Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.409
Peer-review started: September 13, 2023
First decision: December 8, 2023
Revised: December 14, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: February 27, 2024
Processing time: 165 Days and 3.8 Hours
Advancements in laparoscopic technology and a deeper understanding of intra
To investigate a standardized cranial-dorsal strategy for LLH, focusing on important anatomical markers, surgical skills, and ICG staining methods.
Thirty-seven patients who underwent ICG fluorescence-guided LLH at Qujing Second People's Hospital between January 2019 and February 2022 were retrospectively analyzed. The cranial-dorsal approach was performed which involves dissecting the left hepatic vein cephalad, isolating the Arantius ligament , exposing the middle hepatic vein, and dissecting the parenchyma from the dorsal to the foot in order to complete the anatomical LLH. The surgical methods, as well as intra- and post-surgical data, were recorded and analyzed. Our hospital’s Medical Ethics Committee approved this study (Ethical review: 2022-019-01).
Intraoperative blood loss during LLH was 335.68 ± 99.869 mL and the rates of transfusion and conversion to laparotomy were 13.5% and 0%, respectively. The overall incidence of complications throughout the follow-up (median of 18 months; range 1-36 months) was 21.6%. No mortality or severe complications (level IV) were reported.
LLH has the potential to become a novel, standardized approach that can effectively, safely, and simply expose the middle hepatic vein and meet the requirements of precision surgery.
Core Tip: Current laparoscopic hepatectomy (LH) approaches require advanced skills and pose challenges in anatomical landmark identification. Advancements in understanding intrahepatic anatomy and laparoscopic technology will improve LH procedures. By combining a cranial-dorsal approach along the middle vein with the use of indocyanine green staining, we introduce a novel method for laparoscopic left hemihepatectomy. Our new approach is feasible, streamlining the procedure for surgeons and assistants. Our preliminary results indicate that our approach might represent a significant improvement in LH outcomes. Enhanced LH approaches potentially improve patient outcomes.
- Citation: Wang XR, Li XJ, Wan DD, Zhang Q, Liu TX, Shen ZW, Tong HX, Li Y, Li JW. Laparoscopic left hemihepatectomy guided by indocyanine green fluorescence: A cranial-dorsal approach. World J Gastrointest Surg 2024; 16(2): 409-418
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/409.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.409
Advancements in comprehending intrahepatic anatomy, coupled with rapid progress in laparoscopic technology, mark a transformative phase, ushering laparoscopic hepatectomy (LH) into an era of meticulous anatomical resection[1]. Recently, the indocyanine green (ICG) fluorescence navigation technique has been applied in clinical practice and has emerged as the most effective method for identifying hepatic regions[2]. Moreover, intraoperative ICG navigation might help overcome the limitations of LH[3]. Laparoscopic left hemihepatectomy (LLH) is an established and standardized procedure[4], supported by numerous relevant surgical guidelines and standards[5]. We began to explore the cranial-dorsal strategy along the middle hepatic vein (MHV) in 2017. This strategy is safe and feasible for LLH, with certain advantages[6]. This study aimed to investigate LLH performed with a cranial-dorsal strategy under ICG fluorescence guidance, with a specific focus on the anatomical landmarks and surgical skills required for LLH.
Data from thirty-seven patients who underwent ICG fluorescence-guided LLH at Qujing Second People's Hospital between January 2019 and February 2022 were retrospectively analyzed. The surgical methods, as well as intra- and post-surgical data, were recorded and analyzed. The patients’ baseline characteristics are listed in Table 1. Our hospital’s Medical Ethics Committee approved this study.
| Characteristics | Total (n = 37) |
| Age (yr) | 55.00 [26.00; 78.00] |
| Sex | |
| Female | 16 (43.24) |
| Male | 21 (56.76) |
| BMI (kg/m2) | 22.16 ± 2.52 |
| Hepatitis B/C virus status | |
| B | 18 (48.65) |
| C | 0 (0.00) |
| Neither | 19 (51.35) |
| Diagnosis | |
| HCC | 12 (32.43) |
| HL | 21 (56.76) |
| FNH | 2 (5.41) |
| HH | 2 (5.41) |
| Cirrhosis | |
| No | 33 (89.19) |
| Yes | 4 (10.81) |
| Comorbidities | |
| No | 17 (45.95) |
| Yes | 20 (54.05) |
| Transfusion | |
| No | 32 (86.49) |
| Yes | 5 (13.51) |
| Complications | |
| No | 29 (78.38) |
| Yes | 8 (21.62) |
| Clavien complication grade | |
| I | 4 (10.81) |
| II | 3 (8.11) |
| III | 1 (2.70) |
| IV | 0 (0.00) |
| Intra-surgical blood loss (mL) | 335.68 ± 99.869 |
| Operative time (min) | 217.92 ± 74.19 |
| Post-surgical hospital stay (d) | 8.00 [5.00; 12.00] |
| Child–Pugh grade | |
| A | 31 (83.78) |
| B | 6 (16.22) |
| Previous surgery | |
| No | 37 (100.00) |
| Yes | 0 (0.00) |
| ICG staining method (+) | |
| No | 15 (40.54) |
| Yes | 22 (59.46) |
The inclusion criteria were as follows: (1) The lesion was confined to the left lobe of the liver; (2) Complete follow-up profile; (3) Patients who underwent ICG fluorescence-guided LLH; and (4) The cranial-dorsal approach was performed.
The exclusion criteria were as follows: (1) Previous surgery; (2) Long-term use of immunosuppressive medications; (3) History of antitumor therapy before surgery; and (4) Patients with a psychiatric history.
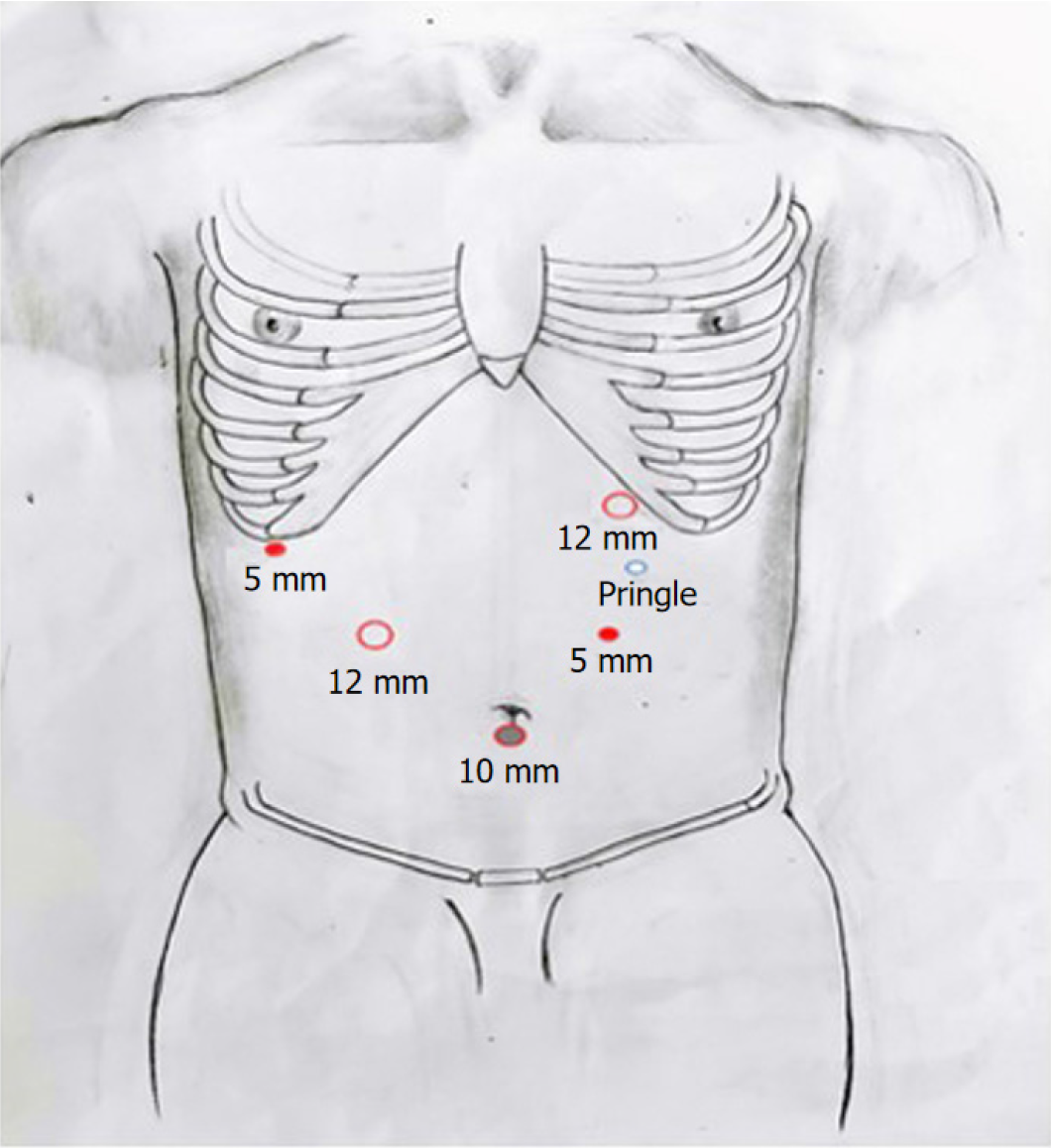
Trocar pore distribution, anesthesia, and position: Patients were placed in the supine position and were administered a combination of intravenous and inhalation anesthetics. Figure 1 illustrates the placement of the five-hole trocars. The main surgeon was positioned on the left side of the patient, while the assistant was on the right side. Carbon dioxide was injected into the abdominal cavity through the cannula needle hole below the navel, inducing a pneumoperitoneum with a pressure maintained between 11-13 mmHg. To control blood inflow, an intermittent Pringle maneuver was performed using a patented hilar occlusion device (Patent number: 200920127107.7) with an interval of 10 min. The maneuver was repeated after an interval of 5 min[7]. Dexamethasone (10 mg) was administered intravenously before the initial occlusion.
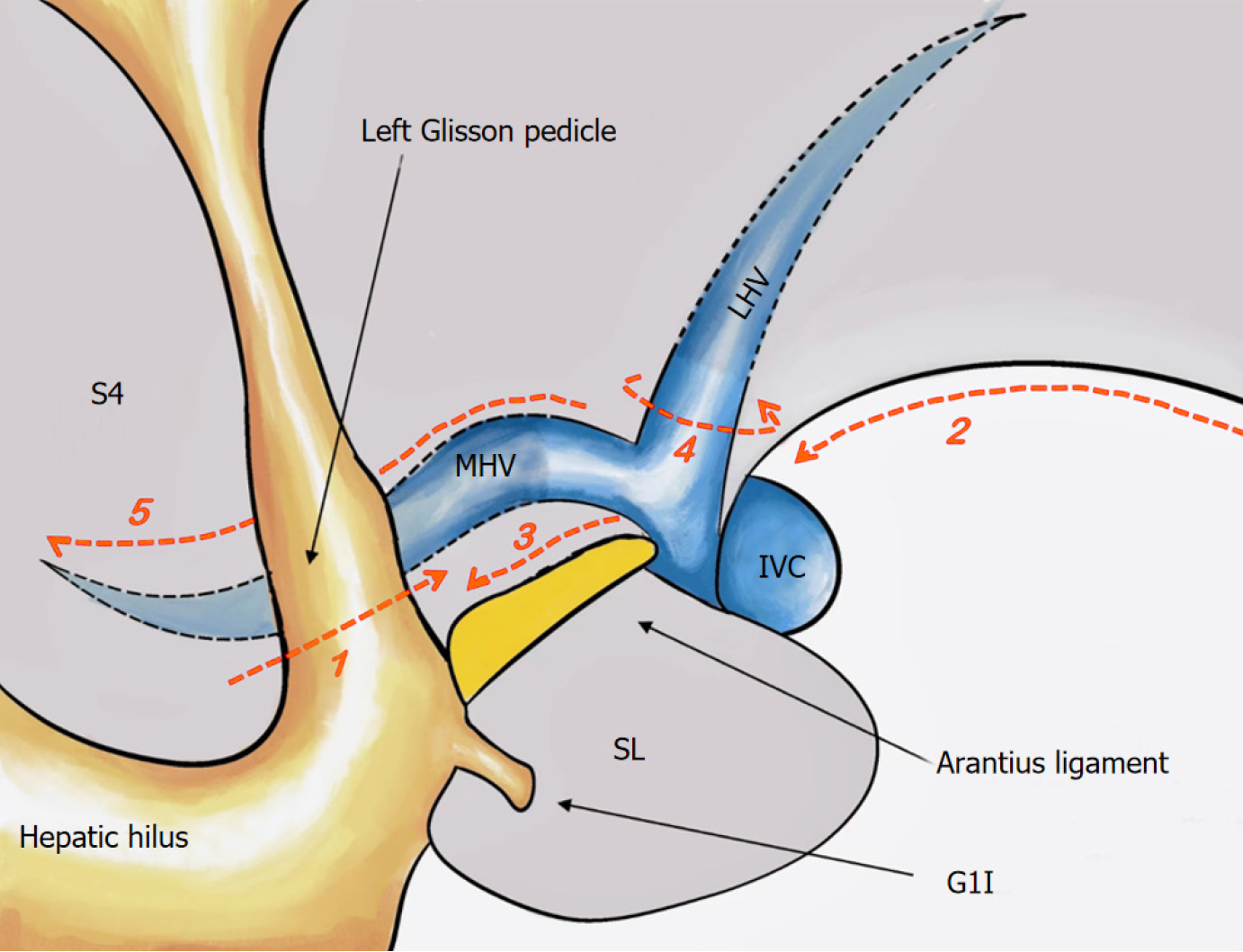
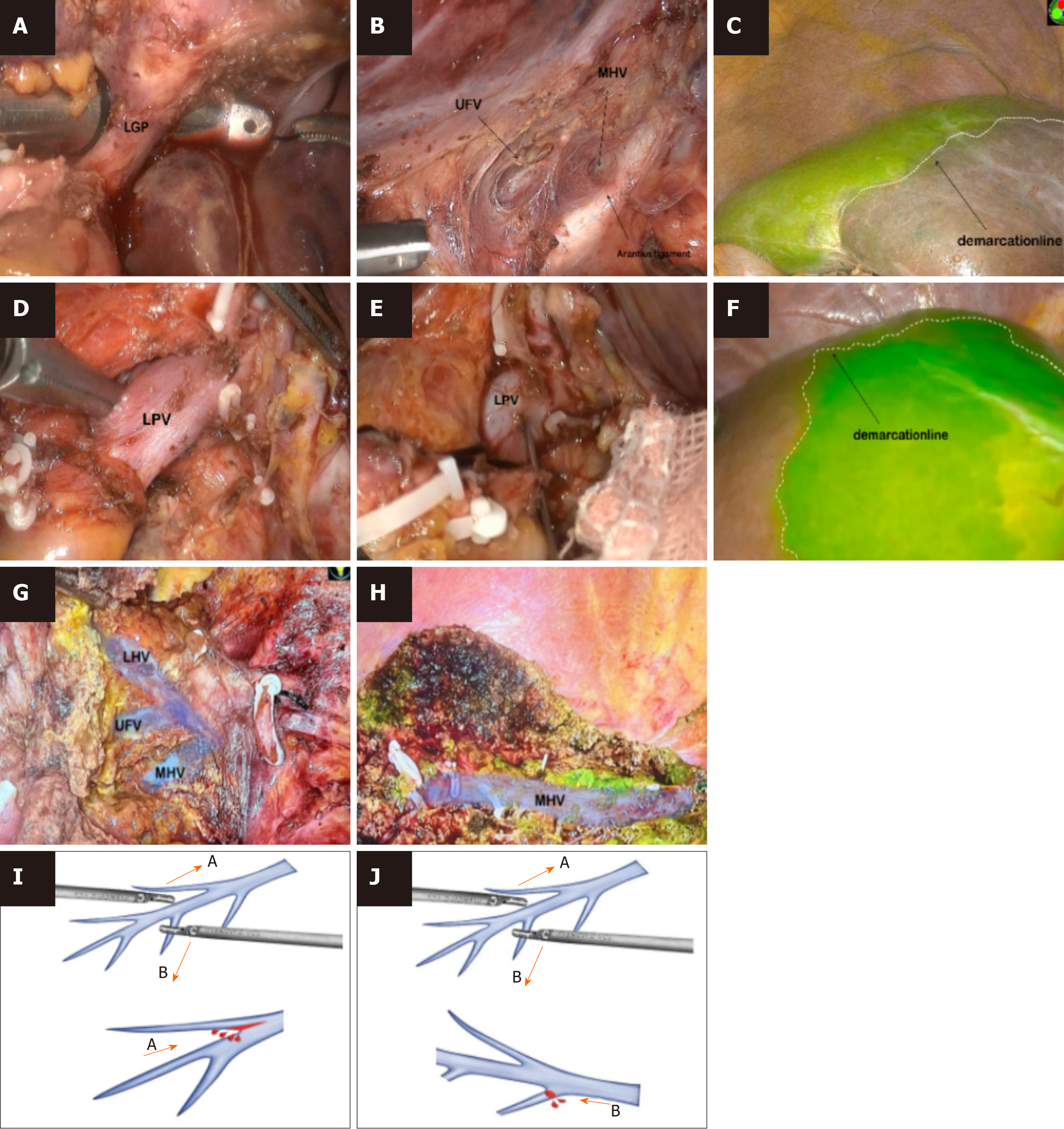
Treatment of the first hepatic hilum and ICG staining: Figure 2 shows the standard procedure of the cranial-dorsal strategy along the Arantius ligament in LLH. The hepatic portal plate was lowered, and both sides of the left Glissonean pedicle (GP) were dissected ventral to the Arantius ligament. Then, the laparoscopic instrument, called “Goldfinger”, was inserted within the capsule space over the Arantius ligament (Figures 3A and B), while carefully isolating the extrahepatic left GP in order to avoid damage to the duct of the left caudate and right hepatic lobes. Fifteen patients underwent ICG-negative staining (Figure 3A-D). A linear cutter was used to cut the left hepatic pedicle. Following the appearance of an obvious ischemic line between the left and right liver lobes, ICG was injected into the peripheral blood circulation for negative staining. Twenty-two patients underwent ICG-positive staining after removing the sheath from the extrahepatic pedicle and separating the GP (Figure 3E-H). The left and middle hepatic arteries were divided for ligation, and the left portal vein (LPV) was punctured for ICG injection (Figure 3E). The hepatic resection plane was determined after successful staining (Figure 3F), and the LPV was ligated and severed.
Hepatic mobilization and the second hepatic hilum treatment: The ligaments, including the round, sickle, and left perihepatic ligaments, were mobilized and the second hepatic hilum was exposed, while focusing on protecting the phrenic vein. The assistant created a favorable surgical field by lifting the hepar toward the ventral side. The Arantius plate was completely peeled from the hepatic parenchyma along Laennec’s gap, exposing the Arantius ligament ventrum. The cranial-dorsal strategy was used to expose the left hepatic vein (LHV) root and the position in which the LHV merged with the inferior vena cava. Forceps-assisted separation of the LHV was followed, using a linear cutter to suspend and sever the LHV. Notably, the hepatic diaphragmatic membrane must be opened if the LHV and MHV are confluent and have a common trunk.
Hepatic parenchyma transection: A fluorescence-stained boundary was used to highlight the hepatic anterior surface, and the Arantius ligament was used to mark the hepatic dorsal side. The hepatic parenchyma was transected from cranial to ventral and dorsal to ventral sides, adhering to the “clipped with ultrasonic knife” rule. When hepatic venous bleeding occurred, the bleeding point was assessed after blood drainage, and small ethmoid bleeding was treated with hemostatic material for compression. If the MHV branch was damaged, a Hemolock was used to clamp it. If the main trunk of the MHV was damaged, 5-0 Prolene was used to actively suture the bleeding vessels to avoid stenosis or a larger tear, and the first hilus was temporarily blocked if necessary. A 1–3 mm vascular incision was performed using an ultrasonic scalpel, and the blood vessels (≥ 3 mm) were clamped using a Hemolock and then severed. The surrounding hepatic parenchyma was completely crushed, and the entire circumference of the vasculature was stripped. Before severing, it was confirmed that there were no additional vessels or branches on the dorsal side. To separate the liver completely, the hepatic parenchyma was severed from the foot. As the procedure progressed, the MHV was gradually exposed at 180° to the hepatic section (Figure 3H). Choledochotomy, choledochoscopic exploration, and calculus removal were performed in patients with choledocholithiasis, followed by the conventional placement of a T-tube.
All analyses in this study were conducted using SPSS 23.0 statistical software. Counting data are expressed as n (%), and measurement data with a normal distribution are expressed as mean ± SD, while those with a non-normal distribution are expressed as median (Q1, Q3).
The intraoperative blood loss during LLH was 335.68 ± 99.869 mL, and the rates of transfusion and conversion to laparotomy were 13.5% and 0%, respectively. Compared with a previously reported LLH group[8], blood loss in the current study was much lower, demonstrating satisfactory results. The rate of midway opening operation in this study was 0%, which was better than that reported in previous studies[9,10]. All staining procedures were successfully completed, exposing the MHV at 180° across the hepatic sections throughout each procedure.
The incidence of complications was 21.6%. Ascites occurred in one case, necessitating abdominal puncture and drainage, and bile leakage occurred in three cases, successfully treated by continuous drainage with a pre-indwelling drain tube. None of the patients developed bile leakage requiring drain puncture. Two patients experienced lung infections, and two patients had infections in the abdominal cavity; however, none developed infections around the incision. After performing bacterial cultures on bile or drain fluid, infections were treated with antibiotics selected based on drug sensitivity tests. No deaths or severe complications (level IV) were observed. All patients with complications were discharged after significant symptom improvement.
All patients (37) were successfully followed up for a median period of 18 months (range, 1–36 months). The intra-surgical stone clearance rate in patients with hepatolithiasis was 100%, and the combined rate of stone and cholangitis recurrence was 4.76%. The 1- and 3-year overall survival rates of the patients with hepatic malignancies were 90% and 60%, respectively. Tumor recurrence was observed in three patients: one underwent reoperation, and two underwent a combination of radiofrequency ablation and targeted therapy. Two patients died due to tumor recurrence, metastasis, and liver failure. No patient developed residual stones; only one case showed stone recurrence 11 months later and eventually underwent endoscopic retrograde cholangiopancreatography following stone removal.
According to Makuuchi, hepatic resection involves sectioning the main hepatic pedicle of the target hepatic section and fully exposing the main hepatic vein[9]. Moreover, complete resection of the tumor-containing portal vein and affected bile duct drainage areas is required[11]. Conversely, LH requires advanced skills, and the complexity of intraoperative ultrasound examination and the loss of tactile sensation to the laparoscopic forceps limit its application, making it challenging to localize the tumor and confirm the demarcation line[12]. In addition, one of the challenges of LH is determining the plane of hepatic parenchyma transection due to difficulties in implementing methods such as hepatic staining, which are typically used during open hepatectomy. Over the past two decades, hepatobiliary surgeons have increasingly focused on ICG[13]. Dissecting the hepatic parenchyma along the borders of the fluorescently stained region enables anatomical LH. The hepatic vein serves as a landmark of the intrahepatic plane as it marks the boundary between the hepatic lobes and sections. The MHV and GP-guided strategy can assist in achieving an anatomical LLH[14]. However, the direction of MHV exposure throughout the entire process remains controversial. In 2017, our research team began investigating the cranial-dorsal strategy along the MHV, which is safe and feasible for anatomical LLH and presents specific benefits[6,15]. Hence, this study underscores the significance of combining the cranial-dorsal strategy with ICG fluorescence staining for ensuring safe and standardized surgical techniques and maintaining high-quality operative assurance.
LLH can reduce bleeding and prevent disorientation by exposing the MHV. Following the unique caudal view advantage of laparoscopy, the hepatic parenchyma is typically transected from the caudal to cranial direction[16]. When the MHV branches are damaged on the distal side, lacerations easily spread to the central side, and the operative field is obscured, resulting in difficult repair and massive bleeding (Figure 3I). In addition, the tenting sign[17] might be observed, which makes it difficult to expose the MHV within the entire hepatic section after the surgeon loses the sense of direction. The exposure of the MHV via the cranial strategy corresponds to the branching direction of the hepatic vein. Bleeding caused by the laceration can be reduced by moving the ultrasonic scalpel from the central to the distal side of the vein (Figure 3J) using small forceps[18-20]. In this study, MHVs were exposed in all patients. During the hepatic parenchyma transection from the demarcation line of the MHV exposed at the hepatic section to the hepatic surface, the GP was almost never observed and could be easily separated. Using this strategy, the dorsal side of the LHV frequently appears lined with other veins, such as the umbilical fissure vein (UFV), which should not be considered as the MHV without further confirmation (Figure 3G). According to our experience, the UFV runs ventrally when the left hepatic artery is lifted; therefore, it is necessary to keep looking at the dorsal side of the MHV. Surgeons should continue separating the caudal side and identify the first large vein once it is exposed on the dorsal side of the LHV by cranial dissection. In this study, MHVs were easily recognized through additional section openings, guided by ICG fluorescent staining. If the vein was dorsally oriented and found in the hepatic area stained by ICG, it was deemed to be the MHV.
The first study describing the application of fluorescence imaging for sectional mapping during hepatic surgery was published in 2008 by Aoki et al[21]. Ishizawa et al[22] reported the use of the Aoki method in 2012 for LH with fluorescence imaging, categorizing the results as “positive” and “negative”. Negative staining was achieved by administering 5–10 mL of ICG (0.025 mg/mL) following ligation of the left GP. Positive staining was accomplished by injecting 5–10 mL of ICG directly into the LPV using a disposable intravenous infusion needle (0.7 × 23.5 TWLB black). ICG should be administered slowly to prevent backflow into branches serving other sections. In our study, the effect of staining was satisfactory, and the MHV was completely exposed in all patients.
The postoperative complications of hepatectomy are directly related to patient prognosis, requiring prompt and appropriate treatment[23]. Due to deep surgical site infection after hepatectomy, bile leakage is a common complication. In our study, four patients experienced grade A biliary leakage, and no patients developed grade B or C biliary leakages. This incidence is lower than that reported in the literature[24], demonstrating that our strategy can reduce the incidence of biliary leakage. If the bile leakage volume is small and limited, the drain is kept open, or the drain tube is appropriately replaced while awaiting the localization of bile leakage or the formation of a sinus tract. In severe cases, simultaneous endoscopic nasobiliary drainage can be performed, and right hepatic duct injury is a severe side effect. In our experience, it is best to transect the hepatic parenchyma to some extent after the LHP has been ligated and suspended to search for the right duct before the left pedicle is severed. Moreover, injury and stenosis of the duct caused by the disconnection of the endoscopic linear cutter should be prevented. Attention should be paid to the joint direction of the right-bent endoscopic linear cutter. In the present study, no right hepatic duct injuries were observed. Hemostasis and blood transfusion should be performed if active bleeding is observed. If bleeding worsens, decisive intervention or surgical exploration should be performed immediately to ensure the best treatment outcome for the patient[24]. To reduce hepatic ischemia and congestion, the structural integrity of the inflow and outflow tracts should be considered during surgery, as well as the function of the hepatic reserve and residual hepatic volume[24]. After surgery, comprehensive fluid management, prompt evaluation, and prompt treatment should be ensured to prevent complications.
Treatment of the second hepatic portal is particularly important when following the cranial-dorsal approach in LLH. Fast and accurate identification of the MHV is crucial. To determine the MHV, preoperative imaging data can be used to preliminarily determine the variation and cooperation of the hepatic vein. If conditions permit, three-dimensional visualization can further improve surgical planning. In this study, during the operation, the authors chose fluorescence staining-assisted technology for identification of the hepatic vein, after which the target liver was stained. The vessels in the target liver were within the staining range, thereby assisting the surgeon in determining the veins exposed in the field of vision and facilitating the experience of exposing the visual field of the assistant during liver resection, which may also be related to the patient’s position, as well as the direction of liver resection. At the same time, there was no significant difference in the treatment of blood vessels in the liver section between the main knife and foot sides. In addition, our preliminary data indicate that the cranial-dorsal approach is more advantageous than the conventional approach in terms of bleeding volume, surgical time, and exposure of the MHV during liver transection.
Although our strategy has many advantages, it also has certain drawbacks. In our study, surgeons have adjusted the changes in trocar holes and surgical position. The main operation hole of traditional LLH is hole D, placed on the line of the hepatic transection. In contrast, hole B, the main operation hole of LLH via the cranial-dorsal strategy, is positioned closer to the second hepatic hilum. Traditional LLH does not require the LHV to be cut off or severed prior to hepatic transection; whereas in our technique, the LHV had to be completely severed and dissociated before separating hepatic parenchyma and exposing the MHV root. If the MHV and LHV are confluent, it is necessary to open the diaphragmatic hepatic capsule, dissociate it, and cut off the LHV from the hepatic tissue. This strategy is feasible when the tumor is near or overlaps the second hilum, affecting its exposure. Therefore, appropriate patient selection is required, and surgeons should be proficient in laparoscopic liver resection. This approach also carries a risk of hepatic vein root injury, particularly when performed by less-experienced surgeons.
In conclusion, the cranial-dorsal strategy has the potential to become a novel and standardized approach for LLH, and can effectively, safely, and simply expose the MHV to meet the requirements of precision surgery. However, due to the small sample size, this study lacks a comparison of different ICG staining methods, which will be addressed in future studies.
The improved understanding of intrahepatic anatomy and rapid advances in laparoscopic technology have brought laparoscopic hepatectomy (LH) to the era of precise anatomical resection. Recently, the indocyanine green (ICG) fluorescence navigation technique has been applied in clinical practice and has emerged as the most effective method for identifying hepatic regions. Moreover, intraoperative ICG navigation might help overcome the limitations of LH.
This study investigated the effectiveness of standardized cranial-dorsal strategy for LLH with the aid of ICG fluorescence guidance.
The study aimed to investigate a standardized cranial-dorsal strategy for LLH, focusing on important anatomical markers, surgical skills, and ICG staining methods and the indicators of operation.
A retrospective study design was employed, involving 37 patients who underwent ICG fluorescence-guided LLH. The cranial-dorsal approach was performed. Data on relevant indicators were collected preoperatively, intraoperatively, and postoperatively, and an analysis of surgical outcomes was conducted.
The comparison results showed that intraoperative blood loss during LLH was 335.68 ± 99.869 mL and the rates of transfusion and conversion to laparotomy were 13.5% and 0%, respectively. The overall incidence of complications throughout the follow-up was 21.6%. No severe complications or mortality were reported.
The study findings demonstrate that LLH has the potential to become a novel, standardized approach that can effectively, safely, and simply expose the middle hepatic vein and meet the requirements of precision surgery.
The results highlight the effectiveness of standardized cranial-dorsal strategy for LLH with the aid of ICG fluorescence guidance. The cranial-dorsal strategy has the potential to become a novel and standardized approach for LLH, and can effectively, safely, and simply expose the MHV to meet the requirements of precision surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jani K, India S-Editor: Wang JL L-Editor: Webster JR P-Editor: Zheng XM
| 1. | Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 2. | Inoue Y, Arita J, Sakamoto T, Ono Y, Takahashi M, Takahashi Y, Kokudo N, Saiura A. Anatomical Liver Resections Guided by 3-Dimensional Parenchymal Staining Using Fusion Indocyanine Green Fluorescence Imaging. Ann Surg. 2015;262:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Qi C, Zhang H, Chen Y, Su S, Wang X, Huang X, Fang C, Li B, Xia X, He P. Effectiveness and safety of indocyanine green fluorescence imaging-guided hepatectomy for liver tumors: A systematic review and first meta-analysis. Photodiagnosis Photodyn Ther. 2019;28:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Yan Y, Cai X, Geller DA. Laparoscopic Liver Resection: A Review of Current Status. J Laparoendosc Adv Surg Tech A. 2017;27:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Okuda Y, Honda G, Kurata M, Kobayashi S, Sakamoto K. Dorsal approach to the middle hepatic vein in laparoscopic left hemihepatectomy. J Am Coll Surg. 2014;219:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Kim JH. Ventral Approach to the Middle Hepatic Vein During Laparoscopic Hemihepatectomy. Ann Surg Oncol. 2019;26:290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 8. | Guan BY, Yang GJ, Liu TX. The application experience of laparoscopic accurate liver resection under the guidance of indocyanine green fluorescence staining (in Chinese). Fuqiangjing Waike Zazhi. 2020;25:919-924. [DOI] [Full Text] |
| 9. | Makuuchi M. Surgical treatment for HCC--special reference to anatomical resection. Int J Surg. 2013;11 Suppl 1:S47-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Yeo D, Perini MV, Muralidharan V, Christophi C. Focal intrahepatic strictures: a review of diagnosis and management. HPB (Oxford). 2012;14:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ariizumi SI, Katagiri S, Kotera Y, Yamashita S, Omori A, Kato T, Shibuya G, Egawa H, Takasaki K, Yamamoto M. Improved Mortality, Morbidity, and Long-Term Outcome After Anatomical Hepatectomy With the Glissonean Pedicle Approach in Patients With Hepatocellular Carcinoma: 30 Years' Experience at a Single Institute. Ann Surg. 2022;275:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Ito D, Ishizawa T, Hasegawa K. Laparoscopic positive staining of hepatic segments using indocyanine green-fluorescence imaging. J Hepatobiliary Pancreat Sci. 2020;27:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lu H, Gu J, Qian XF, Dai XZ. Indocyanine green fluorescence navigation in laparoscopic hepatectomy: a retrospective single-center study of 120 cases. Surg Today. 2021;51:695-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Monden K, Alconchel F, Berardi G, Ciria R, Akahoshi K, Miyasaka Y, Urade T, García Vázquez A, Hasegawa K, Honda G, Kaneko H, Hoon Kim J, Tanabe M, Yamamoto M, Wakabayashi G; Study group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP surgery). Landmarks and techniques to perform minimally invasive liver surgery: A systematic review with a focus on hepatic outflow. J Hepatobiliary Pancreat Sci. 2022;29:66-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Chiba N, Abe Y, Nakagawa M, Koganezawa I, Yokozuka K, Kobayashi T, Hikita K, Ozawa Y, Sano T, Tomita K, Tsutsui R, Kawachi S. The "Tenting Sign of the Hepatic Vein" Is Important for Laparoscopic Anatomical Hepatectomy Along the Major Hepatic Vein. J Gastrointest Surg. 2020;24:1448-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Sureka B, Sharma N, Khera PS, Garg PK, Yadav T. Hepatic vein variations in 500 patients: surgical and radiological significance. Br J Radiol. 2019;92:20190487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Kim JH. Laparoscopy-specific ventral approach in laparoscopic hemihepatectomy. J Surg Oncol. 2017;116:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Ome Y, Honda G. [Basics in laparoscopic live resection: Mobilization, parenchymal dissection, and bleeding control]. Rinsyogeka. 2020;75:88-100. |
| 20. | Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021;274:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 21. | Aoki T, Yasuda D, Shimizu Y, Odaira M, Niiya T, Kusano T, Mitamura K, Hayashi K, Murai N, Koizumi T, Kato H, Enami Y, Miwa M, Kusano M. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008;32:1763-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Ishizawa T, Zuker NB, Kokudo N, Gayet B. Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch Surg. 2012;147:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1413] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 24. | Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, Fan ST, Nimura Y, Figueras J, Vauthey JN, Rees M, Adam R, Dematteo RP, Greig P, Usatoff V, Banting S, Nagino M, Capussotti L, Yokoyama Y, Brooke-Smith M, Crawford M, Christophi C, Makuuchi M, Büchler MW, Weitz J. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011;13:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |