Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.481
Peer-review started: November 30, 2023
First decision: December 18, 2023
Revised: December 30, 2023
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 27, 2024
Processing time: 87 Days and 6.5 Hours
Individuals with refractory ascites in the context of liver cirrhosis typically face an adverse prognosis. The transjugular intrahepatic portosystemic shunt (TIPS) is an efficacious intervention, but there is a lack of reliable tools for postoperative pro
To investigate the associations between the Child-Pugh score, model for end-stage liver disease (MELD) score, and serum cystatin C (Cys C) level and post-TIPS pro
A retrospective analysis was conducted on 75 patients with liver cirrhosis and refractory ascites who underwent TIPS at our institution from August 2019 to August 2021. These patients were followed up regularly for two years, and the death toll was meticulously documented. The patients were allocated into a survival group (n = 45 patients) or a deceased group (n = 30 patients) based on their prognosis status. The clinical data of the two groups were collected, and Child-Pugh scores and MELD scores were calculated for analysis. Spearman correlation analysis was carried out to evaluate the correlation of prognosis with Child-Pugh grade, MELD score, and Cys C level. Additionally, a multiple-factor analysis utilizing the Cox proportional hazard model was used to identify independent risk factors affecting the post-TIPS prognosis of patients with liver cirrhosis and refractory ascites. The receiver operating characteristic curve (ROC) ascertained the predictive value of the Cys C concentration, Child-Pugh grade, and MELD score for the prognosis of liver cirrhosis with refractory ascites in post-TIPS patients.
During a 2-year follow-up period, among 75 patients with liver cirrhosis and refractory ascites who underwent TIPS treatment, 30 patients (40.00%) passed away. The deceased cohort exhibited heightened aspartate aminotransferase, alanine aminotransferase, total bilirubin, Scr, prothrombin time, Cys C, international normalized ratio, Child-Pugh, and MELD scores compared to those of the survival cohort, while Alb and Na+ levels were attenuated in the deceased group (P < 0.05). Spearman analysis revealed moderate to high positive correlations between prognosis and Child-Pugh score, MELD score, and Cys C level (r = 0.709, 0.749, 0.671, P < 0.05). Multivariate analysis using the Cox proportional hazard model demonstrated that the independent risk factors for post-TIPS prognosis in patients with liver cirrhosis and refractory ascites were Cys C (HR = 3.802; 95%CI: 1.313-11.015), Child-Pugh (HR = 3.030; 95%CI: 1.858-4.943), and MELD (HR = 1.222; 95%CI: 1.073-1.393) scores. ROC analysis confirmed that, compared to those of the classic prognostic models for Child-Pugh and MELD scores, the predictive accuracy of Cys C for post-TIPS prognosis in patients with liver cirrhosis and refractory ascites was slightly lower. This analysis yielded sensitivity and specificity values of 83.33% and 82.22%, respectively. The area under the curve value at this juncture was 0.883, with an optimal cutoff value set at 1.95 mg/L.
Monitoring the serum Cys C concentration is valuable for assessing the post-TIPS prognosis in patients with liver cirrhosis and refractory ascites. Predictive models based on serum Cys C levels, as opposed to Scr levels, are more beneficial for evaluating the condition and prognosis of patients with ascites due to cirrhosis.
Core Tip: Cirrhosis is a predominant contributor to global morbidity and mortality. This study sought to probe the relationship between Child-Pugh and model for end-stage liver disease (MELD) scores, as well as serum cystatin C (Cys C) levels, and the prognosis in patients with liver cirrhosis and refractory ascites who have undergone transjugular intrahepatic portosystemic shunt (TIPS). Child-Pugh and MELD scores have been extensively utilized for assessing the prognosis of cirrhosis, whereas serum Cys C levels serve as a potential biomarker reflecting renal function. The outcomes of this investigation may furnish valuable insights into the prognostic assessment of patients with liver cirrhosis and refractory ascites undergoing TIPS treatment.
- Citation: Hu XG, Yang XX, Lu J, Li G, Dai JJ, Wang JM, Deng Y, Feng R. Correlation between serum markers and transjugular intrahepatic portosystemic shunt prognosis in patients with cirrhotic ascites. World J Gastrointest Surg 2024; 16(2): 481-490
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/481.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.481
Cirrhosis is an end-stage condition characterized by liver damage that arises from multiple chronic liver diseases and constitutes a foremost contributor to worldwide morbidity and mortality[1,2]. With a diverse and complicated etiology, cirrhosis prevails across the globe, involving causative factors such as obesity, nonalcoholic fatty liver disease, excessive alcohol consumption, hepatitis B or C infections, autoimmune disorders, and cholestatic diseases[3,4]. Portal hyper
A transjugular intrahepatic portosystemic shunt (TIPS) is a medical technique in which a stent is inserted through the jugular vein to establish an artificial conduit within the liver. This conduit redirects blood flow from the portal vein to the systemic circulation[10]. The TIPS is employed to treat PH complications by mitigating portal venous pressure[11]. In recent years, with advancements in TIPS technology, its indications have expanded, and it has found widespread application in the treatment of cirrhosis and its complications[12]. The “Guidelines for the Diagnosis and Treatment of Cirrhosis Ascites” also recommend TIPS as a second-line treatment for refractory ascites in cirrhosis patients[13]. Conse
Therefore, this study focusing on patients with liver cirrhosis refractory ascites who underwent TIPS treatment aimed to explore the association between the prognosis after TIPS and the Child-Pugh and model for end-stage liver disease (MELD) scores, as well as Cys C levels. This investigation was conducted to identify more accurate prognostic indicators and refine treatment strategies for this specific patient cohort.
Our research retrospectively evaluated 75 patients suffering from liver cirrhosis and refractory ascites who underwent TIPS treatment at our facility between August 2019 and August 2021. The criteria for inclusion were as follows: (1) Meeting the diagnostic criteria for patients with liver cirrhosis and refractory ascites as outlined in the “Diagnosis and Treatment Guidelines for Ascites and Related Complications in Cirrhosis”[16]; (2) Meeting the indications for TIPS surgery; and (3) Obtaining informed consent from both patients and their families for participation in the study. The exclusion criteria were as follows: (1) Concurrent malignancy; (2) Severe liver and kidney dysfunction; (3) Coagulation abnormalities; and (4) Incomplete clinical data. This study received approval from the institutional ethics committee, and comprehensive information, including the study's purpose and procedures, was conveyed to all patients and their families. Consent was obtained through the completion of informed consent forms, with the patients signifying their voluntary agreement to participate in the study.
Combining passive and active follow-up methods, the patient follow-up encompassed diverse modalities, including home visits and telephonic verification. The follow-up concluded upon the occurrence of either death or liver transplant. The patients’ survival outcomes were documented, and if a patient could not be reached for follow-up on three con
Collection of baseline data: General information and past medical history, including sex, age, body mass index (BMI), systolic blood pressure, diastolic blood pressure, smoking history, and history of hypertension and CHD, were obtained from all enrolled subjects. The cohort of survivors consisted of 22 males and 23 females, ranging in age from 20 to 64 years, with a mean age of 42.3 ± 10.6 years. The deceased group comprised 17 males and 13 females aged between 22 and 65 years, with an average age of 43.1 ± 10.2 years. There were no statistically significant differences in age, sex, BMI, blood pressure, smoking history, hypertension, or coronary heart disease history between the two cohorts (all P > 0.05), suggesting comparability.
Detection of clinical indices: Comparisons of the serum glutamic oxalacetic transaminase [GOT or aspartate aminotransferase (AST)], glutamic pyruvic transaminase [GPT or alanine aminotransferase (ALT)], alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBil), Alb, and Scr levels; blood urea nitrogen (BUN) level; Na+ level; blood platelet count (PLT); prothrombin time (PT); and Cys C concentration were performed between the two groups. Specific methods: On the morning of the examination day, 5 mL of venous blood from the elbow was collected while the patients were in a fasting state. The blood samples were subjected to centrifugation at a speed of 3000 revolutions per minute and a centrifugal radius of 10 centimeters for a duration of 8 minutes. After the upper clear liquid was separated, an automatic biochemical analyzer (produced by Beckman Coulter (China) Limited., model AU5800) was used to measure AST, ALT, ALP, GGT, TBil, Alb, Scr, BUN, Na+, PLT, PT, and Cys C levels in the serum. The reagent kits used were obtained from Shanghai Kehua Bio-Engineering Co., Ltd., and Shanghai Ruikang Biotechnology Co., Ltd. The procedures were carried out in alignment with SOP. All blood samples were tested within 2 h following collection.
Classic prognostic models: Calculations were made for the international normalized ratio (INR), Child-Pugh score, and MELD score as follows:
INR[17]: Calculated using the formula PT/international sensitivity index (ISI).
Child-Pugh scores[18] were calculated using the formula Alb (> 35 g/L, 1 point; 28-35 g/L, 2 points; < 28 g/L, 3 points); TBil (< 34 μmol, 1 point; 34-51 μmol, 2 points; > 51 μmol, 3 points); PT (≤ 14 s, 1 point; 15-17 s, 2 points; ≥ 18 s, 3 points); ascites (none, 1 point; easily removable, 2 points; difficult to remove, 3 points); and hepatic encephalopathy (none, 1 point; grade I-II, 2 points; grade III-IV, 3 points). The total score ranges from 1 to 15 points and is categorized as follows: Grade A (5-8 points), Grade B (9-11 points), or Grade C (12-15 points).
MELD scores[19] were calculated using the Formula 3.8 × In (TBil) (mg/dL) + 9.6 × In (Scr) (mg/dL) + 11.2In (INR) (mg/dL) + 6.4 × etiology, and the calculated outcome was rounded to the nearest integer.
The statistical analysis was performed with the assistance of SPSS 22.0 software. Measurement data are presented as “mean ± SD” and were analyzed using the t test, while enumeration data are presented as “%” and were evaluated via the χ² test. Spearman correlation analysis was used to evaluate the correlation between the Child-Pugh score, MELD score, and Cys C level and patient prognosis. Additionally, a multiple-factor analysis employing the Cox proportional hazard model was applied to ascertain the independent risk factors impacting the post-TIPS prognosis of liver cirrhosis patients with refractory ascites. The receiver operating characteristic (ROC) curve was used to evaluate the ability of the Cys C concentration, Child-Pugh grade, and MELD score to predict the prognosis for patients with liver cirrhosis and refractory ascites after surgery. When P < 0.05, the difference was considered to be statistically significant.
After a 2-year follow-up, 30 deaths (40.00%) occurred among the 75 patients diagnosed with liver cirrhosis and refractory ascites who underwent TIPS treatment, 45 of whom (60.00%) survived. Within the deceased cohort, the AST (60.72 ± 14.09), ALT (45.19 ± 10.52), TBil (54.07 ± 16.76), Scr (72.21 ± 12.86), PT (17.83 ± 2.83), Cys C (2.43 ± 0.47), INR (1.84 ± 0.39), Child-Pugh (12.87 ± 1.29), and MELD (17.46 ± 2.98) values were higher than those in the survival cohort, for which AST (54.29 ± 11.57), ALT (121.90 ± 30.37), TBil (44.93 ± 13.64), Scr (66.04 ± 10.38), PT (16.12 ± 3.09), Cys C (1.68 ± 0.36), INR (1.57 ± 0.32), Child-Pugh (10.09 ± 1.45), and MELD (11.58 ± 2.34) values were recorded. Moreover, the Alb (24.67 ± 7.91) and Na+ (126.74 ± 12.35) levels in the deceased group were lower than those in the survival group, for which Alb (29.52 ± 8.53) and Na+ (136.24 ± 18.67) levels were recorded. The difference between the two cohorts was statistically significant (P < 0.05), as displayed in Table 1.
| Group | Survival group (n = 45 cases) | Deceased group (n = 30 cases) | t/χ2 | P value | |
| Age (yr) | 51.42 ± 7.28 | 53.25 ± 7.09 | 1.078 | 0.285 | |
| Sex | Male | 26 | 16 | 0.144 | 0.704 |
| Female | 19 | 14 | |||
| Combined background disease | Presence | 15 | 12 | 0.347 | 0.556 |
| Absence | 30 | 18 | |||
| Family history | Presence | 4 | 5 | 1.031 | 0.310 |
| Absence | 41 | 25 | |||
| Smoking history | Presence | 12 | 11 | 0.847 | 0.358 |
| Absence | 33 | 19 | |||
| Drinking history | Presence | 10 | 10 | 1.136 | 0.286 |
| Absence | 35 | 20 | |||
| AST (U/L) | 54.29 ± 11.57 | 60.72 ± 14.09 | 2.160 | 0.034 | |
| ALT (U/L) | 40.31 ± 9.86 | 45.19 ± 10.52 | 2.044 | 0.045 | |
| ALP (U/L) | 121.90 ± 30.37 | 129.74 ± 32.37 | 1.067 | 0.290 | |
| GGT (U/L) | 64.86 ± 16.25 | 70.38 ± 18.39 | 1.367 | 0.176 | |
| Tbil (μmol/L) | 44.93 ± 13.64 | 54.07 ± 16.76 | 2.593 | 0.012 | |
| Alb (g/L) | 29.52 ± 8.53 | 24.67 ± 7.91 | 2.482 | 0.015 | |
| Scr (μmol/L) | 66.04 ± 10.38 | 72.21 ± 12.86 | 2.290 | 0.025 | |
| BUN (μmol/L) | 7.23 ± 2.07 | 7.41 ± 2.18 | 0.361 | 0.719 | |
| Na+ (mmol/L) | 136.24 ± 18.67 | 126.74 ± 12.35 | 2.450 | 0.017 | |
| PLT (× 109/L) | 60.03 ± 12.47 | 56.42 ± 10.19 | 1.318 | 0.192 | |
| PT (s) | 16.12 ± 3.09 | 17.83 ± 2.83 | 2.427 | 0.018 | |
| Cys C (mg/L) | 1.68 ± 0.36 | 2.43 ± 0.47 | 7.813 | 0.000 | |
| INR | 1.57 ± 0.32 | 1.84 ± 0.39 | 3.278 | 0.002 | |
| Child-Pugh score (points) | 10.09 ± 1.45 | 12.87 ± 1.29 | 8.494 | 0.000 | |
| MELD score (points) | 11.58 ± 2.34 | 17.46 ± 2.98 | 9.547 | 0.000 | |
Spearman analysis was used to investigate the correlation between prognosis and variables such as Child-Pugh score, MELD score, and Cys C. The results suggested that Child-Pugh (r = 0.709, P < 0.05), MELD (r = 0.749, P < 0.05), and Cys C (r = 0.671, P < 0.05) had moderate and high positive associations with prognosis, as displayed in Table 2.
| Indicator | Child-Pugh scores | MELD scores | Cys C | |||
| r | P value | r | P value | r | P value | |
| Prognosis | 0.709 | 0.000 | 0.749 | 0.000 | 0.670 | 0.000 |
The findings of the multifactor analysis employing the Cox proportional hazard model showed that the independent risk factors for post-TIPS prognosis in patients with liver cirrhosis and refractory ascites were Cys C (hazard ratio (HR): 3.802; 95%CI: 1.313-11.015), Child-Pugh scores (HR: 3.030; 95%CI: 1.858-4.943), and MELD scores (HR: 1.222; 95%CI: 1.073-1.393; Table 3).
| Factor | B | Sb | Wald χ2 | P value | HR (95%CI) |
| AST | -0.022 | 0.017 | 1.627 | 0.202 | 0.978 (0.946-1.012) |
| ALT | 0.041 | 0.027 | 2.320 | 0.128 | 1.042 (0.988-1.099) |
| TBil | -0.017 | 0.016 | 1.179 | 0.278 | 0.983 (0.953-1.014) |
| Alb | -0.012 | 0.033 | 0.131 | 0.717 | 0.988 (0.927-1.054) |
| Scr | -0.042 | 0.023 | 3.243 | 0.072 | 0.959 (0.916-1.004) |
| Na+ | -0.028 | 0.021 | 1.766 | 0.184 | 0.972 (0.933-1.013) |
| PT | 0.001 | 0.095 | 0.000 | 0.991 | 1.001 (0.831-1.206) |
| Cys C | 1.336 | 0.543 | 6.057 | 0.014 | 3.802 (1.313-11.015) |
| INR | 1.282 | 0.714 | 3.228 | 0.072 | 3.604 (0.890-14.597) |
| Child-Pugh scores | 1.109 | 0.250 | 19.723 | 0.000 | 3.030 (1.858-4.943) |
| MELD scores | 0.201 | 0.067 | 9.099 | 0.003 | 1.222 (1.073-1.393) |
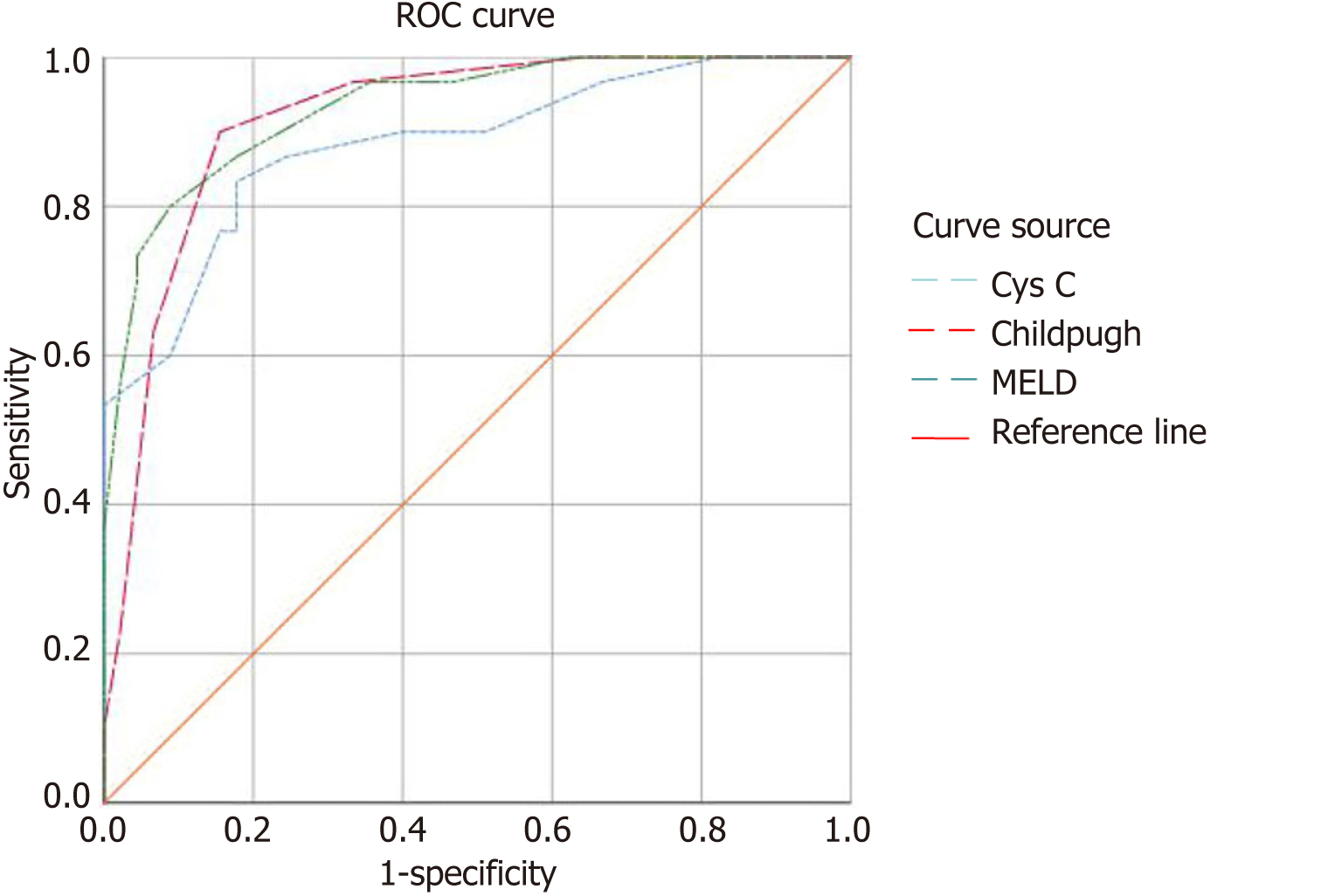
The findings of the ROC analysis revealed that the classic prognostic models based on the Child-Pugh and MELD scores exhibited marginally superior diagnostic efficacy in predicting the post-TIPS prognosis in patients with liver cirrhosis and refractory ascites. For the Child-Pugh score, the sensitivity and specificity were 90% and 84.244%, respectively, with an area under the curve (AUC) of 0.919 and an optimal cutoff value of 11.5 points. For the MELD score, the sensitivity and specificity were 86.67% and 82.22%, respectively, with an AUC of 0.934 and an optimal cutoff value of 13.5 points. In contrast, Cys C exhibited a slight decrease in diagnostic efficiency for predicting post-TIPS prognosis in patients with liver cirrhosis and refractory ascites, with a sensitivity and specificity of 83.33% and 82.22%, respectively; an AUC of 0.883; and an optimal cutoff value of 1.95 mg/L (Table 4 and Figure 1).
| Risk factor | ROC curve | Optimal cutoff value | Sensitivity (%) | Specificity (%) | ||
| AUC | 95%CI | P value | ||||
| Cys C | 0.883 | 0.803-0.963 | 0.000 | 1.95 mg/L | 83.33 (25/30) | 82.22 (37/45) |
| Child-Pugh scores | 0.919 | 0.856-0.981 | 0.000 | 11.50 points | 90.00 (27/30) | 84.44 (38/45) |
| MELD scores | 0.934 | 0.879-0.988 | 0.000 | 13.50 points | 86.67 (26/30) | 82.22 (37/45) |
The primary initiating factor for refractory ascites is liver cirrhosis with PH. This condition can cause bacterial translocation, vascular dilation, renal perfusion insufficiency, and water-sodium retention, eventually resulting in ascites and progression to refractory ascites. According to current and international guidelines, liver transplantation is an efficacious treatment. Nevertheless, in light of the scarcity of available donor livers, TIPS placement is advocated as a viable alter
Cys C, characterized by its low molecular weight and nonglycated nature, is stably synthesized by the body’s nucleus cells. Its metabolic processes are predominantly confined to the renal system, wherein it undergoes free filtration in the glomeruli and subsequent reabsorption and breakdown in the proximal tubules. Cys C synthesis remains relatively steady and is scarcely influenced by factors such as sex, age, or activity. Initially, the NRS-2002 was used to assess early renal function impairment in patients[27]. Several previous investigations have revealed that the measurement of Cys C is a valuable instrument for the timely detection of moderate renal dysfunction, especially in individuals with cirrhosis (especially those in Child-Pugh class C) or female patients[15,28-30]. A study by Seo et al[31] demonstrated that Cys C levels are an independent predictor of mortality in cirrhosis patients with ascites, whereas Scr levels are not. Notably, a study by Torner et al[32] demonstrated for the first time that in TIPS patients, creatinine is a better predictor of mortality in males, while Cys C is a better predictor of mortality in females. Nevertheless, there is a significant dearth of research on Cys C in patients who have undergone TIPS treatment. The Child-Pugh score is a commonly applied clinical grading criterion for quantitatively assessing liver reserve function in patients with cirrhosis. The MELD score is a recognized indicator of cirrhosis severity and serves as a predictive factor for the incidence and mortality rate among patients under
In summary, for patients with liver cirrhosis and refractory ascites with a poor prognosis after TIPS, elevated serum Cys C levels were observed. These levels displayed a moderate to high positive correlation with classic prognostic models, such as the Child-Pugh and MELD scores. The Cox proportional hazard model confirmed that the serum Cys C concentration was an independent risk factor influencing the prognosis in liver cirrhosis patients with refractory ascites post-TIPS (HR = 3.802; 95%CI: 1.313-11.015). Given their convenience, minimal susceptibility to sex, age, activity, and inflammation, as well as their ability to furnish reliable data, measurements of Cys C can potentially function as specific inflammatory biomarkers for the assessment of prognosis in individuals affected by these medical conditions. Furthermore, ROC curve analysis validated the value of the serum Cys C concentration in assessing the prognosis of liver cirrhosis patients with refractory ascites post-TIPS, with an optimal cutoff value of 1.95 mg/L. This has remarkable guiding implications for follow-up and treatment. However, the utility of predictive models based on Cys C needs confirmation in prospective large-scale studies.
The transjugular intrahepatic portosystemic shunt (TIPS) is a frequently employed interventional technique for the management of hepatic ascites, yet uncertainties persist regarding its prognosis. Serum marker levels may play a crucial role in predicting the prognosis of patients with hepatic ascites who are undergoing TIPS procedures.
Currently, there is a lack of comprehensive research on the correlation between serum marker levels and the prognosis for TIPSs in hepatic ascites patients, resulting in a limited understanding of this topic. Consequently, the purpose of this study was to comprehensively explore the associations between various serum markers and the prognosis in individuals with hepatic ascites who underwent TIPS, with the aim of enhancing the precision of prognostic assessment and guiding treatment strategies.
This study aimed to investigate the associations between Child-Pugh score, model for end-stage liver disease (MELD) score, serum cystatin C (Cys C) level, and post-TIPS prognosis in patients with liver cirrhosis refractory ascites.
We conducted a retrospective study involving 75 patients with decompensated liver cirrhosis and refractory ascites, all of whom underwent TIPS at our institution from August 2019 to August 2021. Over a two-year period, comprehensive follow-up assessments were undertaken, and patient outcomes were meticulously recorded. Clinical data, including Child-Pugh and MELD scores, were systematically collected. Spearman correlation analysis was used to evaluate the associations between the Child-Pugh score, MELD score, and Cys C level. The Cox proportional hazards model was used to identify independent risk factors influencing patient prognosis. Receiver operating characteristic curves were generated to assess the ability of Cys C levels, Child-Pugh scores, and MELD scores to predict the prognosis subsequent to TIPS treatment.
After 2 years of TIPS treatment, 40.00% of the 75 patients with refractory ascites due to liver cirrhosis passed away. Increased aspartate aminotransferase, alanine aminotransferase, total bilirubin, serum creatinine (Scr), prothrombin time, Cys C, international normalized ratio, Child-Pugh, and MELD scores were observed in the deceased group, while albumin and Na+ levels decreased (P < 0.05). The Child-Pugh score, MELD score, and Cys C concentration were iden
Monitoring the serum Cys C concentration holds great value in assessing the prognosis in patients with refractory ascites due to liver cirrhosis after TIPS treatment. Compared to the use of Scr levels, predictive models based on serum Cys C levels are more beneficial for evaluating the condition and prognosis of patients with ascites due to cirrhosis.
This study provides evidence of the correlation between serum biomarkers and the prognosis of patients undergoing TIPS, which may serve as a prognostic indicator. Nevertheless, additional validation and extension of the relationship between serum biomarkers and TIPS prognosis necessitate further inquiry. This entails conducting larger-sample clinical trials and exploring additional factors that may influence prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Paula Ceballos M, Argentina S-Editor: Yan JP L-Editor: A P-Editor: Zhang YL
| 1. | Wilson R, Williams DM. Cirrhosis. Med Clin North Am. 2022;106:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 402] [Article Influence: 36.5] [Reference Citation Analysis (10)] |
| 3. | Bajaj JS, Kamath PS, Reddy KR. The Evolving Challenge of Infections in Cirrhosis. N Engl J Med. 2021;384:2317-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 824] [Article Influence: 206.0] [Reference Citation Analysis (1)] |
| 5. | Karagiannakis DS, Voulgaris T, Siakavellas SI, Papatheodoridis GV, Vlachogiannakos J. Evaluation of portal hypertension in the cirrhotic patient: hepatic vein pressure gradient and beyond. Scand J Gastroenterol. 2018;53:1153-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Tonon M, Piano S. Cirrhosis and Portal Hypertension: How Do We Deal with Ascites and Its Consequences. Med Clin North Am. 2023;107:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Jeong SW. [Ascites]. Korean J Gastroenterol. 2018;72:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Larrue H, Vinel JP, Bureau C. Management of Severe and Refractory Ascites. Clin Liver Dis. 2021;25:431-440. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Rajesh S, George T, Philips CA, Ahamed R, Kumbar S, Mohan N, Mohanan M, Augustine P. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26:5561-5596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Parker R. Role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Clin Liver Dis. 2014;18:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zhang JB, Chen J, Zhou J, Wang XM, Chen S, Chu JG, Liu P, Ye ZD. Systematic review and meta-analysis of trans-jugular intrahepatic portosystemic shunt for cirrhotic patients with portal vein thrombosis. World J Clin Cases. 2021;9:5179-5190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Angeli P. The first Chinese guidelines on the Management of Ascites and its Related Complications in Cirrhosis: a great goal for a great country. Hepatol Int. 2019;13:395-398. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Abd El Wahab AM, Awadeen A, Mansour MM, Shemies R. The diagnostic and prognostic utility of serum cystatin C and angiopoietin 2 in patients with liver cirrhosis complicated by acute kidney injury. Ther Apher Dial. 2023;27:419-427. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Hepatology; Chinese Medical Association; Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, Linghu E, Zhuang H. Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int. 2019;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Hirsh J, Poller L. The international normalized ratio. A guide to understanding and correcting its problems. Arch Intern Med. 1994;154:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Favaloro EJ. Optimizing the Verification of Mean Normal Prothrombin Time (MNPT) and International Sensitivity Index (ISI) for Accurate Conversion of Prothrombin Time (PT) to International Normalized Ratio (INR). Methods Mol Biol. 2017;1646:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3667] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 20. | Singh V, De A, Mehtani R, Angeli P, Maiwall R, Satapathy S, Singal AK, Saraya A, Sharma BC, Eapen CE, Rao PN, Shukla A, Shalimar, Choudhary NS, Alcantara-Payawal D, Arora V, Aithal G, Kulkarni A, Roy A, Shrestha A, Mamun Al Mahtab, Niriella MA, Siam TS, Zhang CQ, Huei LG, Yu ML, Roberts SK, Peng CY, Chen T, George J, Wong V, Yilmaz Y, Treeprasertsuk S, Kurniawan J, Kim SU, Younossi ZM, Sarin SK. Asia-Pacific association for study of liver guidelines on management of ascites in liver disease. Hepatol Int. 2023;17:792-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 22. | Lin CL, Tseng KC, Chen KY, Liao LY, Kao JH. Factors predicting outcomes of hepatitis B-related cirrhosis patients with long-term antiviral therapy. J Formos Med Assoc. 2020;119:1483-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Khan A, Maheshwari S, Gupta K, Naseem K, Chowdry M, Singh S. Rate, reasons, predictors, and burden of readmissions after transjugular intrahepatic portosystemic shunt placement. J Gastroenterol Hepatol. 2021;36:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kumada T, Toyoda H, Yasuda S, Miyake N, Ito T, Tanaka J. Long-term prognosis with or without nucleot(s)ide analogue therapy in hepatitis B virus-related decompensated cirrhosis. J Viral Hepat. 2021;28:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Balcar L, Tonon M, Semmler G, Calvino V, Hartl L, Incicco S, Jachs M, Bauer D, Hofer BS, Gambino CG, Accetta A, Brocca A, Trauner M, Mandorfer M, Piano S, Reiberger T; Baveno Cooperation: an EASL consortium. Risk of further decompensation/mortality in patients with cirrhosis and ascites as the first single decompensation event. JHEP Rep. 2022;4:100513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Torp N, Israelsen M, Madsen B, Lutz P, Jansen C, Strassburg C, Mortensen C, Knudsen AW, Sorensen GL, Holmskov U, Schlosser A, Thiele M, Trebicka J, Krag A. Level of MFAP4 in ascites independently predicts 1-year transplant-free survival in patients with cirrhosis. JHEP Rep. 2021;3:100287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Shlipak MG, Inker LA, Coresh J. Serum Cystatin C for Estimation of GFR. JAMA. 2022;328:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Mindikoglu AL, Opekun AR, Mitch WE, Magder LS, Christenson RH, Dowling TC, Weir MR, Seliger SL, Howell CD, Raufman JP, Rana A, Goss JA, Khaderi SA, Vierling JM. Cystatin C Is a Gender-Neutral Glomerular Filtration Rate Biomarker in Patients with Cirrhosis. Dig Dis Sci. 2018;63:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Randers E, Ivarsen P, Erlandsen EJ, Hansen EF, Aagaard NK, Bendtsen F, Vilstrup H. Plasma cystatin C as a marker of renal function in patients with liver cirrhosis. Scand J Clin Lab Invest. 2002;62:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Gerbes AL, Gülberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Seo YS, Park SY, Kim MY, Kim SG, Park JY, Yim HJ, Jang BK, Park SH, Kim JH, Suk KT, Kim JD, Kim TY, Cho EY, Lee JS, Jung SW, Jang JY, An H, Tak WY, Baik SK, Hwang JS, Kim YS, Sohn JH, Um SH. Serum cystatin C level: An excellent predictor of mortality in patients with cirrhotic ascites. J Gastroenterol Hepatol. 2018;33:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Torner M, Mangal A, Scharnagl H, Jansen C, Praktiknjo M, Queck A, Gu W, Schierwagen R, Lehmann J, Uschner FE, Graf C, Strassburg CP, Fernandez J, Stojakovic T, Woitas R, Trebicka J. Sex specificity of kidney markers to assess prognosis in cirrhotic patients with TIPS. Liver Int. 2020;40:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Bayona Molano MDP, Barrera Gutierrez JC, Landinez G, Mejia A, Haskal ZJ. Updates on the Model for End-Stage Liver Disease Score and Impact on the Liver Transplant Waiting List: A Narrative Review. J Vasc Interv Radiol. 2023;34:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Suksamai A, Chaiprasert A, Chirapongsathorn S. Serum cystatin C as a predictor of 90-day mortality among patients admitted with complications of cirrhosis. JGH Open. 2021;5:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |