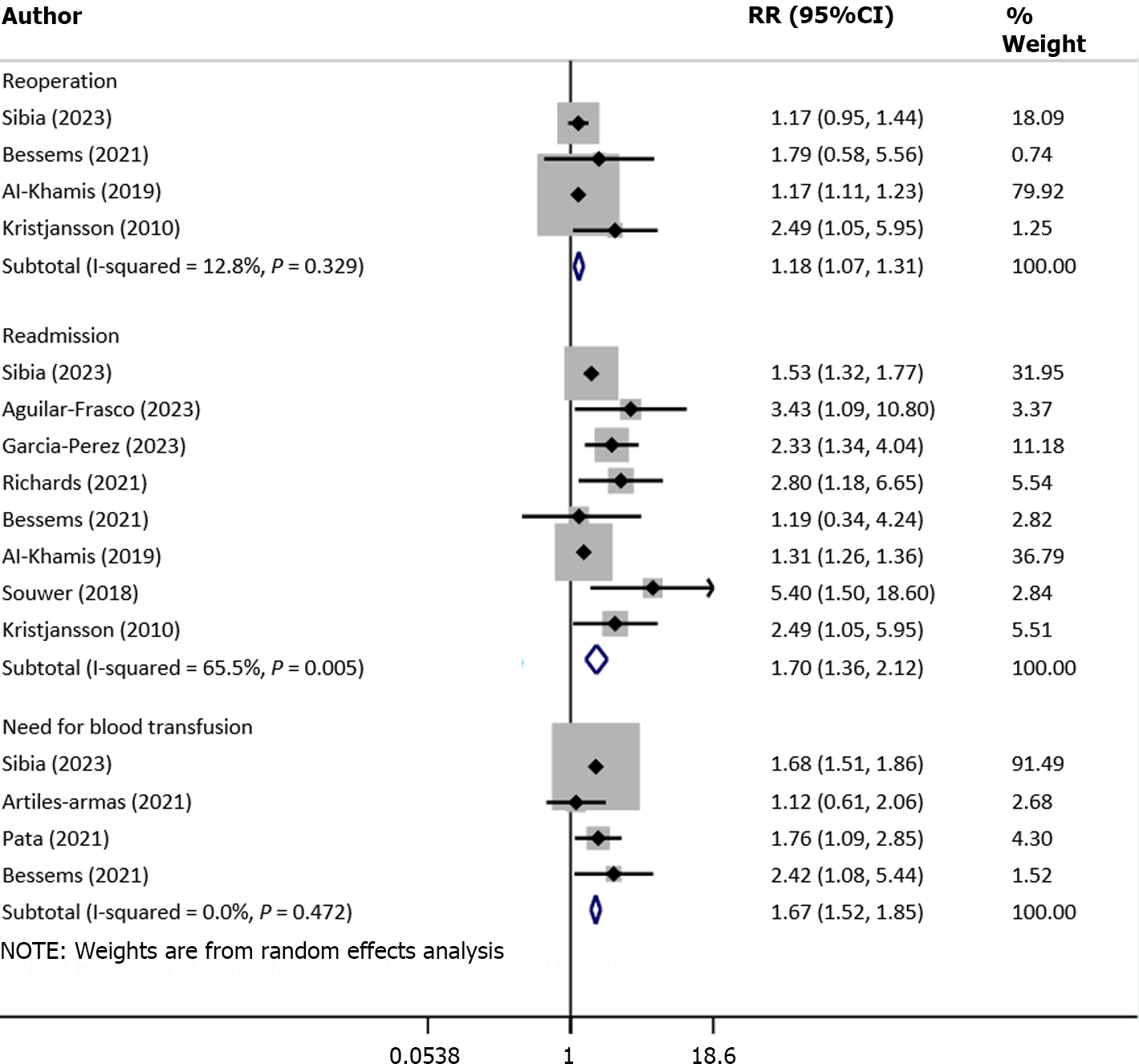
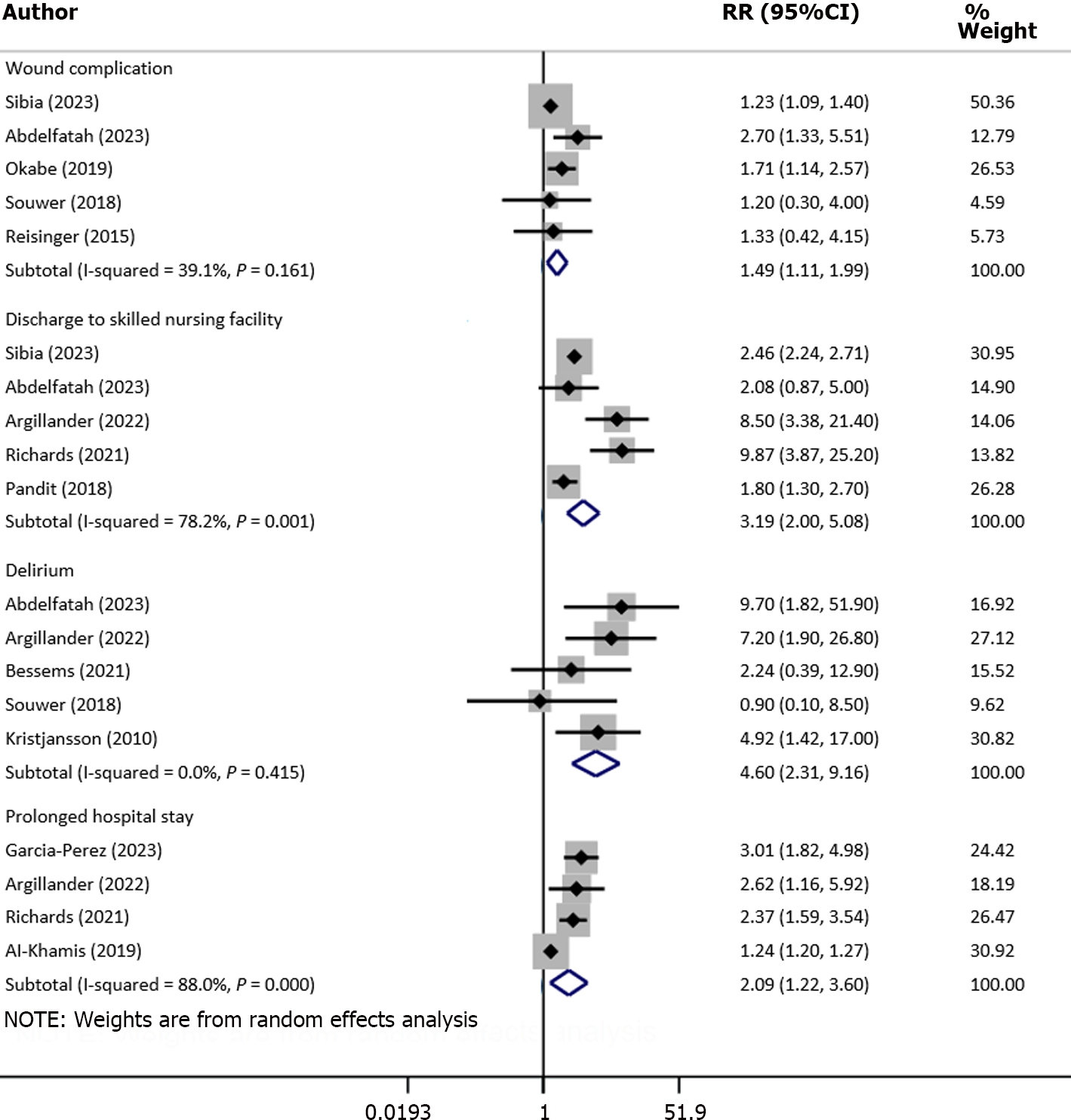
More specifically, the risk of reoperation (RR: 1.18, 95%CI: 1.07–1.31; n = 4, I2 = 12.8%), readmission (RR: 1.70, 95%CI: 1.36–2.12; n = 8, I2 = 65.6%) and need for blood transfusion (RR: 1.67, 95%CI: 1.52–1.85; n = 4, I2 = 0.0%) was higher in frail compared with nonfrail patients (Figure 4). Similarly, the risk of wound complications (RR: 1.49, 95%CI: 1.11–1.99; n = 5, I2 = 39.1%), delirium (RR: 4.60, 95%CI: 2.31–9.16; n = 5, I2 = 0.0%) and discharge to skilled nursing facility or rehabilitation Centre (RR: 3.19, 95%CI: 2.0–5.08; n = 5, I2 = 78.2%) were all higher in frail patients (Figure 5).
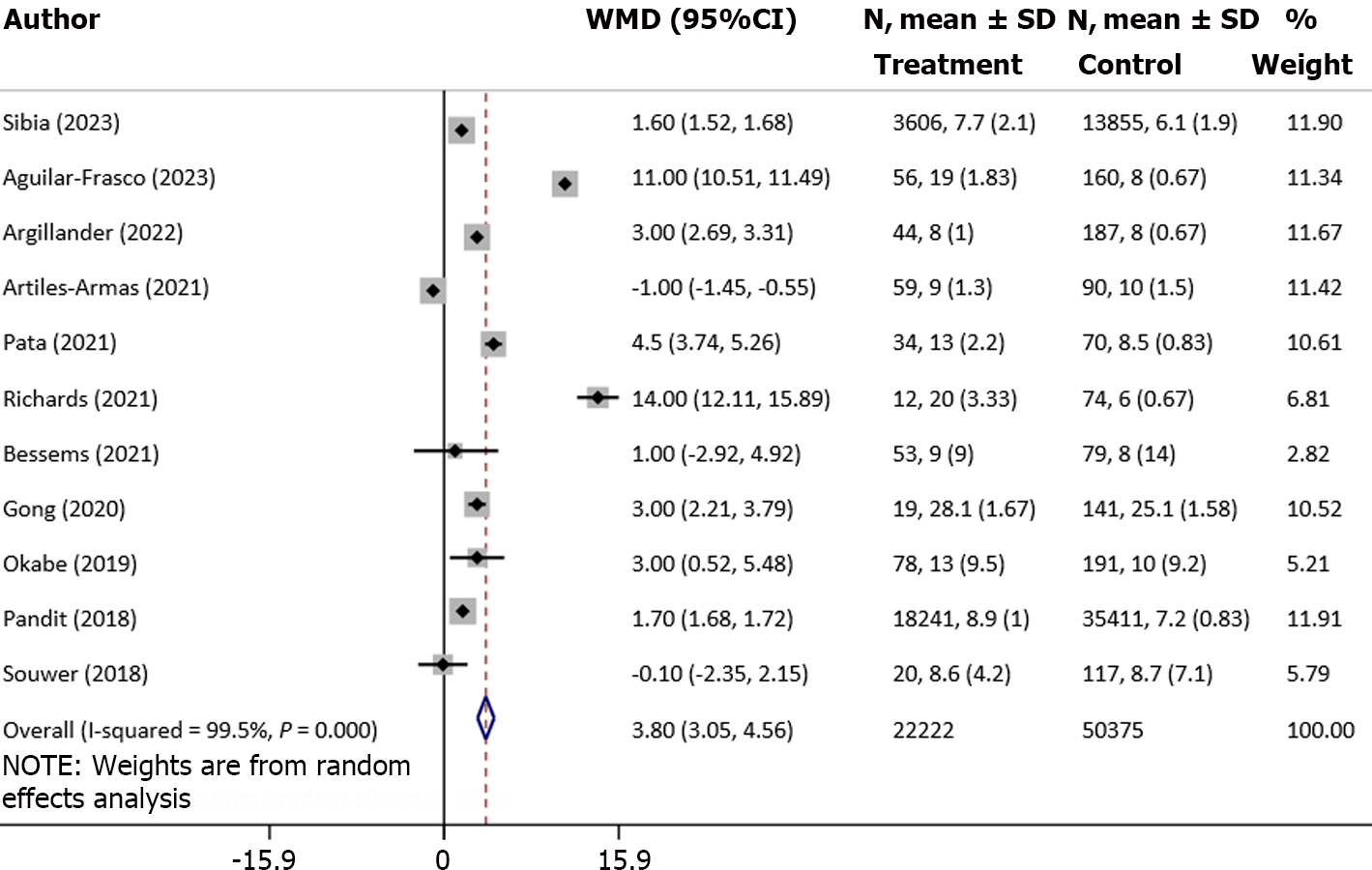
There was a significant increase in the length of hospital stay (in days) in frail patients, compared with normal/nonfrail patients (WMD: 3.80, 95%CI: 3.05-4.56; n = 11, I2 = 99.5%) (Figure 6). The risk of prolonged hospitalization (RR: 2.09, 95%CI: 1.22–3.60; n = 4, I2 = 88.0%) was also higher in frail patients (Figure 5).
Our meta-analysis showed a significant association between preoperative frailty and increased risk of mortality and complications, including reoperation, readmission, need for blood transfusion, wound complications, delirium, and risk of prolonged hospitalization. The increased mortality risks, identified at multiple time points, at 30 d, 90 d, and 1 year of follow-up, further emphasize the enduring impact of frailty on survival prospects. Frail patients frequently face challenges related to their weakened cardiovascular, respiratory and immune systems, making them less resilient when it comes to the physical demands of surgery and its subsequent recovery[40-42]. This fragility may result in a heightened risk of postoperative complications, ultimately contributing to elevated mortality rates.
The association between frailty and an increased incidence of postoperative complications is equally noteworthy. Frail patients in our study faced a nearly twofold higher risk of encountering any complication and the risk of major complications was nearly threefold higher. Colorectal cancer surgery is a complex and invasive procedure that can place significant physical and physiological stress on patients. Our results show that frailty is associated with a higher incidence of surgical complications such as wound infections. Our conclusions agree with previous studies that showed that the weakened physiological state of frail patients can impair the healing process, making them more susceptible to these surgical challenges[43,44]. Frailty may disrupt the stress response, potentially leading to imbalanced inflammation, delayed wound healing, and impaired tissue recovery[45,46]. These interconnected processes set off a chain of events that can increase the susceptibility of frail patients to various complications. Additionally, a compromised immune system of these patients makes them vulnerable to infections frequently associated with surgical procedures[47].
We also found an increased risk of prolonged hospital stay in frail patients that could also be attributed to the increased risk of complications that may eventually require longer hospitalization. In addition to the impact on the patient, extended hospitalization also strains the healthcare system. This strengthens the importance of more robust support systems to address the multifaceted needs of frail patients during their hospitalization.
Our findings strongly advocate the inclusion of a frailty assessment in the preoperative evaluation of candidates for colorectal cancer surgery. Early identification of frail patients can enable the implementation of personalized interventions to optimize their perioperative care, reduce potential complications, and enhance overall outcomes[48,49]. This suggestion emanates from a recent systematic review by Guo et al[48] that included nine studies with 1313 cancer patients. The review found that prehabilitation for frailty reduced the risk of complications and the average length of hospital stay. However, the intervention did not have a significant impact on 30-d and 3-month mortality and readmission rates. These findings emphasize the importance of collaborative efforts involving surgical teams, geriatric specialists, and other relevant healthcare providers to ensure the comprehensive management of frail patients.
The findings of our review have implications for the healthcare team. It emphasizes the importance of a collaborative effort to minimize stressors, carefully monitor vital signs, and customize anesthetic and fluid management. Effective communication among healthcare professionals is paramount in ensuring that everyone is aware of the patient’s frailty status and potential risks. In the postoperative phase, nurses could play a critical role in monitoring patients for complications, such as infections or delirium, and advocating for appropriate pain management strategies. Additionally, they could actively participate in designing rehabilitation plans, offering patient and family education, and contributing to quality improvement efforts. Emotionally supporting frail patients and facilitating access to psychological services is one of the important aspects of the comprehensive care that nursing personnel can provide.
The finding that frailty correlates with adverse outcomes necessitates a thoughtful integration of this information into the crucial preoperative consent and decision-making process between patients and surgeons. During the informed consent discussions, surgeons should meticulously elaborate on the implications of frailty, elucidating the heightened risks associated with postoperative complications and prolonged recovery times. This discourse should not only encompass a comprehensive assessment of the individual’s frailty level but also foster shared decision-making, allowing patients to actively engage in treatment choices based on their values and preferences. Importantly, setting realistic expectations becomes paramount, creating awareness among patients about the challenges posed by frailty and the potential limitations of surgery. Additionally, preoperative interventions, like physical therapy and nutritional support, can be used to enhance patients’ resilience. The documentation of these discussions in medical records could ensure transparency, will aid continuity of care, and reinforce a patient-centric approach to managing colorectal cancer surgery in frail individuals.
There are several frailty assessment tools to choose from to evaluate and quantify frailty in older individuals. The best tool choice will depend on various factors, including the context of use, the population being assessed, and the specific goals of the assessment. The choice of the best frailty assessment tool will also depend on the specific needs of the healthcare setting, available resources, and the expertise of the assessors. Considering the reliability, validity and feasibility of a tool in the given context is important. Additionally, combining multiple tools or approaches may provide a more comprehensive understanding of frailty in older individuals.
We acknowledge several limitations in this meta-analysis. Firstly, the studies included displayed some heterogeneity in terms of patient populations, surgical techniques, and outcome definitions. This diversity may have introduced variations in the results for certain outcomes. Additionally, the variability in the methods used for assessing frailty across the studies could have led to inconsistent categorization of frailty, potentially affecting the strength of the observed associations. Moreover, the included studies were retrospective and may have involved selection bias when considering frail patients. This may have affected the internal validity of the results. The presence of unmeasured confounding variables, such as differences in comorbidities or socioeconomic factors, might have influenced the observed outcomes.
This meta-analysis emphasizes the crucial role of frailty as a predictive factor for adverse postoperative outcomes in colorectal cancer surgery. The identified associations between frailty and elevated risks of mortality, complications and other adverse events highlight the urgency of implementing comprehensive strategies tailored to the specific requirements of frail individuals. The integration of frailty assessment into routine clinical practice has the potential to not only enhance patient care but also guide treatment decisions, ultimately improving surgical outcomes for this vulnerable patient population.
Colorectal cancer presents a significant healthcare challenge. The management is centered on surgical procedures, with or without neoadjuvant therapy. While advancements in surgical techniques have improved outcomes, recent evidence highlights the critical role of preoperative frailty in influencing postoperative results. Our review aimed to update existing evidence on the impact of preoperative frailty on survival and other key clinical outcomes in subjects with colorectal cancer undergoing elective surgery.
To update existing evidence, through inclusion of contemporary studies, in order to guide clinical practice.
To identify and include all relevant studies to analyze and document the association of frailty with short-term postoperative outcomes in patients undergoing colorectal cancer surgery.
A comprehensive literature search was conducted using PubMed, EMBASE and Scopus to identify observational studies involving adults (age ≥ 18 years) undergoing planned colorectal surgery for primary carcinoma and/or secondary metastasis. Included studies utilized recognized frailty assessment tools and featured a comparator group of nonfrail patients. Pooled effect sizes, along with 95% confidence intervals, were reported.
A total of 24 studies were included. Frailty was found to be associated with increased risk of mortality at 30 d, 90 d and 1 year of follow-up. Frail patients had increased risk of overall complications as well as major complications, compared with the nonfrail patients. The risk of need for reoperation, readmission, need for blood transfusion, wound complications, delirium, risk of prolonged hospitalization and discharge to skilled nursing facility or rehabilitation center was higher in frail patients.
In patients undergoing colorectal cancer surgery, frailty was associated with a significant increase in perioperative complications, longer hospital stay, higher risk of reoperation and increased mortality rate.
This finding of this meta-analysis emphasizes the crucial role of frailty as a predictive factor for adverse postoperative outcomes in colorectal cancer surgery. They further call for integration of frailty assessment into routine clinical practice to enhance patient care and guide treatment decisions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yap RV, Philippines S-Editor: Fan JR L-Editor: Kerr C P-Editor: Cai YX
| 1. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1067] [Article Influence: 177.8] [Reference Citation Analysis (1)] |
| 2. | Stintzing S. Management of colorectal cancer. F1000Prime Rep. 2014;6:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Minnella EM, Liberman AS, Charlebois P, Stein B, Scheede-Bergdahl C, Awasthi R, Gillis C, Bousquet-Dion G, Ramanakuma AV, Pecorelli N, Feldman LS, Carli F. The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncol. 2019;58:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Lynch ML, Brand MI. Preoperative evaluation and oncologic principles of colon cancer surgery. Clin Colon Rectal Surg. 2005;18:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1488] [Cited by in RCA: 1312] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 6. | Bortz WM 2nd. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283-M288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 325] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Aubertin-Leheudre M, Woods AJ, Anton S, Cohen R, Pahor M. Frailty Clinical Phenotype: A Physical and Cognitive Point of View. Nestle Nutr Inst Workshop Ser. 2015;83:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lee ACH, Lee SM, Ferguson MK. Frailty Is Associated With Adverse Postoperative Outcomes After Lung Cancer Resection. JTO Clin Res Rep. 2022;3:100414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Tsai HH, Yu JC, Hsu HM, Chu CH, Hong ZJ, Feng AC, Fu CY, Dai MS, Liao GS. The impact of frailty on breast cancer outcomes: evidence from analysis of the Nationwide Inpatient Sample, 2005-2018. Am J Cancer Res. 2022;12:5589-5598. [PubMed] |
| 10. | Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 11. | Cai M, Gao Z, Liao J, Jiang Y, He Y. Frailty affects prognosis in patients with colorectal cancer: A systematic review and meta-analysis. Front Oncol. 2022;12:1017183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Michaud Maturana M, English WJ, Nandakumar M, Li Chen J, Dvorkin L. The impact of frailty on clinical outcomes in colorectal cancer surgery: a systematic literature review. ANZ J Surg. 2021;91:2322-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 14. | Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta- Analysis. [cited 10 October 2023]. Available from: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf. |
| 15. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40493] [Article Influence: 1446.2] [Reference Citation Analysis (2)] |
| 16. | McGovern J, Grayston A, Coates D, Leadbitter S, Hounat A, Horgan PG, Dolan RD, McMillan DC. The relationship between the modified frailty index score (mFI-5), malnutrition, body composition, systemic inflammation and short-term clinical outcomes in patients undergoing surgery for colorectal cancer. BMC Geriatr. 2023;23:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 17. | Sibia US, Badve SB, Istl AC, Klune JR, Riker AI. Impact of Frailty Upon Surgical Decision-Making for Left-Sided Colon Cancer. Ochsner J. 2023;23:120-128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Abdelfatah E, Ramos-Santillan V, Cherkassky L, Cianchetti K, Mann G. High Risk, High Reward: Frailty in Colorectal Cancer Surgery is Associated with Worse Postoperative Outcomes but Equivalent Long-Term Oncologic Outcomes. Ann Surg Oncol. 2023;30:2035-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Aguilar-Frasco JL, Moctezuma-Velázquez P, Rodríguez-Quintero JH, Castro E, Armillas-Canseco F, Hernández-Gaytán CA, Pastor-Sifuentes FU, Moctezuma-Velázquez C. Preoperative frailty assessment in older patients with colorectal cancer: use of clinical and radiological tool. Langenbecks Arch Surg. 2023;408:19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Garcia-Perez E, Aguirre-Larracoechea U, Portugal-Porras V, Azpiazu-Landa N, Telletxea-Benguria S. Frailty assessment has come to stay: Retrospective analysis pilot study of two frailty scales in oncological older patients undergoing colorectal surgery. Rev Esp Anestesiol Reanim (Engl Ed). 2023;70:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Argillander TE, Schäfer S, van Westreenen HL, Kamper A, van der Zaag-Loonen HJ, van Duijvendijk P, van Munster BC. The predictive value of preoperative frailty screening for postoperative outcomes in older patients undergoing surgery for non-metastatic colorectal cancer. J Geriatr Oncol. 2022;13:888-891. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Nakao T, Shimada M, Yoshikawa K, Tokunaga T, Nishi M, Kashihara H, Takasu C, Wada Y, Yoshimoto T, Yamashita S, Iwakawa Y. The correlation of immunoscore and frailty in colorectal cancer. Int J Clin Oncol. 2022;27:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Niemeläinen S, Huhtala H, Andersen J, Ehrlich A, Haukijärvi E, Koikkalainen S, Koskensalo S, Kössi J, Mattila A, Pinta T, Uotila-Nieminen M, Vihervaara H, Hyöty M, Jämsen E. The Clinical Frailty Scale is a useful tool for predicting postoperative complications following elective colon cancer surgery at the age of 80 years and above: A prospective, multicentre observational study. Colorectal Dis. 2021;23:1824-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Artiles-Armas M, Roque-Castellano C, Fariña-Castro R, Conde-Martel A, Acosta-Mérida MA, Marchena-Gómez J. Impact of frailty on 5-year survival in patients older than 70 years undergoing colorectal surgery for cancer. World J Surg Oncol. 2021;19:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Pata G, Bianchetti L, Rota M, Marengoni A, Chiesa D, Cassinotti E, Palmisano S, Colombi M, Del Zotto G, Romanelli G, Calza S, Boni L, De Manzini N, Fumagalli Romario U. Multidimensional Prognostic Index (MPI) score has the major impact on outcome prediction in elderly surgical patients with colorectal cancer: The FRAGIS study. J Surg Oncol. 2021;123:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Tamura K, Matsuda K, Fujita Y, Iwahashi M, Mori K, Yamade N, Hotta T, Noguchi K, Sakata Y, Takifuji K, Iwamoto H, Mizumoto Y, Yamaue H. Optimal Assessment of Frailty Predicts Postoperative Complications in Older Patients with Colorectal Cancer Surgery. World J Surg. 2021;45:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Richards SJG, Cherry TJ, Frizelle FA, Eglinton TW. Pre-operative frailty is predictive of adverse post-operative outcomes in colorectal cancer patients. ANZ J Surg. 2021;91:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Bessems SAM, Konsten JLM, Vogelaar JFJ, Csepán-Magyar R, Maas HAAM, van de Wouw YAJ, Janssen-Heijnen MLG. Frailty screening by Geriatric-8 and 4-meter gait speed test is feasible and predicts postoperative complications in elderly colorectal cancer patients. J Geriatr Oncol. 2021;12:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Mima K, Miyanari N, Morito A, Yumoto S, Matsumoto T, Kosumi K, Inoue M, Mizumoto T, Kubota T, Baba H. Frailty is an independent risk factor for recurrence and mortality following curative resection of stage I-III colorectal cancer. Ann Gastroenterol Surg. 2020;4:405-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Gong W, Qi X. Association of Frailty with Delayed Recovery of Gastrointestinal Function after Elective Colorectal Cancer Resections. J Invest Surg. 2020;33:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Al-Khamis A, Warner C, Park J, Marecik S, Davis N, Mellgren A, Nordenstam J, Kochar K. Modified frailty index predicts early outcomes after colorectal surgery: an ACS-NSQIP study. Colorectal Dis. 2019;21:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Okabe H, Ohsaki T, Ogawa K, Ozaki N, Hayashi H, Akahoshi S, Ikuta Y, Ogata K, Baba H, Takamori H. Frailty predicts severe postoperative complications after elective colorectal surgery. Am J Surg. 2019;217:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Pandit V, Khan M, Martinez C, Jehan F, Zeeshan M, Koblinski J, Hamidi M, Omesieta P, Osuchukwu O, Nfonsam V. A modified frailty index predicts adverse outcomes among patients with colon cancer undergoing surgical intervention. Am J Surg. 2018;216:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Souwer ETD, Verweij NM, van den Bos F, Bastiaannet E, Slangen RME, Steup WH, Hamaker ME, Portielje JEA. Risk stratification for surgical outcomes in older colorectal cancer patients using ISAR-HP and G8 screening tools. J Geriatr Oncol. 2018;9:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 383] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 36. | Ommundsen N, Wyller TB, Nesbakken A, Jordhøy MS, Bakka A, Skovlund E, Rostoft S. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 37. | Neuman HB, Weiss JM, Leverson G, O’Connor ES, Greenblatt DY, Loconte NK, Greenberg CC, Smith MA. Predictors of short-term postoperative survival after elective colectomy in colon cancer patients ≥ 80 years of age. Ann Surg Oncol. 2013;20:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen HO, Bakka A, Wyller TB. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 40. | Clegg A, Young J. The frailty syndrome. Clin Med (Lond). 2011;11:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 42. | Doody P, Lord JM, Greig CA, Whittaker AC. Frailty: Pathophysiology, Theoretical and Operational Definition(s), Impact, Prevalence, Management and Prevention, in an Increasingly Economically Developed and Ageing World. Gerontology. 2023;69:927-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 43. | Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2058] [Cited by in RCA: 2734] [Article Influence: 227.8] [Reference Citation Analysis (1)] |
| 44. | Ferris AE, Harding KG. Are chronic wounds a feature of frailty? Br J Gen Pract. 2020;70:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Ng TP, Lu Y, Choo RWM, Tan CTY, Nyunt MSZ, Gao Q, Mok EWH, Larbi A. Dysregulated homeostatic pathways in sarcopenia among frail older adults. Aging Cell. 2018;17:e12842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Pansarasa O, Pistono C, Davin A, Bordoni M, Mimmi MC, Guaita A, Cereda C. Altered immune system in frailty: Genetics and diet may influence inflammation. Ageing Res Rev. 2019;54:100935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis. 2011;2:466-473. [PubMed] |
| 48. | Guo Y, Ding L, Miao X, Jiang X, Xu T, Xu X, Zhu S, Xu Q, Hu J. Effects of prehabilitation on postoperative outcomes in frail cancer patients undergoing elective surgery: a systematic review and meta-analysis. Support Care Cancer. 2022;31:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 49. | Chang MC, Choo YJ, Kim S. Effect of prehabilitation on patients with frailty undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Treat Res. 2023;104:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |