Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.768
Peer-review started: December 17, 2023
First decision: January 10, 2024
Revised: January 13, 2024
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 27, 2024
Processing time: 95 Days and 16.5 Hours
Resection of hepatic metastasis from neuroendocrine tumors (NETs) improves quality of life and prolongs 5-year survival. Ablation can be utilized with surgery to achieve complete resection. Although several studies report long-term out
To determine if intra-operative ablation during hepatectomy increases risk of ad
A retrospective analysis of the hepatectomy National Surgical Quality Impro
Of the 966 patients included in the study, 298 (30.9%) underwent ablation during hepatectomy. There were 78 (11.7%) patients with SSIs in the hepatectomy alone group and 39 (13.1%) patients with a SSIs in the hepatectomy with ablation group. Bile leak occurred in 41 (6.2%) and 14 (4.8%) patients in the two groups, respec
Intraoperative ablation with hepatic resection for NETs is safe in the perioperative period without significant increased risk of infection, bleeding, or bile leak. Surgeons should utilize this modality when appropriate to a
Core Tip: There are no definitive guidelines for managing metastatic neuroendocrine tumors (NETs) to the liver. Liver ablation is often used as an adjunct to surgical resection; however its effect on perioperative outcomes is unknown. In this retrospective National Surgical Quality Improvement Program study, patients undergoing liver ablation in conjunction with surgical resection were compared to patients undergoing hepatectomy alone. The aim of the study was to determine if ablation during hepatectomy increases the risk of adverse perioperative outcomes such as surgical site infections, bile leaks, and bleeding. We demonstrate that ablation is safe and does not increase the risk of adverse peroperative outcomes in patients undergoing hepatectomy for NET liver metastasis.
- Citation: Ostapenko A, Stroever S, Eyasu L, Kim M, Aploks K, Dong XD, Seshadri R. Role of ablation therapy in conjunction with surgical resection for neuroendocrine tumors involving the liver. World J Gastrointest Surg 2024; 16(3): 768-776
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/768.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.768
Neuroendocrine tumors (NETs) are epithelial tumors that can arise from most organs. They are indolent, slow growing neoplasms that are frequently discovered at a late stage when they become symptomatic from hormonal excretion by metastasizing to the liver. It is estimated that almost 80%-90% of these tumors are inoperable at the time of presentation[1]. However, several studies have demonstrated that resection of hepatic metastasis from NETs improves both quality of life and prolongs 5-year survival[2,3]. These studies demonstrated that aggressive management of hepatic neuroendocrine metastases with adjunct modalities such as transarterial embolization, chemoembolization, and thermal ablation significantly prolong long-term survival and improve patient outcomes[2,4]. Despite these findings there is hesitancy among surgeons to utilize radiofrequency ablation due to fear of bleeding, abdominal infections, and biliary tree injury[5,6].
Of the available image guided ablative therapies, ethanol, microwave, radiofrequency, and cyro-ablation are the most common. These adjuncts have been utilized for primary malignancies such as hepatocellular carcinoma (HCC) and cholangiocarcinoma and are now accepted as the curative treatment option for early HCC by most guidelines[4,7]. Si
We performed a cross sectional study utilizing ACS-NSQIP database and Hepatectomy Procedure Targeted database for 2015-2019. The variables from the Procedure Targeted database were merged with the standard public use file using the CASEID variable. We included patients undergoing hepatic resection for neuroendocrine liver metastasis who were between the age of 18 and 90. Patients were separated into two study groups: Those who underwent an intra-operative ablation concurrently with a hepatectomy and those undergoing hepatectomy alone.
The aim of this study was to determine if intra-operative ablation increases the risk of adverse outcomes in patients undergoing hepatic resection for neuroendocrine liver metastasis. We included microwave, radiofrequency ablation, and alcohol ablation. The adverse outcomes of interest included SSIs, bleeding, bile leak, and readmission. We evaluated for the risk factors predictive of the adverse outcomes that were significantly different between the groups as possible confounders which could contribute to the outcomes of interest.
StataSE was used for statistical analysis. Descriptive statistics including mean ± SD for normally distributed continuous variables, median/interquartile range for skewed continuous variables, and number/percentage for categorical variables. We assessed univariate differences in outcomes between patients who underwent ablation concurrently with resection and those with resection alone using the chi-square test for categorical variables. Variables that were statistically asso
The patients were categorized by gender, age, race, ethnicity, comorbidities (BMI, diabetes), pre-operative factors such as steroid use, albumin, and neoadjuvant chemotherapy, wound class assigned to the surgery, and specific tumor characteristics (Table 1). There were 966 patients included in the study. There were 298 patients (30.8%) who had intraoperative ablation concurrently with hepatic resection and 688(69.2%) had hepatic resection alone.
| Characteristics | Overall number (%) | Surgery only, n = 668 (69.2%) | Surgery + ablation treatment, n = 298 (30.8%) | P value |
| Male | 475 (49.2) | 319 (47.8) | 156 (52.4) | 0.19 |
| Age | 59.7 ± 11.22 | 59.8 (11.5) | 59.7 (10.7) | 0.95 |
| BMI | 28.8 ± 6.22 | 28.5 (6.2) | 29.4 (6.3) | 0.0336 |
| Race | < 0.0011 | |||
| White | 687 (71.1) | 444 (66.5) | 243 (81.5) | |
| Black/African American | 82 (8.5) | 55 (8.2) | 27 (9.1) | |
| Other | 36 (3.7) | 32 (4.8) | 4 (1.3) | |
| Unknown | 161 (16.7) | 137 (20.5) | 24 (8.1) | |
| Ethnicity | < 0.0011 | |||
| Hispanic | 36 (3.7) | 27 (4.0) | 9 (3.0) | |
| Not hispanic | 787 (81.5) | 518 (77.5) | 269 (90.3) | |
| Unknown | 143 (14.8) | 123 (18.4) | 20 (6.7) | |
| Wound class | 0.025 | |||
| I | 104 (10.8) | 80 (12.0) | 24 (8.1) | |
| II | 810 (83.9) | 546 (81.7) | 264 (88.6) | |
| III/IV | 52 (5.4) | 42 (6.3) | 10 (3.4) | |
| Diabetes | 171 (17.7) | 120 (18.0) | 51 (17.1) | 0.75 |
| Steroid use | 36 (3.7) | 23 (3.4) | 13 (4.4) | 0.49 |
| Serum albumin | 17.6 ± 18.42 | |||
| Operative approach | 0.0151 | |||
| Minimally invasive | 134 (13.9) | 107 (16.0) | 27 (9.1) | |
| Unplanned open | 36 (3.7) | 24 (3.6) | 12 (4.0) | |
| Planned open | 795 (82.4) | 536 (80.4) | 259 (86.9) | |
| Operative time | 243.98 ± 101.22 | 238.9 (102.9) | 255.4 (96.4) | 0.0191 |
| Neoadjuvant Chemotherapy | 0.83 | |||
| None | 777 (80.4) | 539 (80.7) | 238 (79.9) | |
| Systemic | 83 (8.6) | 55 (8.2) | 28 (9.4) | |
| Other | 106 (11.0) | 74 (11.1) | 32 (10.7) | |
| Number of Metastasis | < 0.0011 | |||
| 1-2 | 459 (50.4) | 388 (61.9) | 71 (25.0) | |
| 3-4 | 182 (20.0) | 113 (18.0) | 69 (24.3) | |
| 5-6 | 85 (9.3) | 42 (6.7) | 43 (15.1) | |
| 7-8 | 63 (6.9) | 25 (4.0) | 38 (13.4) | |
| > 8 | 122 (13.4) | 59 (9.4) | 63 (22.2) | |
| Size of lesion | < 0.0011 | |||
| < 2 cm | 249 (26.9) | 166 (26.0) | 83 (29.0) | |
| 2-5 cm | 410 (44.3) | 263 (41.2) | 147 (51.4) | |
| > 5 cm | 266 (28.8) | 210 (32.9) | 56 (19.6) | |
| Extent of Resection | < 0.0011 | |||
| Total right lobectomy | 120 (12.4) | 105 (15.8) | 15 (5.0) | |
| Total left lobectomy | 67 (6.9) | 50 (7.5) | 17 (5.7) | |
| Trisegmentectomy | 58 (6.0) | 47 (7.0) | 11 (3.7) | |
| Partial lobectomy | 721 (74.6) | 466 (69.8) | 255 (85.6) |
The rates of adverse outcomes are listed in Table 2. There were no significant differences in most adverse outcomes between patients who underwent intra-osperative ablation vs hepatectomy alone on univariate regression analysis. How
| Outcome | Surgical treatment only, n = 668 (69.2%) | Surgery + ablation treatment, n = 298 (30.8%) | P value |
| Death | 7 (1.1) | 3 (1.0) | 1.00 |
| Significant bleed | 117 (17.5) | 33 (11.1) | 0.0111 |
| Bile leak | 41 (6.2) | 14 (4.8) | 0.38 |
| Myocardial infarction | 3 (0.5) | 1 (0.3) | 1.00 |
| Pulmonary embolism | 11 (1.7) | 4 (1.3) | 1.00 |
| Pneumonia | 17 (2.5) | 8 (2.7) | 0.90 |
| Sepsis | 21 (3.1) | 16 (5.4) | 0.096 |
| Liver failure | 23 (3.4) | 4 (1.3) | 0.089 |
| Return to operating room | 24 (3.6) | 7 (2.4) | 0.31 |
| Readmission | 73 (10.9) | 34 (11.4) | 0.83 |
| Surgical site infection | |||
| Superficial | 27 (4.0) | 10 (3.4) | 0.61 |
| Deep incisional | 4 (0.6) | 1 (0.3) | 1.00 |
| Organ space | 48 (7.2) | 30 (10.1) | 0.13 |
| Wound | 4 (0.6) | 1 (0.3) | 1.00 |
| Any | 78 (11.7) | 39 (13.1) | 0.54 |
We performed a univariate analysis of variables predictive of bile leaks, readmission, bleeding, organ space SSIs, and any surgical infection. There were 55 (5.7%) patients who experienced a bile leak. None of the variables in our model, includ
There were 150 (15.5%) significant bleeding occurrences that required blood transfusion (Supplementary Table 3). We determined several factors predictive of bleeding. A minimally invasive surgical approach (OR = 0.45, P = 0.015), partial lobectomy (OR = 0.50, P = 0.005), and ablation (OR = 0.59, P = 0.011) were protective from bleeding. On the other hand, neoadjuvant chemotherapy (OR = 2.55, P < 0.001), wound class II (OR = 3.38, P = 0.006), and lesion size between 2-5 cm (OR = 2.56, P = 0.005) all increased risk of significant bleeding. Furthermore, patients undergoing hepatecomy for lesions greater than 5 cm had 8.05 times the odds of bleeding than patients with lesions smaller than 2 cm (OR = 8.05, P < 0.001). However, extensive hepatectomy such as trisegmentectomy or total left hepatectomy did not confer increased risk of bleeding.
There were insufficient occurrences of superficial, deep, and wound dehiscence for regression modeling. We therefore only performed univariate analysis for organ space infections and any SSIs. There were 78 (8.1%) organ space infections (Supplementary Table 4). Most of the variables included in the model were not predictive of infections; however Hispanic ethnicity (OR = 3.97, P < 0.001) and presence of more than 8 metastases (OR = 2.16, P = 0.013) increased the risk of organ space SSI. These findings were similar for any SSI (Supplementary Table 5). We therefore performed a multivariable ana
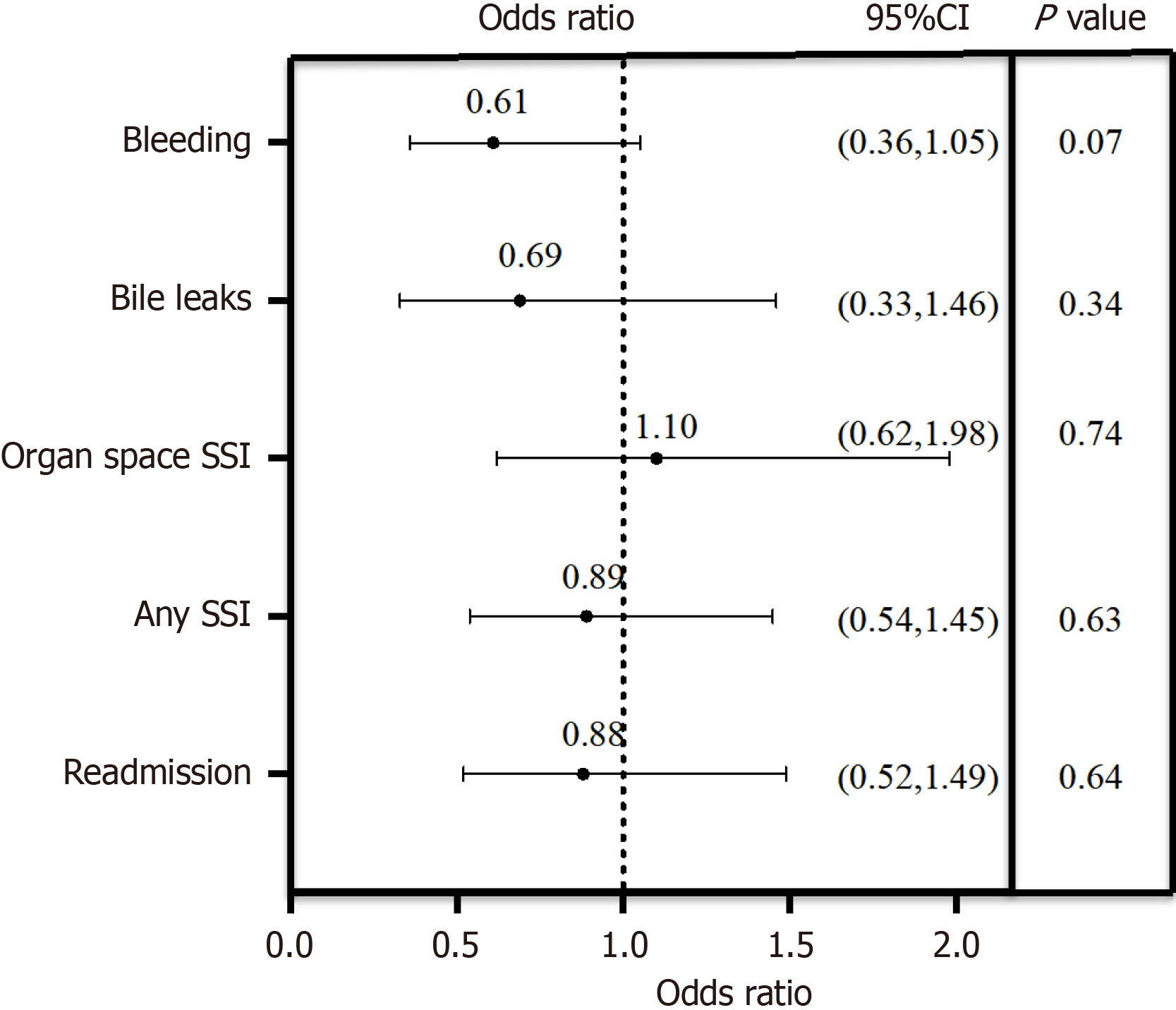
On multivariate logistic regression analysis, there were no differences in adverse outcomes between patients undergoing intraoperative ablation and those undergoing hepatectomy alone (Figure 1). After controlling for potential confounding covariates that were identified in the univariate analysis, we determined the odds of readmission (OR = 0.88, P = 0.64), bile leaks (OR = 0.69, P = 0.34), organ space SSIs (OR = 1.10, P = 0.74), or any SSIs (OR = 0.89, P = 0.63), which were not significantly different between the two groups. Although ablation was associated with significant bleeding on univariate analysis, after controlling for the predictive covariates in the multivariate analysis it was no longer predictive of bleeding (OR = 0.61, P = 0.07).
Management of liver malignancies is complex and is primarily driven by the pathology, size, location, and number of tumors. The surgical approach to liver malignancies has changed dramatically over the last few decades with the introduction and utilization of new technology, which has facilitated operative planning and improved surgical precision[10]. This has resulted in a marked decline in many perioperative complications[11]. Several adjunct modalities to surgery have been utilized to improve surgical outcomes, including portal vein embolization, stereotactic body radiation therapy, and thermal/chemical ablation. Ablation utilization has been rising over time for both primary liver and biliary tumors as well as metastatic colorectal disease[12-15]. The reported incidence of significant complications from ablation modalities ranges between 6%-11%[15,16]. Most studies report bleeding, abdominal infection, biliary track damage, portal vein thrombosis, and pleural effusion/pneumothorax as the most frequent complications, causing hesitancy in widespread utilization of ablation[5,15,17]. Despite this, the use of ablation therapy has been applied to patients with a variety of disease pathologies, including metastatic NETs to the liver.
Treatment algorithms for hepatic NETs metastases have changed over time. Non-operative management with che
Given the evidence for aggressive surgical control of metastatic NETs, we set out to explore the perioperative risks of ablation in conjunction with hepatic resection. Several studies report the overall morbidity of ablation used concurrently with resection to be between 20% and 42%[2,18]. However, these studies were limited by insufficient sample sizes for multivariate analysis as well as use of composite outcomes; as a result, it is difficult to determine whether there is an association between ablation therapy and specific adverse outcomes. Studies describing ablation monotherapy in patients with unresectable NETs hepatic metastasis were similarly restricted by sample size and use of composite outcomes, as well as being limited by lack of generalizability[19,20]. In our study we report the frequencies of each adverse outcome and demonstrate that intra-operative ablation does not increase risk of common complications when compared to hepatectomy alone.
This study represents the largest sample of patients to date with hepatic NET metastases treated simultaneously with ablation and surgery. Of the 966 patients included in the study, 298 (30.8%) underwent ablation concurrently with a resection. In principle, ablation is combined with surgical resection in patients with bilateral tumors, for tumors deep in the liver parenchyma, or when resections of many individual lesions would significantly decrease the future liver remnant[2]. We therefore expected patients in the ablation group to have large tumors, require extensive hepatectomy, or have greater number of lesions. Although the ablation group had fewer extensive hepatic resections (total lobectomy or trisemgmentectomies), there were more patients with > 8 tumors and with greater number of smaller lesions. This was unsurprising, as previous studies reported higher efficacy of ablation for smaller lesions[18,20,21].
The rate of significant bleeding in our study was 15.5%. The reported rates in the literature vary widely between 1.1% and 25%, likely due to the subjective nature of “significant bleeding”[22,23]. Unsurprisingly, minimally invasive ap
Biliary complications and bile leaks occur in 3% to 12% of hepatic resections, and tend to confer significant morbidity due to need for percutaneous drainage, endoscopic retrograde cholangiopancreatography, or surgical intervention[26-28]. In our study there were 55 (5.7%) bile leaks occurrences, with no significant difference between patients who had ablation and those who underwent hepatectomy alone. Even after controlling for possible predictive covariates in the multivariate analysis, ablation did not significantly increase risk of bile leaks. Capussotti et al[26] reported specific re
There were 177 (12.1%) patients who developed an SSI in our study, the majority of which were organ space infections (8.1%). In patients undergoing percutaneous ablation alone without surgery the frequency of abscesses ranges between 1% and 4%[29-32]. Risk factors most predictive of abscess are transarterial chemoembolization and biliary abnormalities predisposing to ascending infections[33-35]. In our study we did not include pre-operative stent in our analysis. How
There are several limitations in this study that should be addressed. This is a cross-sectional retrospective study and is inferior to randomized control trials. Limitations of ACS-NSQIP data that are widely recognized and frequently described apply to this study and include scope of available variables, no mechanism of external data validation, and short-term 30-day outcomes[36,37]. For example, this study could be enhanced if the primary source of the metastatic NET was avai
Liver metastases from NETs have an indolent course, often remaining undetected until hormonal oversecretion or symptoms from mass effect. Surgical resection remains the best therapy for both survival benefit and symptom control when complete irradiation is achieved. Ablation therapies are efficacious adjuncts to accomplish this goal. In this study we demonstrate that ablation is safe in the perioperative period and does not increase the risk of infection, bleeding, or bile leak in patients undergoing hepatic resection for neuroendocrine liver metastasis. Surgeons should not fear utilizing ablation concurrently with resection, even when attempting complete resection.
Resection of hepatic metastasis from neuroendocrine tumors (NETs) improves quality of life and prolongs 5-year survival. Ablation can be utilized with surgery to achieve complete resection. Although several studies report long-term outcomes for patients undergoing ablation, none have explored perioperative effects of ablation in patients with me
Currently, there is no literature on whether perioperative ablation increases risk of adverse outcomes in patients un
Our objective was to determine if intra-operative ablation during hepatectomy increases risk of adverse outcomes such as surgical site infections (SSIs), bleeding, and bile leak.
We performed a retrospective analysis of the hepatectomy module of the National Surgical Quality Improvement Pro
Of the 966 patients included in the study, 298 (30.9%) underwent ablation during hepatectomy. There were 78 (11.7%) patients with SSIs in the hepatectomy alone group and 39 (13.1%) patients with a SSIs in the hepatectomy with ablation group. Bile leak occurred in 41 (6.2%) and 14 (4.8%) patients in the two groups, respectively; bleeding occurred in 117 (17.5%) and 33 (11.1%), respectively. After controlling for confounding variables, ablation did not increase risk of SSI (P = 0.63), bile leak (P = 0.34) or bleeding (P = 0.07) when compared to patients undergoing resection alone on multivariate analysis.
Ablation is safe in the perioperative period and does not increase the risk of infection, bleeding, or bile leak in patients undergoing hepatic resection for neuroendocrine liver metastasis.
Future studies should focus on whether size, number, or relationship to vascular structures influences perioperative outcomes in this cohort of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yuan HJ, China S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | Proye C. Natural history of liver metastasis of gastroenteropancreatic neuroendocrine tumors: place for chemoembolization. World J Surg. 2001;25:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, Pitt HA. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776-83; discussion 783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Vogl TJ, Naguib NN, Zangos S, Eichler K, Hedayati A, Nour-Eldin NE. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol. 2009;72:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, Loyer E, Vallone P, Fiore F, Scordino F, De Rosa V, Orlando R, Pignata S, Daniele B, Izzo F. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Dong J, Yang S, Zeng J, Cai S, Ji W, Duan W, Zhang A, Ren W, Xu Y, Tan J, Bu X, Zhang N, Wang X, Meng X, Jiang K, Gu W, Huang Z. Precision in liver surgery. Semin Liver Dis. 2013;33:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Stroever SJ, Ostapenko AD, Casasanta MJ. Racial Disparities and Upward Trend in Bowel Preparation for Elective Colectomy in the National Surgical Quality Improvement Program Procedure Targeted Dataset: 2012 to 2018. Ann Surg Open. 2021;2:e092. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, Hatanaka T, Takawa M, Nagamatsu H, Imai Y. Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | McDermott S, Gervais DA. Radiofrequency ablation of liver tumors. Semin Intervent Radiol. 2013;30:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Dimick JB, Wainess RM, Cowan JA, Upchurch GR Jr, Knol JA, Colletti LM. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Massarweh NN, Park JO, Farjah F, Yeung RS, Symons RG, Vaughan TL, Baldwin LM, Flum DR. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2010;210:441-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Mayo SC, Heckman JE, Shore AD, Nathan H, Parikh AA, Bridges JF, Anders RA, Anaya DA, Becker NS, Pawlik TM. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol. 2014;110:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Park EK, Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim JW, Cho CK. A comparison between surgical resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Ann Surg Treat Res. 2014;87:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 359] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 17. | Schullian P, Johnston E, Laimer G, Putzer D, Eberle G, Amann A, Effenberger M, Maglione M, Freund MC, Loizides A, Bale R. Frequency and risk factors for major complications after stereotactic radiofrequency ablation of liver tumors in 1235 ablation sessions: a 15-year experience. Eur Radiol. 2021;31:3042-3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Atwell TD, Charboneau JW, Que FG, Rubin J, Lewis BD, Nagorney DM, Callstrom MR, Farrell MA, Pitot HC, Hobday TJ. Treatment of neuroendocrine cancer metastatic to the liver: the role of ablative techniques. Cardiovasc Intervent Radiol. 2005;28:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Gut P. Liver metastases in gastroenteropancreatic neuroendocrine tumours - treatment methods. Prz Gastroenterol. 2020;15:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Stiles ZE, Behrman SW, Glazer ES, Deneve JL, Dong L, Wan JY, Dickson PV. Predictors and implications of unplanned conversion during minimally invasive hepatectomy: an analysis of the ACS-NSQIP database. HPB (Oxford). 2017;19:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB (Oxford). 2014;16:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Hatzidakis A, Zervakis N, Krokidis M. Fatal arterial hemorrhage after microwave ablation of multiple liver metastases: The lessons learned. Interv Med Appl Sci. 2013;5:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Shi QM, Xue C, He YT, Hu XB, Yu ZJ. Massive abdominal hemorrhage after radiofrequency ablation of recurrent hepatocellular carcinoma with successful hemostasis achieved through transarterial embolization: a case report. J Int Med Res. 2020;48:300060519898012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Capussotti L, Ferrero A, Viganò L, Sgotto E, Muratore A, Polastri R. Bile leakage and liver resection: Where is the risk? Arch Surg. 2006;141:690-4; discussion 695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Reed DN Jr, Vitale GC, Wrightson WR, Edwards M, McMasters K. Decreasing mortality of bile leaks after elective hepatic surgery. Am J Surg. 2003;185:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Tsao JI, Loftus JP, Nagorney DM, Adson MA, Ilstrup DM. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Ann Surg. 1994;220:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Karavokyros I, Orfanos S, Angelou A, Meropouli A, Schizas D, Griniatsos J, Pikoulis E. Incidence and Risk Factors for Organ/Space Infection after Radiofrequency-Assisted Hepatectomy or Ablation of Liver Tumors in a Single Center: More than Meets the Eye. Front Surg. 2017;4:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Ramanathan R, Borrebach J, Tohme S, Tsung A. Preoperative Biliary Drainage Is Associated with Increased Complications After Liver Resection for Proximal Cholangiocarcinoma. J Gastrointest Surg. 2018;22:1950-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Su XF, Li N, Chen XF, Zhang L, Yan M. Incidence and Risk Factors for Liver Abscess After Thermal Ablation of Liver Neoplasm. Hepat Mon. 2016;16:e34588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, Lim JH, Paik SW, Yoo BC, Choi MS, Kim S. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Odisio BC, Richter M, Aloia TA, Conrad C, Ahrar K, Gupta S, Vauthey JN, Huang SY. Use of Prophylactic Antibiotics to Prevent Abscess Formation Following Hepatic Ablation in Patients with Prior Enterobiliary Manipulation. J Gastrointest Surg. 2016;20:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Iida H, Aihara T, Ikuta S, Yamanaka N. Risk of abscess formation after liver tumor radiofrequency ablation: a review of 8 cases wtih a history of enterobiliary anastomosis. Hepatogastroenterology. 2014;61:1867-1870. [PubMed] |
| 35. | Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Tzeng CW, Cooper AB, Vauthey JN, Curley SA, Aloia TA. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford). 2014;16:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Fagenson AM, Gleeson EM, Pitt HA, Lau KN. Minimally Invasive Hepatectomy in North America: Laparoscopic Versus Robotic. J Gastrointest Surg. 2021;25:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |