Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.571
Peer-review started: October 29, 2023
First decision: December 6, 2023
Revised: December 14, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 27, 2024
Processing time: 119 Days and 1.1 Hours
The efficacy and safety of anti-tumor necrosis factor-α (TNF-α) monoclonal antibody therapy [adalimumab (ADA) and infliximab (IFX)] with therapeutic drug monitoring (TDM), which has been proposed for inflammatory bowel disease (IBD) patients, are still controversial.
To determine the efficacy and safety of anti-TNF-α monoclonal antibody therapy with proactive TDM in patients with IBD and to determine which subtype of IBD patients is most suitable for proactive TDM interventions.
As of July 2023, we searched for randomized controlled trials (RCTs) and observational studies in PubMed, Embase, and the Cochrane Library to compare anti-TNF-α monoclonal antibody therapy with proactive TDM with therapy with reactive TDM or empiric therapy. Pairwise and network meta-analyses were used to determine the IBD patient subtype that achieved clinical remission and to determine the need for surgery.
This systematic review and meta-analysis yielded 13 studies after exclusion, and the baseline indicators were balanced. We found a significant increase in the number of patients who achieved clinical remission in the ADA [odds ratio (OR) = 1.416, 95% confidence interval (CI): 1.196-1.676] and RCT (OR = 1.393, 95%CI: 1.182-1.641) subgroups and a significant decrease in the number of patients who needed surgery in the proactive vs reactive (OR = 0.237, 95%CI: 0.101-0.558) and IFX + ADA (OR = 0.137, 95%CI: 0.032-0.588) subgroups, and the overall risk of adverse events was reduced (OR = 0.579, 95%CI: 0.391-0.858) according to the pairwise meta-analysis. Moreover, the network meta-analysis results suggested that patients with IBD treated with ADA (OR = 1.39, 95%CI: 1.19-1.63) were more likely to undergo TDM, especially in comparison with patients with reactive TDM (OR = 1.38, 95%CI: 1.07-1.77).
Proactive TDM is more suitable for IBD patients treated with ADA and has obvious advantages over reactive TDM. We recommend proactive TDM in IBD patients who are treated with ADA.
Core Tip: The efficacy and safety of anti-tumor necrosis factor-α monoclonal antibody therapy [adalimumab (ADA) and infliximab] with therapeutic drug monitoring (TDM), which has been proposed for inflammatory bowel disease (IBD) patients, are still controversial. In this study, we found that proactive TDM was more suitable for IBD patients treated with ADA and had obvious advantages over reactive TDM.
- Citation: Zheng FY, Yang KS, Min WC, Li XZ, Xing Y, Wang S, Zhang YS, Zhao QC. Is tumor necrosis factor-α monoclonal therapy with proactive therapeutic drug monitoring optimized for inflammatory bowel disease? Network meta-analysis. World J Gastrointest Surg 2024; 16(2): 571-584
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/571.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.571
The introduction of biologics has played a central role in stimulating the development of the “targeted therapy” paradigm, which is now the basis for treating inflammatory bowel disease (IBD) patients and facilitating their clinical remission. Anti-tumor necrosis factor-α (TNF-α) monoclonal antibodies are still the classic treatment option and are widely used as biologic agents, and they include infliximab (IFX), adalimumab (ADA), etanercept, etc[1-3]. However, 13%-40% of patients are primarily nonresponsive to anti-TNF-α monoclonal antibody therapy, and another 23%-46% of patients have secondary response loss over time[4]. To avoid acquired insensitivity, therapeutic drug monitoring (TDM) of anti-TNF-α monoclonal antibody therapy has been proposed for patients, which involves measuring serum agent concentrations (usually trough values) and anti-drug antibody concentrations as a potential strategy for optimizing anti-TNF-α therapy.
TDM can also be applied in IBD patients with stable disease to maintain trough concentrations within a known therapeutic window to ensure a complete response, which is called proactive TDM[5,6]. Proactive TDM may have better therapeutic value than reactive TDM and empiric therapy; however, this topic is still controversial[7]. Two clinical practice guidelines have recently been published on this issue, and both support the application of reactive TDM, but their recommendations for proactive TDM differ[8,9]. Additional evidence is needed to resolve these discrepancies. While previous studies followed rigorous guidelines[10], they did not consider endpoints such as anti-TNF-α monoclonal antibody development and anti-TNF therapy discontinuation or the comparison of proactive vs reactive TDM.
Therefore, the purpose of this systematic review and network meta-analysis was to determine the efficacy and safety of anti-TNF-α monoclonal antibody therapy with proactive TDM in patients with IBD and to determine which subtype of IBD patients is most suitable for proactive TDM interventions.
The current study was performed in accordance with the guidelines established by the Cochrane Collaboration[11] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[12]. The study was registered on the PROSPERO website under registration No. CRD42023451642[13].
The following databases were searched for relevant literature with ulcerative colitis (UC), anti-TNF therapy, and TDM as the subject and text terms: PubMed, Embase, and The Cochrane Center Register of Controlled Trial. There were no publication or language restrictions. Taking the PubMed database as an example, the following search terms were used: [“Colitis, Ulcerative” (Mesh)] OR [Idiopathic Proctocolitis (Title/Abstract)] OR [Ulcerative Colitis (Title/Abstract)] OR [Colitis Gravis (Title/Abstract)] OR [Inflammatory Bowel Disease, Ulcerative Colitis Type (Title/Abstract)]. Obviously irrelevant studies were excluded based on their titles and abstracts. Two authors (Zheng FY and Yang KS) independently screened the full texts for incorporation. Disagreements and disputes were resolved by discussion with a third ex
Studies that included adult patients with IBD who received anti-TNF-α monoclonal antibody therapy with proactive TDM as the intervention group and patients who received both empiric therapy and reactive TDM as the maintenance management group were included. Both randomized controlled trials (RCTs) and observational studies were included, and whether the anti-TNF-α monoclonal antibody was IFX or ADA was recorded. Studies including only IBD patients were excluded, as were pharmacokinetic studies. One-arm therapy studies, studies with no useful data (no quantitative data for meta-analysis), and studies with child subjects were also excluded. The preset efficacy outcomes were clinical remission, the need for surgery, treatment discontinuation, endoscopic remission, clinical relapse, and the presence of anti-drug antibodies; the safety outcomes included adverse events, acute infusion reactions, and delayed hypersensitivity.
Two investigators selected the studies and extracted the data independently, and any differences between the two investigators was resolved by discussion with a third researcher. Baseline characteristic information of the included studies was recorded in self-designed original data sheets. Two authors independently assessed risk of bias in RCTs using the Cochrane risk of bias tool[14] and nonrandomized studies using the Newcastle Ottawa scale (NOS)[15]. RCTs were considered by Cochrane risk of bias tool, and as long as there was not too much red (high risk) acquired, the study can be included. Nonrandomized studies were considered by NOS score and those scored over 4 were acceptable. In all cases, discrepancies were resolved with a third reviewer as needed until a consensus was reached.
Although our sample size was relatively small, we hoped to conduct a relatively complete network meta-analysis, and outcomes with one more study reported were included in our network meta-analysis. We used a random-effects model to avoid heterogeneity. Pooled estimates were indicated as odds ratios (ORs) for dichotomous outcomes and as standardized mean differences for continuous outcomes, with their 95% confidence intervals (CIs). Heterogeneity among included studies was assessed using the χ2 test, with significance defined as P < 0.05 and the I2 statistic ≥ 50%[16]. We planned subgroup analyses based on different types of disease [IBD, UC, or Crohn’s disease (CD)], study type (RCT or observational), comparison (proactive vs empiric or proactive vs reactive), and anti-TNF-α monoclonal antibody type (IFX or ADA). Furthermore, meta-regression P < 0.05 was used to determine whether a specific factor was the source of heterogeneity[17]. Furthermore, we performed Begg’s and Egger’s tests to assess publication bias for available comparisons, and P < 0.05 indicated the presence of publication bias. We also used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scale[18] to evaluate the quality of the outcomes from pairwise meta-analysis.
For network meta-analysis, we estimated a random-effects model to prevent inconsistencies; similarly, OR with corresponding 95%CI was also used to confirm the significance of the network meta-analysis results. Inconsistency between indirect sources of evidence was statistically assessed using a global (design-by-treatment inconsistency model) and a local method (back calculation)[19,20]. We estimated the mean rank and relative treatment rankings for each intervention node according to surface under the cumulative ranking curve (SUCRA) values and produced rank plots for the results of the clinical remission, need of surgery, and adverse events. SUCRA values ranges from 0%-100%; for example, a higher SUCRA value indicates a better clinical response rate in patients receiving therapy with proactive TDM. Furthermore, we produced comparison-adjusted funnel plots to explore publication bias for the network meta-analysis outcomes. All analyses were performed using RevMan version 5.3 and Stata/SE version 15.1.
For this work, after a literature search of the three electronic databases and the removal of duplicates, 1013 publications were screened by checking titles and abstracts. After excluding the studies that could not be included, 852 publications were removed, and 161 articles were assessed for eligibility. After a detailed review of the full-text literature, a total of 13 original studies[21-33] were included (Figure 1), with 2328 patients assigned to the proactive TDM group and 2213 assigned to the maintenance management group.
The summary baseline characteristics, including disease type, study type, comparison, and anti-TNF-α monoclonal antibody type, were recorded (Table 1; Supplementary Table 1). The baseline indicators included male sex (%), CD (%), age, baseline remission (%), active smoker status (%), duration of disease, prior surgery (%), and C-reactive protein concentration, and they were balanced. All the studies that we included had acceptable quality results in the assessment of risk of bias (Supplementary Table 2, Supplementary Figure 1).
| Disease type | Study type | Comparison | Anti-TNF-α monoclonal antibody type | Ref. |
| IBD | RCT | Proactive vs empiric | IFX | Vande Casteele et al[21] |
| Observational study | Proactive vs empiric | IFX | Sánchez-Hernández et al[22] | |
| Lee et al[25] | ||||
| Guidi et al[29] | ||||
| Kelly et al[30] | ||||
| Bossuyt et al[31] | ||||
| IFX + ADA | Ponte et al[23] | |||
| ADA | Papamichael et al[28] | |||
| Capoulas et al[27] | ||||
| Proactive vs reactive | ADA | Papamichael et al[26] | ||
| IFX | Papamichael et al[28] | |||
| UC only | Observational study | Proactive vs empiric | IFX | Fernandes et al[24] |
| RCT | Proactive vs empiric | ADA | Panés et al[33] | |
| CD only | Observational study | Proactive vs empiric | IFX | Fernandes et al[24] |
| RCT | Proactive vs reactive | ADA | D'Haens et al[32] | |
| RCT | Proactive vs empiric | ADA | Panés et al[33] | |
| Baseline indicator | OR1/SMD2 (95%CI) | P, I2 | Balanced or not | |
| Male sex, n (%) | 1.106 (0.936, 1.307)1 | 0.283, 16.9 | Yes | |
| CD, n (%) | 1.114 (0.872, 1.422)1 | 0.299, 16.6 | Yes | |
| Age, yr, median (%) | -0.042 (-0.432, 0.348)2 | 0.000, 96.23 | Yes | |
| Baseline remission, n (%) | 1.263 (0.780, 2.046)1 | 0.406, 0.0 | Yes | |
| Active smoker, n (%) | 0.974 (0.633, 1.499)1 | 0.141, 45.1 | Yes | |
| Duration of disease, y, median (%) | -0.034 (-0.216, 0.148)2 | 0.003, 72.33 | Yes | |
| Prior surgery, n (%) | 1.075 (0.690, 1.675)1 | 0.923, 0.0 | Yes | |
| CRP concentration (mg/L) (%) | 0.463 (-0.171, 1.097)1 | 0.000, 98.23 | Yes | |
We used clinical remission, the need for surgery, treatment discontinuation, endoscopic remission, clinical relapse, and the presence of anti-drug antibodies as indicators of efficacy outcomes. Ten studies[21,23,24,29-33] reported data about clinical remission, and no significant difference was found (OR = 1.281, 95%CI: 0.972-1.688), with substantial heterogeneity (P = 0.002, I2 = 65.9%). According to our subgroup analysis of clinical remission, significant differences were detected in UC patients from the disease type group (OR = 1.563, 95%CI: 1.063-2.298; P = 0.058, I2 = 64.8%), RCT group (OR = 1.393, 95%CI: 1.182-1.641; P = 0.771, I2 = 0.0%), and ADA group (OR = 1.416, 95%CI: 1.196-1.676; P = 0.793, I2 = 0.0%), which favored the proactive TDM group. Moreover, meta-regression revealed that differences in disease type might be the main cause of the clinical heterogeneity (P = 0.028). Furthermore, publication bias was detected in the overall outcome and IBD subgroups, with low to high GRADE scores among the overall outcomes (Table 2).
| Outcome type | Subgroup type | Study (n) | OR (95%CI) | P, I2 (heterogeneity) | P value from meta-regression | Publication bias (Begg’s, Egger’s) | Grade | |
| Efficacy outcome | Clinical remission | Total (%) | 10 | 1.281 (0.972, 1.688) | 0.002, 65.92 | 0.194, 0.0004 | Moderate | |
| Disease type | ||||||||
| IBD (%) | 5 | 0.887 (0.671, 1.174) | 0.390, 2.8 | 0.0283 | 0.050, 0.0904 | Moderate | ||
| UC (%) | 3 | 1.563 (1.063, 2.298)1 | 0.058, 64.82 | 0.602, 0.112 | Low | |||
| CD (%) | 2 | 2.412 (0.889, 6.544) | 0.032, 78.12 | 0.317, - | Low | |||
| Study type | ||||||||
| RCT | 4 | 1.393 (1.182, 1.641)1 | 0.771, 0.0 | 0.861 | 0.497, 0.467 | High | ||
| Observational (%) | 6 | 1.305 (0.691, 2.464) | 0.000, 78.82 | 0.851, 0.376 | Moderate | |||
| Comparison | ||||||||
| Proactive vs empiric (%) | 8 | 1.330 (0.959, 1.843) | 0.003, 68.22 | 0.746 | 0.805, 0.755 | Moderate | ||
| Proactive vs reactive (%) | 2 | 1.074 (0.461, 2.501) | 0.036, 77.22 | 0.317, - | Low | |||
| Monoclonal type | ||||||||
| IFX (%) | 6 | 1.368 (0.724, 2.585) | 0.000, 77.72 | 0.954 | 0.851, 0.390 | Moderate | ||
| ADA (%) | 3 | 1.416 (1.196, 1.676)1 | 0.793, 0.0 | 0.602, 0.404 | Low | |||
| Need of surgery (all observational) | Total (%) | 9 | 0.525 (0.243, 1.130) | 0.001, 71.32 | ||||
| Disease type | ||||||||
| IBD (%) | 7 | 0.354 (0.155, 0.804)1 | 0.007, 66.02 | 0.140 | 0.548, 0.556 | Moderate | ||
| Comparison | ||||||||
| Proactive vs empiric (%) | 7 | 0.694 (0.282, 1.707) | 0.002, 72.12 | 0.353 | 0.293,0.993 | Moderate | ||
| Proactive vs reactive (%) | 2 | 0.237 (0.101, 0.558)1 | 0.302, 6.2 | 0.317, - | Low | |||
| Monoclonal type | ||||||||
| IFX (%) | 6 | 0.571 (0.233, 1.402) | 0.001, 75.32 | 0.672 | 0.851, 0.841 | Moderate | ||
| IFX + ADA (%) | 2 | 0.137 (0.032, 0.588)1 | 0.563, 0.0 | 0.317, - | Low | |||
| Treatment discontinuation (all observational) | Total (%) | 7 | 0.395 (0.130, 1.205) | 0.000, 85.72 | 0.812, 0.677 | Moderate | ||
| Disease type | ||||||||
| IBD | 5 | 0.377 (0.078, 1.831) | 0.000, 90.02 | 0.793 | 0.806, 0.998 | Moderate | ||
| Comparison | ||||||||
| Proactive vs empiric (%) | 5 | 0.494 (0.196, 1.248) | 0.046, 58.82 | 0.412 | 0.462,0.045 | Moderate | ||
| Proactive vs reactive (%) | 2 | 0.394 (0.018, 8.742) | 0.000, 94.92 | 0.317, - | Low | |||
| Monoclonal type | ||||||||
| IFX (%) | 5 | 0.494 (0.142, 1.715) | 0.000, 90.02 | 0.938 | 0.624, 0.705 | Moderate | ||
| ADA (%) | 2 | 0.125 (0.015, 1.027) | 0.808, 0.0 | 0.317, - | Low | |||
| Endoscopic remission | Total (%) | 4 | 1.435 (1.089, 1.890)1 | 0.169, 40.4 | 0.089, 0.093 | Moderate | ||
| Clinical relapse | Total (%) | 2 | 0.513 (0.294, 0.895)1 | 0.294, 9.2 | 1.000, - | Low | ||
| Anti-drug antibodies | Total (%) | 2 | 0.234 (0.116, 0.474) | 0.703, 0.0 | 0.317, - | Low | ||
| Safety | Adverse events | Total (%) | 10 | 0.579 (0.391, 0.858)1 | 0.001, 67.22 | 0.586, 0.377 | Moderate | |
| Disease type | ||||||||
| IBD (%) | 6 | 0.301 (0.157, 0.576)1 | 0.649, 0.0 | 0.0403 | 0.348, 0.427 | High | ||
| UC (%) | 2 | 0.987 (0.817, 1.193) | 0.732, 0.0 | 0.317, - | Low | |||
| CD (%) | 2 | 0.427 (0.107, 1.711) | 0.002, 89.42 | 0.317, - | Very low | |||
| Study type | ||||||||
| RCT (%) | 4 | 0.951 (0.804, 1.124) | 0.839, 0.0 | 0.0113 | 0.174, 0.753 | High | ||
| Observational (%) | 6 | 0.246 (0.146, 0.413)1 | 0.698, 0.0 | 0.348, 0.477 | High | |||
| Comparison | ||||||||
| Proactive vs empiric (%) | 7 | 0.577 (0.346, 0.964)1 | 0.002, 72.0 | 0.872 | 0.453, 0.113 | High | ||
| Proactive vs reactive (%) | 3 | 0.464 (0.175, 1.235) | 0.084, 59.7 | 0.602, 0.253 | Moderate | |||
| Monoclonal type | ||||||||
| IFX (%) | 5 | 0.264 (0.153, 0.455) | 0.428, 0.0 | 0.0213 | 0.142, 0.108 | High | ||
| ADA (%) | 5 | 0.923 (0.760, 1.120) | 0.323, 14.3 | 0.050, 0.0084 | High | |||
| Acute infusion reactions | Total (%) | 4 | 0.572 (0.235, 1.390) | 0.163, 41.4 | 0.308, 0.168 | Moderate | ||
| Delayed hypersensitivity | Total (%) | 2 | 0.719 (0.017, 29.584) | 0.079, 67.7 | 1.000, - | Moderate |
For the need for surgery outcome[22-26,28,30], which was summarized only for observational studies, significant differences were found among the IBD (OR = 0.354, 95%CI: 0.155-0.804), proactive vs reactive (OR = 0.237, 95%CI: 0.101-0.558), and IFX + ADA (OR = 0.137, 95%CI: 0.032-0.588) subgroups. For treatment discontinuation[24,25,27,28,30,31] according to observational studies, the overall OR was 0.395 (95%CI: 0.130 to 1.205), with no significant difference found in the subgroup analysis. Moreover, significant differences in endoscopic remission[30,32,33] (OR = 1.435, 95%CI: 1.089-1.890) and clinical relapse[21,23] outcomes (OR = 0.513, 95%CI: 0.294-0.895) that favored proactive TDM were found, while no significant difference in the presence of anti-drug antibodies[21,30] was found. There was low to substantial heterogeneity, a low risk of publication bias, and low to high GRADE scores among the above outcomes (Table 2). Overall, the efficacy of proactive TDM was better than that of conventional management.
We considered total adverse events, acute infusion reactions, and delayed hypersensitivity as safety outcomes. Ten of the 13 studies[21,22,24,26,28,32,33] reported original data on adverse events, and we noticed that proactive TDM intervention could decrease the risk of adverse effects (OR = 0.579, 95%CI: 0.391-0.858; P = 0.001, I2 = 67.2%). Moreover, significant differences in IBD (OR = 0.301, 95%CI: 0.157-0.576; P = 0.649, I2 = 0.0%), observational studies (OR = 0.246, 95%CI: 0.146-0.413; P = 0.698, I2 = 0.0%), and proactive vs empiric (OR = 0.577, 95%CI: 0.346-0.964; P = 0.002, I2 = 72.0%) subgroups were also found. Furthermore, meta-regression revealed that different types of disease, study types, and anti-TNF-α monoclonal agents were sources of heterogeneity, with P values equal to 0.040, 0.011, and 0.021, respectively. There was little publication bias or low to high GRADE scores among the above safety outcomes (Table 2).
In conclusion, ADA, a anti-TNF-α monoclonal antibody, is more effective than other agents and does not increase the risk of adverse events during proactive TDM intervention. However, it is necessary to conduct a follow-up network meta-analysis on which type of IBD patients are most suitable for proactive TDM intervention.
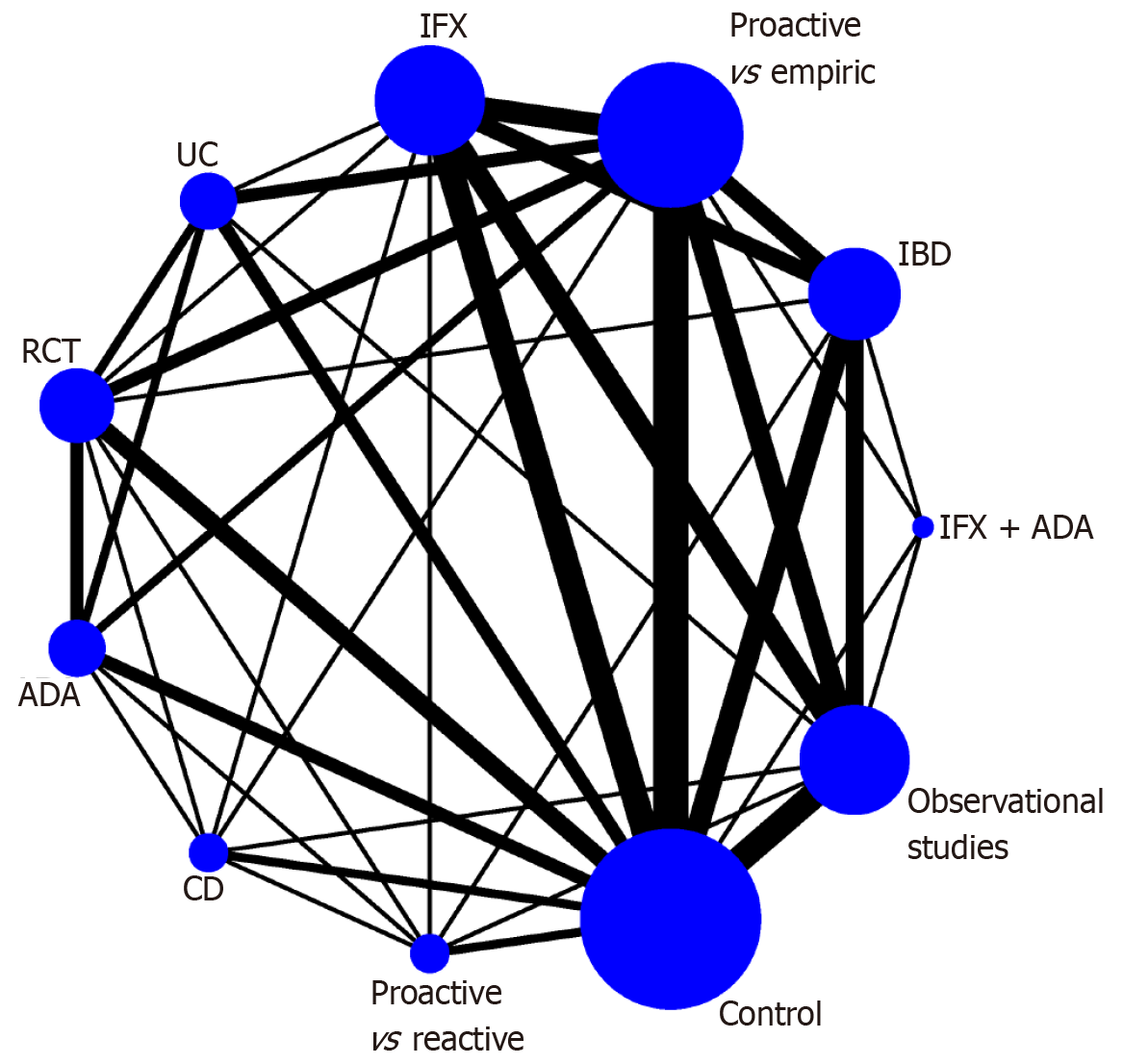
Due to the small sample size, we only used clinical remission, the need for surgery, and adverse events for follow-up network meta-analysis to identify the IBD subtype that is most suitable for the proactive TDM intervention. We constructed a network plot in which there are subgroups for direct comparison, as well as the number of patients studied (Figure 2). For the clinical remission outcome of the network meta-analysis, the CD group was ranked first (OR = 1.50, 95%CI: 1.14-1.97) according to the SUCRA score in comparison with the control group. The rest of the groups were ranked as follows: ADA as anti-TNF-α monoclonal antibody therapy (OR = 1.39, 95%CI: 1.19-1.63); UC (OR = 1.39, 95%CI: 1.17-1.64); RCT (OR = 1.38, 95%CI: 1.19-1.61); proactive vs reactive (OR = 1.38, 95%CI: 1.07-1.77); proactive vs empiric (OR = 1.35, 95%CI: 1.17-1.57); IFX (OR = 1.31, 95%CI: 1.03-1.66); observational studies (OR = 1.29, 95%CI: 1.02-1.63); IFX + ADA(1.17, 95%CI: 0.73-1.89) and IBD(1.22, 95%CI: 0.97-1.54) (Figure 3). No significant differences were found in the other comparisons, and no publication bias was detected from the network funnel plot (Supplementary Figure 2).
When evaluating the need for surgery, we found that ADA, an anti-TNF-α monoclonal agent, ranked first according to the SUCRA score (OR = 0.21; 95%CI: 0.04-1.29), followed by proactive vs reactive TDM; UC, IBD, IFX; proactive vs empiric therapy; and IFX + ADA and CD, with no significant differences (Figure 4A). When evaluating adverse effects, compared with the control group, observational studies ranked first (OR = 0.42, 95%CI: 0.23-0.74), followed by IBD (0.43, 95%CI: 0.20-0.93) and CD (OR = 0.53; 95%CI: 0.29-0.98), proactive vs reactive(OR = 0.53; 95%CI: 0.28-1.01), ADA(OR = 0.55; 95%CI: 0.28-1.08), IFX (OR = 0.57, 95%CI: 0.36-0.89), proactive vs empiric (OR = 0.57, 95%CI: 0.36-0.89), RCT (OR = 0.64, 95%CI: 0.41-1.00), and UC(OR = 0.63; 95%CI: 0.38-1.04) (Figure 4B).
Overall, the results did not significantly differ among the subgroups, and to further identify the type of patients most suitable for proactive TDM interventions, we combined pairwise and network meta-analysis data using cross-hair plots. The combined outcomes showed that the three subgroups, namely, the CD, ADA, and proactive vs reactive groups, had better outcomes for clinical remission (Figure 5A) and did not increase the risk of overall adverse effects (Figure 5B). These outcomes suggest that patients with IBD treated with ADA are more likely to undergo TDM, especially in comparison with patients treated with reactive TDM. However, in terms of which type of IBD is more suitable (UC or CD), the outcomes are debatable.
This systematic review and network meta-analysis followed the PRISMA guidelines and was registered on the PROSPERO website. First, we screened 13 original studies, including four RCTs and nine observational studies, involving a total of 4541 patients with IBD with balanced baseline characteristics (Figure 1, Table 1). Second, from pairwise meta-analysis, we found that proactive TDM was effective and did not increase the risk of adverse events in the subgroup of patients treated with ADA (Table 2). Third, the network meta-analysis results suggested that patients with IBD treated with ADA were more likely to undergo TDM, especially compared to patients who underwent reactive TDM. However, in terms of which type of IBD is most appropriate (UC or CD), the outcomes are debatable (Figures 2-5). In summary, we recommend proactive TDM in IBD patients who are treated with ADA.
In patients with IBD, the use of detectable serum trough concentrations of IFX or ADA was superior to the use of undetectable agents, which was first identified more than a decade ago[34]. Ever since, many studies have revealed exposure-response relationships between various outcomes and anti-TNF agent concentrations[35]. It seems logical to infer that implementing routine TDM to maintain the drug concentration within the therapeutic window improves treatment efficacy[4-6]. Another general consideration is that many TDM assays have long cycles, so anti-TNF dose decisions are usually based on the trough concentrations infused in previous weeks, such as the TAILORIX trial[36]. New point-of-care analysis may help to avoid this situation[37]. The timing of the outcome assessment is another significant factor. Moreover, the proactive optimization of maintenance dosing might prolong the time to loss of response in some patients[38], and induction trough concentration values were lower in IFX primary nonresponders than in responders[39]. It remains to be determined whether this represents a causal relationship and, if so, whether the use of TDM during induction may reduce the primary nonresponse to anti-TNF-α antibodies. The use of multiple immunomodulators in many patients is also relevant. The SONIC trial confirmed the superiority of IFX combined with azathioprine to IFX monotherapy[40]. A recent cutting-edge study demonstrated that proactive TDM, which targets higher exposure concentrations (> 5 µg/mL), can improve disease remission rates and enhance the durability of anti-TNF biologics. The effective management of anti-TNF therapies in children with IBD requires evidence-based precision dosing strategies, including routine TDM and proactive pharmacodynamic assessments[41]. Therefore, TDM may be the most useful measure for patients receiving monoclonal antibody monotherapy.
There are several limitations to our research. First, only short-term outcomes, such as clinical remission, the need for surgery, and treatment discontinuation, were used to determine the efficacy of proactive TDM as a standard of evaluation. Second, a more systematic review of the outcomes, including some long-term results such as discontinuation and the anti-drug antibody concentration, may be better suited to detect the therapy benefits of proactive TDM. This is particularly prominent given the underlying limitations of using clinical remission as an outcome measure, especially given the known incomplete correlation between symptoms and endoscopic activity, especially in patients with IBD. Furthermore, given the effectiveness of anti-TNF therapy, the benefit of TDM may be difficult to detect by endoscopy, especially when evaluated in the short term. Third, our study did not incorporate pediatric-specific data. Children represent a particularly relevant population because of their variability in size, which may not be adequately addressed by body weight-based doses. Although not the focus of this review, other unknown factors include optimal trough concentration ranges and upper limit concentrations, beyond which further increases may be useless. Finally, these thresholds may vary depending on various factors, such as specific outcomes, population (children vs adults, UC vs CD patients), and treatment stage (induction vs maintenance). The optimal frequency of active TDM also remains to be determined, but trough concentration measurements before each infusion are most likely unnecessary.
From our network meta-analysis, we found that proactive TDM had better therapeutic efficacy than reactive TDM, which is an innovative finding. Additionally, the lines of reactive and proactive TDM can quickly blur in many common clinical settings. Physicians employing a TDM-based strategy need to take into account the drug concentration with respect to the inflammatory status of the patient, the underlying pharmacokinetics and pharmacodynamics of the agent, the risk of immunogenicity, and the therapeutic goals for the patient. Physicians should understand the limits of TDM and feel comfortable making therapeutic decisions with imperfect information[42-44]. Furthermore, we also found that ADA may be more suitable for IBD patients who undergo active TDM. Assa et al[45] also performed an RCT including pediatric patients with CD and found that proactive monitoring of ADA trough concentrations and adjustment of doses and intervals resulted in significantly higher rates of corticosteroid-free clinical remission than reactive monitoring. The above results indicate that ADA is more suitable for TDM. Conversely, whether IFX is more stable and more effective still needs to be studied.
In conclusion, proactive TDM is more suitable for IBD patients treated with ADA and has obvious advantages over reactive TDM. The available evidence supports the superiority of the proactive TDM strategy in improving clinical remission rates and suggests that long-term outcomes of proactive TDM associated with a persistent treatment response may be more appropriate for determining the efficacy of TDM. Overall, long-term, better RCTs are needed to determine the efficacy of proactive TDM more definitively to optimize the clinical outcomes of IBD. Future research should include the efficacy of TDM during induction, the regulation of the dosage of monoclonal antibodies, and the application of this research in a pediatric setting.
The efficacy and safety of anti-tumor necrosis factor-α (TNF-α) monoclonal antibody therapy [adalimumab (ADA) and infliximab] with therapeutic drug monitoring (TDM), which has been proposed for inflammatory bowel disease (IBD) patients, are still controversial.
To promote rational drug use in clinical practice.
To determine the efficacy and safety of anti-TNF-α monoclonal therapy with proactive TDM in patients with IBD and to determine which subtype of IBD patients is most suitable for proactive TDM interventions.
Randomized controlled trials and observational studies in three electronic databases to compare TNF-α monoclonal therapy with proactive TDM with therapy with reactive TDM or empiric therapy were included.
Significant differences were frequently found in the proactive TDM subgroups, and these differences did not increase the risk of adverse events. A network meta-analysis suggested that patients with IBD treated with ADA were more likely to undergo TDM, especially in comparison with patients treated with reactive TDM.
TDM is more suitable for IBD patients treated with ADA and has obvious advantages over reactive TDM.
Future research should include the efficacy of TDM during induction, the regulation of the dosage of monoclonal antibodies, and the application of this research in a pediatric setting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin SR, Taiwan; M'Koma AE, United States S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Zheng XM
| 1. | Tappenden P, Ren S, Archer R, Harvey R, James MM, Basarir H, Stevens J, Lobo A, Hoque S. A Model-Based Economic Evaluation of Biologic and Non-Biologic Options for the Treatment of Adults with Moderately-to-Severely Active Ulcerative Colitis after the Failure of Conventional Therapy. Pharmacoeconomics. 2016;34:1023-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol. 2023;29:2733-2746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (4)] |
| 3. | Cheah E, Huang JG. Precision medicine in inflammatory bowel disease: Individualizing the use of biologics and small molecule therapies. World J Gastroenterol. 2023;29:1539-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Reference Citation Analysis (0)] |
| 4. | Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Restellini S, Afif W. Update on TDM (Therapeutic Drug Monitoring) with Ustekinumab, Vedolizumab and Tofacitinib in Inflammatory Bowel Disease. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr, Osterman MT, Saroufim A, Siegel CA, Yarur AJ, Melmed GY, Papamichael K. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am J Gastroenterol. 2021;116:2014-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 7. | Silva-Ferreira F, Afonso J, Pinto-Lopes P, Magro F. A Systematic Review on Infliximab and Adalimumab Drug Monitoring: Levels, Clinical Outcomes and Assays. Inflamm Bowel Dis. 2016;22:2289-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 9. | Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Spinelli A, Panis Y, Doherty G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 530] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 10. | Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology. 2017;153:835-857.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 11. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24605] [Article Influence: 1757.5] [Reference Citation Analysis (3)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39040] [Article Influence: 9760.0] [Reference Citation Analysis (2)] |
| 13. | National Institute for Health and Care Research. Does Proactive Therapeutic Drug Monitoring of Monoclonal Is Superior to Conventional Management in Inflammatory Bowel Disease? A systematic review and network meta-analysis. 2023. Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=451642. |
| 14. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 14820] [Article Influence: 2470.0] [Reference Citation Analysis (0)] |
| 15. | Wells GA, Shea BJ, O'Connell D, Peterson J, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available from: https://xueshu.baidu.com/usercenter/paper/show?paperid=1x710rj0w3780cn0ts3d0gd08q453667. |
| 16. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46194] [Article Influence: 2099.7] [Reference Citation Analysis (3)] |
| 17. | Feng F, Jiang Q, Jia H, Sun H, Chai Y, Li X, Rong G, Zhang Y, Li Z. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14741] [Article Influence: 867.1] [Reference Citation Analysis (0)] |
| 19. | Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 921] [Cited by in RCA: 1420] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 20. | König J, Krahn U, Binder H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat Med. 2013;32:5414-5429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, Simoens S, Rutgeerts P, Gils A, Vermeire S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320-9.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 686] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 22. | Sánchez-Hernández JG, Rebollo N, Martin-Suarez A, Calvo MV, Muñoz F. A 3-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol. 2020;86:1165-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Ponte A, Pinho R, Fernandes S, Rodrigues A, Alberto L, Silva JC, Silva J, Rodrigues J, Sousa M, Silva AP, Proença L, Freitas T, Leite S, Carvalho J. Impact of Histological and Endoscopic Remissions on Clinical Recurrence and Recurrence-free Time in Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:2238-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Fernandes SR, Bernardo S, Simões C, Gonçalves AR, Valente A, Baldaia C, Moura Santos P, Correia LA, Tato Marinho R. Proactive Infliximab Drug Monitoring Is Superior to Conventional Management in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Lee H, Roberts P, Pattni S. PTH-101 Infliximab therapeutic drug monitoring: reactive vs proactive approach – University Hospitals of Leicester (UHL) experience. Gut. 2019;68:83. [DOI] [Full Text] |
| 26. | Papamichael K, Juncadella A, Wong D, Rakowsky S, Sattler LA, Campbell JP, Vaughn BP, Cheifetz AS. Proactive Therapeutic Drug Monitoring of Adalimumab Is Associated With Better Long-term Outcomes Compared With Standard of Care in Patients With Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 27. | Capoulas M, Loba A, Barroso A, Santos C, Gomes MA, Andreozzi V, Félix J. 6ER-010 Therapeutic drug monitoring of tumour necrosis factor α inhibitors in inflammatory bowel disease: evidence from a real world setting. Eur J Hospital Pharm. 2020;27:209. [DOI] [Full Text] |
| 28. | Papamichael K, Chachu KA, Vajravelu RK, Vaughn BP, Ni J, Osterman MT, Cheifetz AS. Improved Long-term Outcomes of Patients With Inflammatory Bowel Disease Receiving Proactive Compared With Reactive Monitoring of Serum Concentrations of Infliximab. Clin Gastroenterol Hepatol. 2017;15:1580-1588.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 29. | Guidi L, Pugliese D, Panici Tonucci T, Berrino A, Tolusso B, Basile M, Cantoro L, Balestrieri P, Civitelli F, Bertani L, Marzo M, Felice C, Gremese E, Costa F, Viola F, Cicala M, Kohn A, Gasbarrini A, Rapaccini GL, Ruggeri M, Armuzzi A. Therapeutic Drug Monitoring is More Cost-Effective than a Clinically Based Approach in the Management of Loss of Response to Infliximab in Inflammatory Bowel Disease: An Observational Multicentre Study. J Crohns Colitis. 2018;12:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Kelly OB, Donnell SO, Stempak JM, Steinhart AH, Silverberg MS. Therapeutic Drug Monitoring to Guide Infliximab Dose Adjustment is Associated with Better Endoscopic Outcomes than Clinical Decision Making Alone in Active Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Bossuyt P, Pouillon L, Claeys S, D'Haens S, Hoefkens E, Strubbe B, Marichal D, Peeters H. Ultra-proactive Therapeutic Drug Monitoring of Infliximab Based on Point of Care Testing in Inflammatory Bowel Disease: Results of a Pragmatic Trial. J Crohns Colitis. 2022;16:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | D'Haens GR, Sandborn WJ, Loftus EV Jr, Hanauer SB, Schreiber S, Peyrin-Biroulet L, Panaccione R, Panés J, Baert F, Colombel JF, Ferrante M, Louis E, Armuzzi A, Zhou Q, Goteti VS, Mostafa NM, Doan TT, Petersson J, Finney-Hayward T, Song AP, Robinson AM, Danese S. Higher vs Standard Adalimumab Induction Dosing Regimens and Two Maintenance Strategies: Randomized SERENE CD Trial Results. Gastroenterology. 2022;162:1876-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 33. | Panés J, Colombel JF, D'Haens GR, Schreiber S, Panaccione R, Peyrin-Biroulet L, Loftus EV Jr, Danese S, Tanida S, Okuyama Y, Louis E, Armuzzi A, Ferrante M, Vogelsang H, Hibi T, Watanabe M, Lefebvre J, Finney-Hayward T, Sanchez Gonzalez Y, Doan TT, Mostafa NM, Ikeda K, Xie W, Huang B, Petersson J, Kalabic J, Robinson AM, Sandborn WJ. Higher vs Standard Adalimumab Induction and Maintenance Dosing Regimens for Treatment of Ulcerative Colitis: SERENE UC Trial Results. Gastroenterology. 2022;162:1891-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 482] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 35. | Moore C, Corbett G, Moss AC. Systematic Review and Meta-Analysis: Serum Infliximab Levels During Maintenance Therapy and Outcomes in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Laharie D, D'Haens G, Nachury M, Lambrecht G, Bossuyt P, Bouhnik Y, Louis E, Janneke van der Woude C, Buisson A, Van Hootegem P, Allez M, Filippi J, Brixi H, Gilletta C, Picon L, Baert F, Vermeire S, Duveau N, Peyrin-Biroulet L. Steroid-Free Deep Remission at One Year Does Not Prevent Crohn's Disease Progression: Long-Term Data From the TAILORIX Trial. Clin Gastroenterol Hepatol. 2022;20:2074-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Van Stappen T, Bollen L, Vande Casteele N, Papamichael K, Van Assche G, Ferrante M, Vermeire S, Gils A. Rapid Test for Infliximab Drug Concentration Allows Immediate Dose Adaptation. Clin Transl Gastroenterol. 2016;7:e206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Mattoo VY, Basnayake C, Connell WR, Ding N, Kamm MA, Lust M, Niewiadomski O, Thompson A, Wright EK. Systematic review: efficacy of escalated maintenance anti-tumour necrosis factor therapy in Crohn's disease. Aliment Pharmacol Ther. 2021;54:249-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Bar-Yoseph H, Levhar N, Selinger L, Manor U, Yavzori M, Picard O, Fudim E, Kopylov U, Eliakim R, Ben-Horin S, Chowers Y, Ungar B. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther. 2018;47:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2362] [Article Influence: 157.5] [Reference Citation Analysis (1)] |
| 41. | Samuels A, Whaley KG, Minar P. Precision Dosing of Anti-TNF Therapy in Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2023;25:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Vaughn BP. A Practical Guide to Therapeutic Drug Monitoring of Biologic Medications for Inflammatory Bowel Disease. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Irving PM, Gecse KB. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology. 2022;162:1512-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 44. | Syversen SW, Jørgensen KK, Goll GL, Brun MK, Sandanger Ø, Bjørlykke KH, Sexton J, Olsen IC, Gehin JE, Warren DJ, Klaasen RA, Noraberg G, Bruun TJ, Dotterud CK, Ljoså MKA, Haugen AJ, Njålla RJ, Zettel C, Ystrøm CM, Bragnes YH, Skorpe S, Thune T, Seeberg KA, Michelsen B, Blomgren IM, Strand EK, Mielnik P, Torp R, Mørk C, Kvien TK, Jahnsen J, Bolstad N, Haavardsholm EA. Effect of Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients With Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial. JAMA. 2021;326:2375-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 45. | Assa A, Matar M, Turner D, Broide E, Weiss B, Ledder O, Guz-Mark A, Rinawi F, Cohen S, Topf-Olivestone C, Shaoul R, Yerushalmi B, Shamir R. Proactive Monitoring of Adalimumab Trough Concentration Associated With Increased Clinical Remission in Children With Crohn's Disease Compared With Reactive Monitoring. Gastroenterology. 2019;157:985-996.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |