Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.689
Peer-review started: December 19, 2023
First decision: January 4, 2024
Revised: January 17, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: March 27, 2024
Processing time: 93 Days and 21.9 Hours
Radical surgery combined with systemic chemotherapy offers the possibility of long-term survival or even cure for patients with pancreatic ductal adenocarcinoma (PDAC), although tumor recurrence, especially locally, still inhibits the treatment efficacy. The TRIANGLE technique was introduced as an extended dissection procedure to improve the R0 resection rate of borderline resectable or locally advanced PDAC. However, there was a lack of studies concerning postoperative complications and long-term outcomes of this procedure on patients with resectable PDAC.
To compare the prognosis and postoperative morbidities between standard pancreaticoduodenectomy (PD) and the TRIANGLE technique for resectable PDAC.
Patients with resectable PDAC eligible for PD from our hospital between June 2018 and December 2021 were enrolled in this retrospective cohort study. All the patients were divided into PDstandard and PDTRIANGLE groups according to the surgical procedure. Baseline characteristics, surgical data, and postoperative morbidities were recorded. All of the patients were followed up, and the date and location of tumor recurrence, and death were recorded. The Kaplan-Meier method and log-rank test were used for the survival analysis.
There were 93 patients included in the study and 37 underwent the TRIANGLE technique. Duration of operation was longer in the PDTRIANGLE group compared with the PDstandard group [440 (410-480) min vs 320 (265-427) min] (P = 0.001). Intraoperative blood loss [700 (500-1200) mL vs 500 (300-800) mL] (P = 0.009) and blood transfusion [975 (0-1250) mL vs 400 (0-800) mL] (P = 0.009) were higher in the PDTRIANGLE group. There was a higher incidence of surgical site infection (43.2% vs 12.5%) (P = 0.001) and postoperative diarrhea (54.1% vs 12.5%) (P = 0.001) in the PDTRIANGLE group. The rates of R0 resection and local recurrence, overall survival, and disease-free survival did not differ significantly between the two groups.
The TRIANGLE technique is safe, with acceptable postoperative morbidities compared with standardized PD, but it does not improve prognosis for patients with resectable PDAC.
Core Tip: We compared the prognosis and postoperative morbidities between standard pancreaticoduodenectomy and the TRIANGLE technique for resectable pancreatic ductal adenocarcinoma (PDAC). The TRIANGLE technique was safe and feasible, with acceptable postoperative complications, and improved the extent of radical resection. However, longer duration of operation, more intraoperative blood loss and higher incidence of postoperative diarrhea indicated that TRIANGLE technique was a more aggressive procedure. Local recurrence, disease-free survival and overall survival did not differ between the two groups. These results suggest that the TRIANGLE technique is not necessary for all resectable PDAC patients.
- Citation: Hang HX, Cai ZH, Yang YF, Fu X, Qiu YD, Cheng H. Comparison of prognosis and postoperative morbidities between standard pancreaticoduodenectomy and the TRIANGLE technique for resectable pancreatic ductal adenocarcinoma. World J Gastrointest Surg 2024; 16(3): 689-699
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/689.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.689
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death, and by 2030, it is expected to become the second most common cause in western countries[1,2]. Currently, the only hope of long-term survival and even cure for PDAC patients is radical resection in combination with systemic chemotherapy[3]. However, despite the continuous advance of surgical techniques and chemotherapy regimens, the survival of PDAC patients is still not optimistic, with a 5-year survival rate of only approximately 10%[4]. Approximately 70% of patients resected will suffer from recurrence within 2 years[5], and 25%-45% of patients with PDAC experience local recurrence following surgical resection[6,7]. To date, there have been a number of prognostic factors identified for recurrence and survival, and positive resection margin has been identified as one of the major factors in terms of local and overall recurrence[8]. Therefore, standardized concepts of radical resection should aim to achieve complete tumor clearance and prevent local recurrence. However, perineural invasion is a distinctive feature of PDAC, which is different from other tumors. PDAC in the pancreatic head may spread to autonomous nerves located alongside the celiac trunk (CT) and superior mesenteric artery (SMA), and consequently, complete resection is compromised at the margins of the medial and posterior resections[9]. Currently, the conventional surgical protocol does not include systematic removal of neural and lymph tissue that is suspected of being infiltrated by tumor but lies between the SMA, CT, and portal vein (PV).
In 2017, a new surgical technique was introduced by a team at Heidelberg University, named the TRIANGLE operation[10]. By using this technique, the tumor, including its associated lymphatic and perineural extensions along the vascular structures, can be completely and radically removed, and arterial resection and reconstruction are not required, which is morbidity-prone. As expected, for patients with initially locally advanced PDAC with stable disease after neoadjuvant therapy, radical surgery was performed and nearly half of them achieved R0 resection. However, there was a lack of studies concerning postoperative complications and long-term oncological outcome of the TRIANGLE technique for patients with resectable PDAC.
Hence, the purpose of the present study was to assess the postoperative morbidities as well as oncological outcomes of the TRIANGLE technique for PDAC patients received pancreaticoduodenectomy (PD) in a single center, and discuss the necessity of the technique for all resectable PDAC patients.
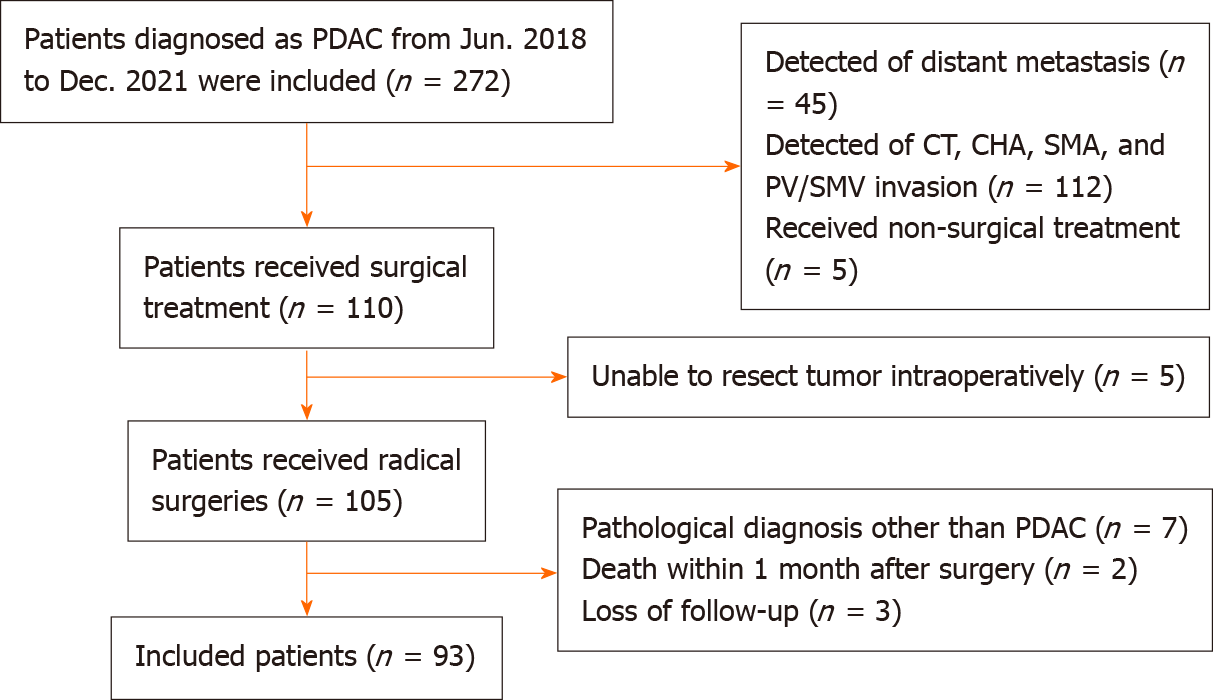
Patients with PDAC who received radical surgery from June 2018 to December 2021 in the Division of Pancreatic Surgery, Department of General Surgery, Nanjing Drum Tower Hospital were analyzed. A flowchart of patient enrollment is shown in Figure 1. This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital (No. 2021-437-01). All procedures were in accordance with the Helsinki Declaration and informed consent of patients was exempted because of the retrospective nature of the study.
Inclusion criteria: (1) PDAC patients who received PD from June 2018 to December 2021 in the Division of Pancreatic Surgery, Department of General Surgery, Nanjing Drum Tower Hospital; (2) no other active cancer; (3) resectable PDAC based on National Comprehensive Cancer Network (NCCN) guidelines; (4) achieved radical resection; (5) confirmation of the diagnosis of PDAC based on histopathological observations after surgery; and (6) completeness of clinical data and survival information.
Exclusion criteria: (1) Received neoadjuvant therapy; (2) multiple organ resection; (3) postoperative pathological examination confirmed other than PDAC; (4) loss of postoperative survival data; and (5) died within 1 months postoperatively.
All the patients enrolled in the study were divided into the PDstandard and PDTRIANGLE groups according to the surgical procedure.
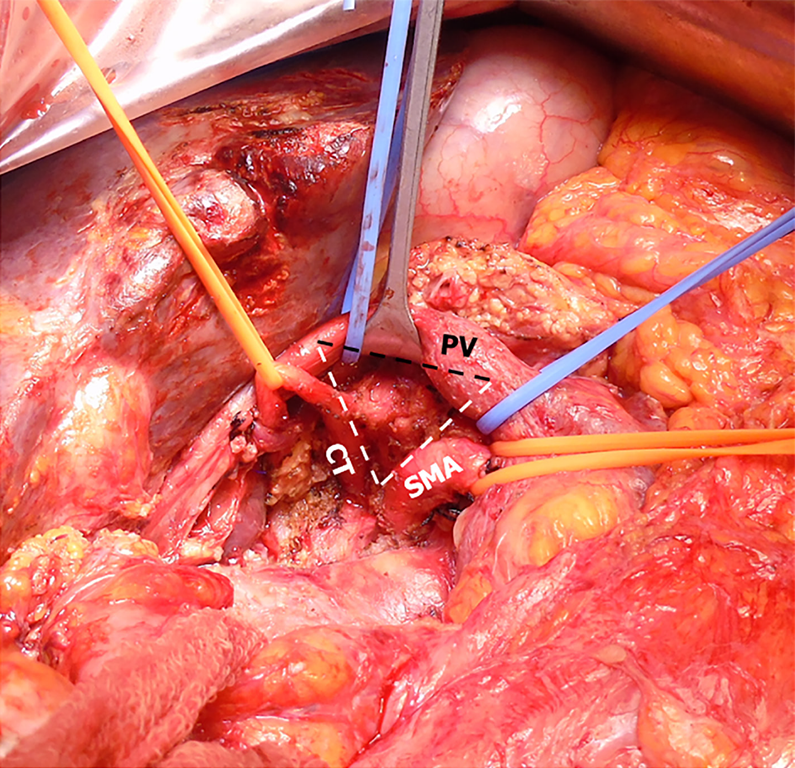
Patients in the PDstandard group received standardized and conventional PD. Patients in the PDTRIANGLE group received the TRIANGLE technique and the major surgical procedure was the same as that described by the Heidelberg University team[9,10]. Laparotomy and exploration of the abdominal cavity were performed to rule out distant metastasis. An extended Kocher’s maneuver completed the mobilization of the pancreatic head, the origins of CT and SMA were palpated and displayed, and if necessary, frozen sections were taken to confirm the tumor resectability. Dissection of the hepatoduodenal ligament was completed and the left, right and proper hepatic arteries were cleared. Lymphadenectomy was subsequently extended along the common hepatic artery towards the CT. Lymphatic and neural tissue that was situated in the TRIANGLE area, which was surrounded by the CT, SMA, and PV, was dissected (Figure 2). During the reconstructive anastomotic procedure, a modified Blumgart technique and a continuously sutured end-to-side method were performed for pancreaticojejunostomy and cholangiojejunostomy respectively, and gastrojejunostomy was performed with or without pylorus preservation.
Prophylactic antibiotics were administered 30 min before incision and continued for 48 h postoperatively. The nutritional risk of every patient was assessed after admission and nutritional support was provided for patients with moderate or severe malnutrition before surgery. After surgery, the diagnosis of postoperative pancreatic fistula (POPF) was based on the amylase measurements and abdominal infection was documented by bacterial culture of drainage fluid, which were both performed 1 d, 3 d, 5 d, and 7 d postoperatively. Drainage tubes were removed early when there was absence of POPF, abdominal infection and intra-abdominal abscess based on postoperative contrast-enhanced computed tomography (CECT).
Age, gender, body mass index (BMI) and nutrition risk screening 2002 score (NRS2002) were recorded as the baseline characteristics. Preoperative concentrations of albumin, hemoglobin and carbohydrate antigen 19-9 (CA19-9) were extracted from the medical laboratory database. Tumor size was reassessed from preoperative CECT images. Operative parameters included duration of operation, intraoperative blood loss and transfusion. All postoperative complications were recorded and graded according to the Clavien-Dindo classification[11]. Additionally, POPF[12], bile leakage[13], delayed gastric emptying (DGE)[14], surgical site infection (SSI)[15], post-pancreatectomy hemorrhage (PPH)[16], and chyle leakage[17] were diagnosed based on the International Study Group of Pancreatic Surgery definitions. Diarrhea was defined as a condition requiring opioid antidiarrheal drug for ≥ 6 months after surgery[18].
Short term oncological outcomes were evaluated based on the pathological work-up, including tumor, node, and metastasis (TNM) staging, which were based on the American Joint Committee on Cancer manual (8th edition), tumor differentiation, resection margin status, and number of extracted lymph nodes. R0 was defined as a distance of at least 1 mm. Structured follow-up protocol included routine clinical status assessment and evaluation of CA19-9 serum level every 3 months, CECT of the abdomen and thorax every 6 months after the operation, and positron emission tomography was also an important supplemental tool if necessary. Overall survival (OS) was defined as the time from the date of surgery to either death from any cause or last follow-up. Disease-free survival (DFS) was defined as the time from the date of resection to the date of tumor recurrence showed by radiological or clinical evidence. Recurrence pattern was recorded including local and distant recurrence. Deadline for follow-up was 31 December, 2022.
SPSS 27.0 software (IBM Corp., Armonk, NY, United States) and PRISM 8 (GraphPad Software, La Jolla, CA, United States) were used for statistical analyses and graph preparation, respectively. Quantitative variables were summarized as mean ± SD or median (interquartile range, IQR) as appropriate and independent t test or Mann-Whitney U test were used for the comparison. Categorical parameters were presented as absolute (frequencies) and statistical analysis was examined with χ2 or Fisher’s exact test. Survival was calculated with the Kaplan-Meier method and the difference of curve pairs was compared using the log-rank test. P < 0.05 based on two-sided testing was considered statistically significant.
We identified 93 patients who underwent PD for PDAC during the study period, 50 male (53.7%) and 43 female (46.3%), with an average age of 65.4 years. In total, 37 patients received PD with clearance of the TRIANGLE area, while the rest received standardized PD. There was no significant difference in age, BMI, NRS2002 score, tumor size, preoperative jaundice, and concentration of CA19-9, albumin, and hemoglobin between the two groups. Demographic and clinical characteristics of the 93 patients are shown in Table 1.
| Characteristics | PDstandard group | PDTRIANGLE group | P value |
| (n = 56) | (n = 37) | ||
| Age (yr) | 65.7 ± 10.1 | 64.2 ± 10.8 | 0.503 |
| Sex (M/F) | 33/23 | 17/20 | 0.219 |
| BMI (kg/m2) | 23.1 ± 3.3 | 23.8 ± 2.9 | 0.250 |
| NRS2002 score, median (IQR) | 4 (3-5) | 4 (3-5) | 0.888 |
| Jaundice, n (%) | 23 (41.1) | 20 (54.1) | 0.219 |
| Preoperative biliary drainage, n (%) | 12 (21.4) | 9 (24.3) | 0.744 |
| Tumor size (cm) | 2.6 ± 0.8 | 2.5 ± 1.1 | 0.604 |
| CA19-9 (U/mL), median (IQR) | 141.0 (25.4-370.5) | 112.6 (16.8-355.9) | 0.580 |
| Albumin (g/L) | 38.4 ± 3.1 | 39.2 ± 2.8 | 0.267 |
| Hb (g/L) | 122.6 ± 14.9 | 127.4 ± 12.1 | 0.110 |
Duration of operation was longer in the PDTRIANGLE group compared with the PDstandard group [440 (410-480) min vs 320 (265-427) min] (P = 0.001). Intraoperative blood loss was higher in the PDTRIANGLE group [700 (500-1200) mL vs 500 (300-800) mL] (P = 0.009) and intraoperative blood transfusion was more common compared with the PDstandard group [975 (0-1250) mL vs 400 (0-800) mL] (P = 0.009).
An overview of postoperative complications is depicted in Table 2 and no postoperative mortality occurred. SSI occurred in 12.5% of the PDstandard group and 43.2% of the PDTRIANGLE group (P = 0.001). There were no significant differences in the occurrence of POPF (PDstandardvs PDTRIANGLE: 17.8% vs 18.9%) (P = 0.114), more specifically, clinically relevant POPF (CR-POPF) (PDstandardvs PDTRIANGLE: 16.1% vs 13.5%) (P = 0.736), bile leakage (PDstandardvs PDTRIANGLE: 10.7% vs 5.4%) (P = 0.470), DGE (PDstandardvs PDTRIANGLE: 21.4% vs 10.8%) (P = 0.263), PPH (PDstandardvs PDTRIANGLE: 10.7% vs 2.7%) (P = 0.237), or chyme leakage (PDstandardvs PDTRIANGLE: 8.9% vs 13.5%) (P = 0.485). Importantly, 54.1% of the patients who underwent TRIANGLE area clearance developed postoperative diarrhea compared with 12.5% in the PDstandard group (P = 0.001).
| Variables | PDstandard group | PDTRIANGLE group | P value |
| (n = 56) | (n = 37) | ||
| Surgical data | |||
| Duration of operation (min), median (IQR) | 320 (265-427) | 440 (410-480) | 0.0011 |
| Intraoperative blood loss (mL), median (IQR) | 500 (300-800) | 700 (500-1200) | 0.0091 |
| Volume of blood transfusion (mL), median (IQR) | 400 (0-800) | 975 (0-1250) | 0.0091 |
| Number of complications, n (%) | 0.0071 | ||
| CDC II | 32 (57.1) | 22 (59.4) | |
| CDC IIIa | 3 (5.4) | 3 (8.1) | |
| CDC IIIb | 0 (0) | 4 (10.8) | |
| CDC Ⅳa | 0 (0) | 1 (2.7) | |
| POPF, n (%) | 19 (17.8) | 7 (18.9) | 0.114 |
| CR-POPF, n (%) | 9 (16.1) | 5 (13.5) | 0.736 |
| Bile leakage, n (%) | 6 (10.7) | 2 (5.4) | 0.470 |
| DGE, n (%) | 12 (21.4) | 4 (10.8) | 0.263 |
| SSI, n (%) | 7 (12.5) | 16 (43.2) | 0.0011 |
| PPH, n (%) | 6 (10.7) | 1 (2.7) | 0.237 |
| Chyme leakage, n (%) | 5 (8.9) | 5 (13.5) | 0.485 |
| Postoperative diarrhea, n (%) | 7 (12.5) | 20 (54.1) | 0.0011 |
| Postoperative length of stay (d) | 18 (14-24) | 18 (14-25) | 0.765 |
Short-term oncological outcomes were assessed based on the pathological reports (Table 3). There were no significant differences in TNM stage, tumor differentiation and status of resection margin. However, more lymph nodes were examined after clearance of the TRIANGLE area [PDstandard: 14 (9-20) vs PDTRIANGLE: 19 (13-24)] (P = 0.010). More importantly, all the resected soft tissues within the TRIANGLE area were not involved by tumor cells according to the pathological reports.
| Variables | PDstandard group | PDTRIANGLE group | P value |
| (n = 56) | (n = 37) | ||
| T stage | 0.600 | ||
| 1 | 9 (16.1) | 6 (16.2) | |
| 2 | 40 (71.4) | 24 (64.7) | |
| 3 | 7 (12.5) | 7 (18.9) | |
| 4 | 0 (0) | 0 (0) | |
| N stage | 0.581 | ||
| 0 | 29 (51.7) | 18 (48.7) | |
| 1 | 14 (25.0) | 17 (45.9) | |
| 2 | 13 (23.3) | 2 (5.4) | |
| M stage | 1.000 | ||
| 0 | 55 (98.3) | 36 (97.3) | |
| 1 | 1 (1.7) | 1 (2.7) | |
| TNM stage | 0.341 | ||
| Ⅰ | 24 (42.8) | 17 (45.9) | |
| II | 19 (33.9) | 17 (45.9) | |
| III | 10 (17.8) | 2 (5.4) | |
| IV | 3 (5.5) | 1 (2.7) | |
| Tumor differentiation | 0.752 | ||
| Poor | 21 (37.5) | 17 (45.9) | |
| Moderate | 35 (62.5) | 17 (45.9) | |
| Well | 0 (0) | 3 (8.2) | |
| Resection margin status | 0.453 | ||
| R0 | 40 (71.4) | 29 (78.3) | |
| R1 | 16 (28.6) | 8 (21.7) | |
| No. of examined lymph nodes | 14 (9-20) | 19 (13-24) | 0.0101 |
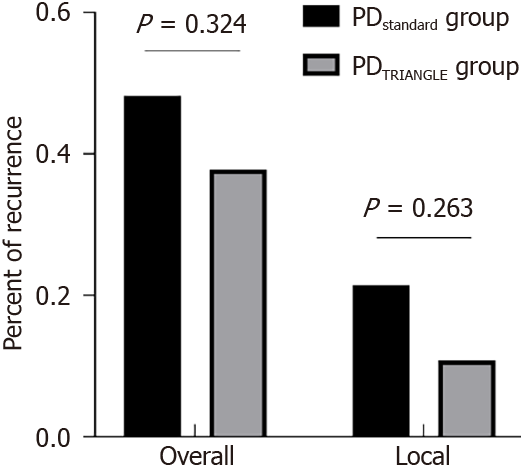
We also compared the long-term oncological outcomes of patients in the different groups (Table 4). The median observation time for patients in the PDstandard group was 33.0 months (range 10.0-54.0 months) and 21.0 months (range 9.0-51.0 months) for patients in the PDTRIANGLE group, but the difference was not significant (P = 0.052). The overall recurrence rate in the PDstandard group was higher than in the PDTRIANGLE group, although the difference was not significant (P = 0.324) (Figure 3). The recurrence site was further analyzed. The rate of local recurrence also showed no significant difference (PDstandardvs PDTRIANGLE: 21.4% vs 10.8%) (P = 0.263) (Figure 3).
| Variables | PDstandard group | PDTRIANGLE group | P value |
| (n = 56) | (n = 37) | ||
| Tumor recurrence, n (%) | 27 (48.2) | 14 (37.8) | 0.324 |
| Local recurrence, n (%) | 12 (21.4) | 4 (10.8) | 0.263 |
| Follow-up duration (months) | |||
| Median | 33 | 21 | 0.052 |
| IQR | 10.0-54.0 | 9.0-51.0 | |
| Adjuvant treatment, n (%) | 47 (83.9) | 29 (78.4) | 0.654 |
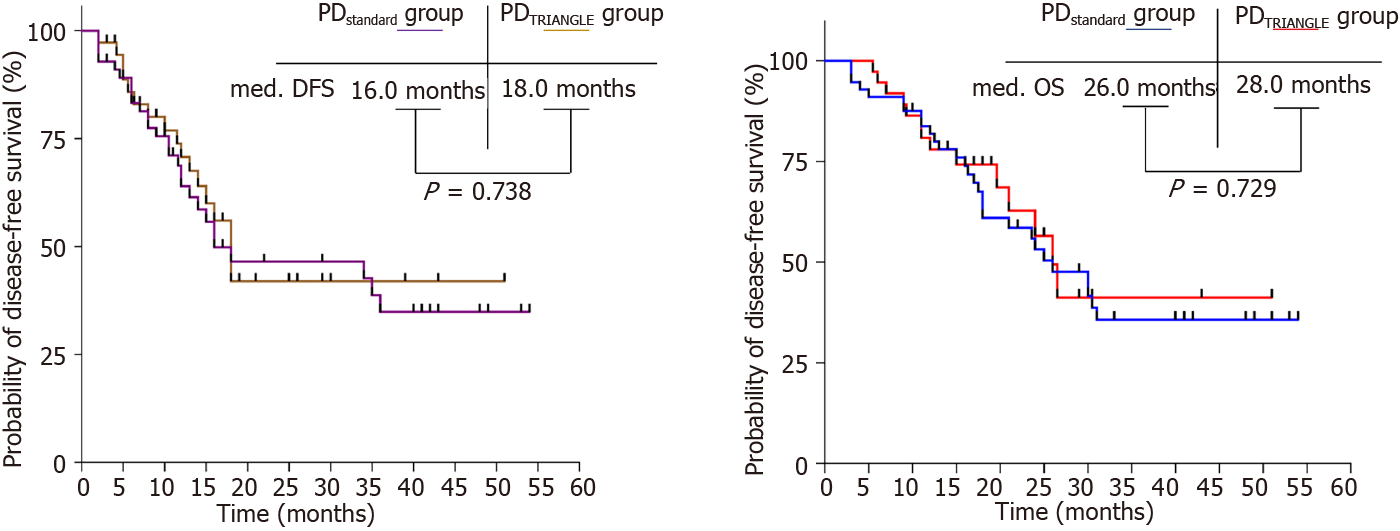
The median DFS of patients in the PDstandard and PDTRIANGLE groups was 16.0 and 18.0 mo, respectively (P = 0.738) (Figure 4A). The median OS of patients in the PDstandard and PDTRIANGLE groups was 26.0 and 28.0 mo, respectively (P = 0.729) (Figure 4B). Based on these results, the TRIANGLE technique can not improve the prognosis for patients with resectable PDAC.
This retrospective study from a single center demonstrated that the TRIANGLE technique was a safe and feasible procedure, with acceptable postoperative complications, for improving the extent of radical resection, compared with standardized PD. However, longer duration of operation, more intraoperative blood loss and higher incidence of postoperative diarrhea in the PDTRIANGLE group indicated that the TRIANGLE technique was a more aggressive procedure. From the perspective of long-term outcome, local recurrence, DFS and OS did not differ between the two groups. The results suggest that routine clearance of the TRIANGLE area was not necessary for all resectable PDAC patients.
Local recurrence has been described as an important pattern of PDAC recurrence in previous studies and positive resection margin was proven to be a high-risk factor. Medial margin around the CT and SMA was most easily involved due to the disperse growth pattern and high neural affinity of PDAC tumor cells[19,20]. To break this dilemma and improve the overall effectiveness of surgical treatment, the TRIANGLE technique was introduced in 2017. Radical clearance of all neural and soft tissues, and lymph nodes that drain the pancreatic head between the CT, SMA, and PV is the primary goal[21,22]. Hackert et al[10] reported that six of 15 patients with locally advanced PDAC achieved R0 resection after neoadjuvant therapy combined with the TRIANGLE operation. Also, no perioperative deaths occurred and 70% of the patients functioned well after discharge, which supports the surgical and oncological outcome of this procedure.
It was noteworthy that no death occurred in patients receiving PD in the PDstandard or PDTRIANGLE group. Likewise, incidence of POPF, bile leakage, DGE and PPH were comparable between the two groups, which indicated the safety of the TRIANGLE technique. Contrary to the initial assumption that extended lymphatic tissues dissection in the TRIANGLE area may increase the rate of chyme leakage, the incidence did not differ in the two groups, in accordance with the results of Klotz et al[21]. However, the rate of SSI in the PDTRIANGLE group was almost three times greater than that in the PDstandard group, which can be explained by the prolonged duration of operation and significantly increased intraoperative blood loss in the PDTRIANGLE group[23-25]. As expected, postoperative diarrhea was more common in patients who underwent the TRIANGLE operation and most of them needed opioids to relieve symptoms compared with the PDstandard group, which was consistent with the results of another study[26]. More importantly, extended neural plexus dissection may sometimes result in intractable diarrhea. Some patients suffer from severe malnutrition, so they are unable to receive postoperative chemotherapy in a timely manner, which affects the overall effect of treatment[27,28]. In response to this problem, we conducted a family nutrition plan to guide patients to provide nutritional support at home for early recovery[29].
In addition to assessing the safety of the TRIANGLE technique, oncological outcomes were another important aspect that should be considered. Zhai et al[30] reported that nine patients with borderline resectable PDAC underwent theTRIANGLE technique in total pancreatectomy, with complete arterial skeletonization and dissection of soft tissues in the TRIANGLE area, and eight patients achieved R0 resection. However, in our study, the rate of R0 resection in the PDTRIANGLE group was higher than in the PDstandard group, although the difference was not significant. The fact that all of the recruited patients had resectable PDAC and the tumor invasion into the TRIANGLE area was less frequent than that in patients with borderline resectable or locally advanced PDAC can be used to explain the outcome. Besides, as the inevitable product of extended radical resection, more lymph nodes were examined in PDTRIANGLE group. While extended lymphadenectomy did not better the oncological outcome as showed in a multi-center prospective randomized controlled trial[31]. From the perspective of long-term efficacy, there were no significant differences concerning recurrence pattern, DFS and OS between the two groups. This indirectly reflects that routine TRIANGLE technique for resectable patients may not reduce the rate of local recurrence and improve prognosis. A multi-center randomized clinical trial from Korea suggested that extended lymphadenectomy with dissection of the nerve plexus around the SMA and CT does not provide a significant survival benefit compared with standard resection in pancreatic head cancer[32]. In another multi-center randomized clinical trial from China, extended pancreatoduodenectomy also did not significantly improve OS[33]. However, it is still controversial whether extended lymphadenectomy and nerve dissection provide a survival benefit. Indeed, some features of extrapancreatic nerve invasion could be judged from preoperative CECT images, such as increased attenuation or mass formation in the peripancreatic fat space[34]. The minimum distance from the tumor boundary to the arteries was another efficient indicator of nerve invasion[35]. Since the nerves and lymph nodes of the pancreatic head and neck usually flow back to the TRIANGLE area, if the aforementioned signs are shown on the CECT images, the TRIANGLE operation may be beneficial to patients with resectable PDAC.
There were some limitations to the present study. First, it was a single-center study and the number of patients was limited. More studies from high-volume centers are needed to support our results. Second, all the patients had resectable PDAC based on the NCCN guidelines, and invasion of the TRIANGLE area was less common compared with that in patients with borderline resectable or locally advanced PDAC. We are actively collecting the data from borderline resectable or locally advanced PDAC patients who underwent pancreatectomy after neoadjuvant therapy for further study. Third, postoperative diarrhea was assessed within 6 months after the operation, but the severity and duration were not traceable, and related parameters such as nutrition score, pancreatic exocrine insufficiency score, and quality of life should be included in further studies.
Routine clearance of the TRIANGLE area did not improve the long-term outcome of patients with resectable PDAC. The acceptable postoperative morbidity compared with standardized PD suggests that this procedure is safe and feasible. More high-quality studies are needed to verify the efficacy of this procedure.
Radical surgery combined with systemic chemotherapy offers the possibility of long-term survival for patients with pancreatic ductal adenocarcinoma (PDAC). The TRIANGLE technique was introduced as an extended dissection procedure to improve the R0 resection rate of borderline resectable or locally advanced PDAC. However, there was a lack of studies concerning postoperative complications and long-term outcomes of this procedure on patients with resectable PDAC.
The TRIANGLE technique is more complex and aggressive than conventional pancreaticoduodenectomy (PD). It is still unclear whether the TRIANGLE technique is necessary for all resectable PDAC patients.
To compare the prognosis and postoperative morbidities between standard PD and the TRIANGLE technique for resectable PDAC.
In this retrospective cohort study, patients with resectable PDAC were divided into PDstandard and PDTRIANGLE groups according to the surgical procedure. Baseline characteristics, surgical data, and postoperative morbidities were recorded. All of the patients were followed up, and date and location of tumor recurrence, and death were recorded. The Kaplan-Meier method and log-rank test were used for the survival analysis.
There were 93 patients included in the study and 37 of them underwent the TRIANGLE technique. Duration of operation was longer in the PDTRIANGLE group, meanwhile, intraoperative blood loss and blood transfusion were higher. There was a higher incidence of surgical site infection and postoperative diarrhea in the PDTRIANGLE group. The rates of R0 resection and local recurrence, overall survival, and disease-free survival did not differ significantly between the two groups.
The TRIANGLE technique was a safe and feasible procedure with acceptable postoperative complications in improving the extent of radical resection, although it was a more aggressive procedure compared with standardized PD. However, routine clearance of the TRIANGLE area did not improve the long-term outcomes for patients with resectable PDAC.
The aim of this study was to evaluate the need for the TRIANGLE technique for all resectable PDAC patients by utilizing patient information from our research center, and conducting research from perspectives of postoperative complications and short- and long-term oncological outcomes. We hope that this study can provide valuable insights into the surgical treatment of resectable PDAC patients.
The authors thank all members of the multidisciplinary team treating hepatobiliary and pancreatic tumors at Nanjing Drum Tower Hospital for their guidance in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sato H, Japan S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9962] [Article Influence: 4981.0] [Reference Citation Analysis (2)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5144] [Article Influence: 467.6] [Reference Citation Analysis (0)] |
| 3. | Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 604] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 4. | Halbrook CJ, Lyssiotis CA, Pasca di Magliano M, Maitra A. Pancreatic cancer: Advances and challenges. Cell. 2023;186:1729-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 584] [Article Influence: 292.0] [Reference Citation Analysis (0)] |
| 5. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 491] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 6. | Jones RP, Psarelli EE, Jackson R, Ghaneh P, Halloran CM, Palmer DH, Campbell F, Valle JW, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ting Y, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Lerch MM, Mayerle J, Tjaden C, Strobel O, Hackert T, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019;154:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Okusaka T. Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chin Clin Oncol. 2022;11:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Zhang XP, Xu S, Gao YX, Zhao ZM, Zhao GD, Hu MG, Tan XL, Lau WY, Liu R. Early and late recurrence patterns of pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: a multicenter study. Int J Surg. 2023;109:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 9. | Schneider M, Strobel O, Hackert T, Büchler MW. Pancreatic resection for cancer-the Heidelberg technique. Langenbecks Arch Surg. 2019;404:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, Berchtold C, Ulrich A, Büchler MW. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford). 2017;19:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 11. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8641] [Article Influence: 540.1] [Reference Citation Analysis (0)] |
| 12. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2965] [Article Influence: 370.6] [Reference Citation Analysis (35)] |
| 13. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1414] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 14. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2332] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 15. | Zhu L, Li T, Yang Y, Tang N, Fu X, Qiu Y. Development and validation of a nomogram for predicting post-operative abdominal infection in patients undergoing pancreaticoduodenectomy. Clin Chim Acta. 2022;534:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1951] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 17. | Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, Asbun HJ, Bockhorn M, Strobel O, Büchler MW, Busch OR, Charnley RM, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Izbicki JR, Lillemoe KD, Neoptolemos JP, Sarr MG, Shrikhande SV, Sitarz R, Vollmer CM, Yeo CJ, Hartwig W, Wolfgang CL, Gouma DJ; International Study Group on Pancreatic Surgery. Definition and classification of chyle leak after pancreatic operation: A consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Kuroki N, Ono Y, Sato T, Inoue Y, Oba A, Ito H, Mise Y, Saiura A, Takahashi Y. Long-Term Outcome of Patients with Postoperative Refractory Diarrhea After Tailored Nerve Plexus Dissection Around the Major Visceral Arteries During Pancreatoduodenectomy for Pancreatic Cancer. World J Surg. 2022;46:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Soer EC, Verbeke CS. Pathology reporting of margin status in locally advanced pancreatic cancer: challenges and uncertainties. J Gastrointest Oncol. 2021;12:2512-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Gola M, Sejda A, Godlewski J, Cieślak M, Starzyńska A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Klotz R, Hackert T, Heger P, Probst P, Hinz U, Loos M, Berchtold C, Mehrabi A, Schneider M, Müller-Stich BP, Strobel O, Diener MK, Mihaljevic AL, Büchler MW. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB (Oxford). 2022;24:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Wei K, Klotz R, Kalkum E, Holze M, Probst P, Hackert T. Safety and efficacy of TRIANGLE operation applied in pancreatic surgery: a protocol of the systematic review and meta-analysis. BMJ Open. 2022;12:e059977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Petit M, Geri G, Salomon E, Victor M, Peschaud F, Vieillard-Baron A, Repessé X. Risk factors for surgical site infection after pancreatic surgery: a better postoperative antibiotic strategy is possible. J Hosp Infect. 2021;107:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Takahashi Y, Takesue Y, Fujiwara M, Tatsumi S, Ichiki K, Fujimoto J, Kimura T. Risk factors for surgical site infection after major hepatobiliary and pancreatic surgery. J Infect Chemother. 2018;24:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Mentor K, Ratnayake B, Akter N, Alessandri G, Sen G, French JJ, Manas DM, Hammond JS, Pandanaboyana S. Meta-Analysis and Meta-Regression of Risk Factors for Surgical Site Infections in Hepatic and Pancreatic Resection. World J Surg. 2020;44:4221-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Téoule P, Tombers K, Rahbari M, Sandra-Petrescu F, Keese M, Rahbari NN, Reißfelder C, Rückert F. [Definition and treatment of superior mesenteric artery revascularization and dissection-associated diarrhea (SMARD syndrome) in Germany]. Chirurg. 2022;93:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Poulia KA, Antoniadou D, Sarantis P, Karamouzis MV. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Rivelsrud M, Paur I, Sygnestveit K, Nilsen RM, Tangvik RJ. Nutritional treatment is associated with longer survival in patients with pancreatic disease and concomitant risk of malnutrition. Clin Nutr. 2021;40:2128-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Carrato A, Cerezo L, Feliu J, Macarulla T, Martín-Pérez E, Vera R, Álvarez J, Botella-Carretero JI. Clinical nutrition as part of the treatment pathway of pancreatic cancer patients: an expert consensus. Clin Transl Oncol. 2022;24:112-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Zhai S, Huo Z, Wang Y, Qian H, Zhao S, Shi Y, Weng Y, Deng X, Shen B. TRIANGLE operation for borderline resectable pancreatic cancer in total pancreatectomy. Transl Cancer Res. 2019;8:2416-2424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Wang W, Lou W, Xu Z, Chen H, Shen Z, Deng X, Peng C, Liu Y, Shen B. Long-term outcomes of standard versus extended lymphadenectomy in pancreatoduodenectomy for pancreatic ductal adenocarcinoma: A Chinese multi-center prospective randomized controlled trial. J Adv Res. 2023;49:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, Kim SG, Kim SW. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Lin Q, Zheng S, Yu X, Chen M, Zhou Y, Zhou Q, Hu C, Gu J, Xu Z, Wang L, Liu Y, Liu Q, Wang M, Li G, Cheng H, Zhou D, Liu G, Fu Z, Long Y, Li Y, Wang W, Qin R, Li Z, Chen R. Standard pancreatoduodenectomy versus extended pancreatoduodenectomy with modified retroperitoneal nerve resection in patients with pancreatic head cancer: a multicenter randomized controlled trial. Cancer Commun (Lond). 2023;43:257-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Khristenko E, Shrainer I, Setdikova G, Palkina O, Sinitsyn V, Lyadov V. Preoperative CT-based detection of extrapancreatic perineural invasion in pancreatic cancer. Sci Rep. 2021;11:1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Guo X, Gao S, Yu J, Zhou Y, Gao C, Hao J. The imaging features of extrapancreatic perineural invasion (EPNI) in pancreatic Cancer: A comparative retrospective study. Pancreatology. 2021;21:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |