Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.396
Peer-review started: August 25, 2023
First decision: September 29, 2023
Revised: November 5, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: February 27, 2024
Processing time: 184 Days and 12.1 Hours
The efficacy of neoadjuvant chemotherapy (NAC) in advanced gastric cancer (GC) is still a controversial issue.
To find factors associated with chemosensitivity to NAC treatment and to provide the optimal therapeutic strategies for GC patients receiving NAC.
The clinical information was collected from 230 GC patients who received NAC treatment at the Central South University Xiangya School of Medicine Affiliated Haikou Hospital from January 2016 to December 2020. Least absolute shrinkage and selection operator logistic regression analysis was used to find the possible predictors. A nomogram model was employed to predict the response to NAC.
In total 230 patients were finally included in this study, including 154 males (67.0%) and 76 females (33.0%). The mean age was (59.37 ± 10.60) years, ranging from 24 years to 80 years. According to the tumor regression grade standard, there were 95 cases in the obvious response group (grade 0 or grade 1) and 135 cases in the poor response group (grade 2 or grade 3). The obvious response rate was 41.3%. Least absolute shrinkage and selection operator analysis showed that four risk factors significantly related to the efficacy of NAC were tumor location (P < 0.001), histological differentiation (P = 0.001), clinical T stage (P = 0.008), and carbohydrate antigen 724 (P = 0.008). The C-index for the prediction nomogram was 0.806. The calibration curve revealed that the predicted value exhibited good agreement with the actual value. Decision curve analysis showed that the nomogram had a good value in clinical application.
A nomogram combining tumor location, histological differentiation, clinical T stage, and carbohydrate antigen 724 showed satisfactory predictive power to the response of NAC and can be used by gastrointestinal surgeons to determine the optimal treatment strategies for advanced GC patients.
Core Tip: Clinical information was collected from 230 gastric cancer patients who received neoadjuvant chemotherapy (NAC) from January 2016 to December 2020. Least absolute shrinkage and selection operator logistic regression analysis was performed to find the possible predictors for a nomogram model for prediction of response to NAC. The nomogram combining tumor location, histological differentiation, clinical T stage, and carbohydrate antigen 724 showed satisfactory predictive power to response of NAC and could be used by gastrointestinal surgeons to identify an optimal treatment strategy for advanced gastric cancer patients.
- Citation: Liu B, Xu YJ, Chu FR, Sun G, Zhao GD, Wang SZ. Development of a clinical nomogram for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer. World J Gastrointest Surg 2024; 16(2): 396-408
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/396.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.396
Gastric cancer (GC) is the fourth most common malignancy in terms of mortality, with approximately 770000 deaths in 2020[1]. However, early GC does not show obvious symptoms, leading to the extremely low early diagnosis rate globally[2]. For advanced GC patients, the 5-year survival rate is as low as 25%-31%[3-6]. Although gastrectomy plus D2 lymph node dissection and postoperative chemotherapy can improve the survival in advanced GC patients, their overall survival (OS) remains low.
Recently, neoadjuvant chemotherapy (NAC) has been proposed by several national and international guidelines as a critical treatment to improve the therapeutic effect in patients with advanced GC[7-9]. NAC is used for the downstaging of the tumor in the hopes of R0 resection for advanced GC patients[10]. The National Comprehensive Cancer Network guideline (version 2021.1) recommends that patients with clinical TNM stage ≥ T2N+ should receive NAC treatment[8]. The fifth edition of Japanese treatment guidelines recommend that patients with the stage from T2 to T4 and lymph node enlargement should receive NAC[9].
Although NAC can reduce tumor burden, decrease tumor stage, increase the radical resection rate, and improve survival outcomes, there are still many controversial points including chemotherapy scheme, chemotherapy frequency, and indications[11]. It was previously reported that NAC depends on the chemotherapeutic response of the tumor to achieve its survival advantage, indicating that patients with complete pathological response to NAC may show long OS and disease-free survival[12-14]. However, patients with low response to chemotherapy and no significant reduction of the tumor after chemotherapy may indicate a poor prognosis. For patients with a low objective response rate to NAC, the treatment not only delays surgery but also causes serious toxic side effects to patients. Therefore, it is very important to predict the sensitivity of NAC for patients with GC and further evaluate whether they are suitable for NAC. For those with poor sensitivity, surgery or other comprehensive treatment should be carried out immediately.
Recently, many studies have been conducted to identify predicting factors for NAC response, and nomogram models have been used for the prediction of advanced GC prognosis after NAC[15-19]. Recently, researchers have built a deep learning radiomic nomogram based on a computed tomography (CT) scan before treatment to solve this problem[20]. Compared with the traditional segmented models, these nomograms showed superior performance. However, most studies have only discussed the prognosis of patients and postoperative complications after NAC. Only a few studies identified some predictors that could predict the effect of NAC before chemotherapy.
Therefore, in this study, we retrospectively analyzed the tumor biological characteristics and clinical parameters that may affect the effect of NAC in patients with advanced GC and established a nomogram model to predict the response of NAC, aiming to provide individualized treatment strategies and maximize the benefits for patients with advanced GC.
This retrospective study was approved by the Research Ethics Committee of Haikou Hospital affiliated to Xiangya Medical College of Central South University. From January 2016 to December 2020, clinical information was extracted from the medical records of 259 patients with advanced GC who received NAC treatment in Haikou Hospital affiliated to Xiangya Medical College of Central South University. Then, the extracted information was analyzed retrospectively. Inclusion criteria were as follows: (1) Patients were diagnosed with GC through gastroscopy and biopsy; (2) GC patients with clinical stage T2N + M0 or T3-4N0/ + M0; (3) Patients who had completed NAC; (4) GC patients received radical gastrectomy after NAC; (5) The chemotherapy regimen was XELOX (capecitabine plus oxaliplatin); and (6) Patients were aged between 18 and 80. The exclusion criteria included: (1) Preoperative chemotherapy was not completed as planned (< 3 cycles); (2) In addition to GC, the patient also suffered from other malignant tumors; (3) Patients with gastric stump cancer; (4) Patients had received radiotherapy, traditional Chinese medicine, or other anti-tumor treatment; (5) Clinical data were incomplete; and (6) Postoperative pathology examination was not adenocarcinoma.
The patients whose clinical stage was T2N + M0 or T3-4N0/+ M0 were treated with laparoscopic exploration. If no distant metastasis such as intraperitoneal metastasis was found during the operation and the tumor could be resected, then chemotherapy was given for 3 cycles on the 1st or 2nd day after the laparoscopic exploration. Adjustments to dosage were made based on the effectiveness and patient tolerability. Two weeks after the completion of NAC, the resectability of the primary tumor site was confirmed again according to endoscopy and enhanced CT examination. Then, the surgery was performed. All enrolled patients received curative tumor resection (total or subtotal gastrectomy, open or laparoscopic surgery) with D2 lymphadenectomy.
The clinical data collected before NAC in this study included age, sex, body mass index, blood group, tumor markers [carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA199, CA724], tumor location, tumor size, depth of invasion, lymph node metastasis, pathological classification, albumin, platelet count, lymphocytes, neutrophils, monocytes, and smoking history. Tumor size, depth of invasion, and lymph node metastasis were evaluated on the basis of enhanced CT with laparoscopic exploration before NAC. The curative effect evaluation standard of NAC was based on the TRG standard as proposed by the National Comprehensive Cancer Network guidelines in 2021[8]. Grade 0 (complete response) is defined as no viable cancer cells, including lymph cells. Grade 1 (near complete response) is defined as single cells or rare small group of cancer cells. Grade 2 (partial response) was interpreted as residual cancer cells with evident tumor regression but more than single cells or rare small groups of cancer cells. Grade 3 (poor or no response) was defined as intermediate extensive residual cancer with no evident tumor regression. We classified grade 0 and grade 1 as obvious response. Grade 2 and grade 3 were classified as poor response. Postoperative complications were defined as events occurring within 30 d after surgery, which were assessed by the Clavien-Dindo classification system[21,22]. The adverse events of NAC were based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
All statistical analyses were performed by SPSS software ver. 22.0 (IBM, Armonk, NY, United States) and R version 4.0.3 software (The R Foundation for Statistical Computing, Vienna, Austria. www.r-project.org).
Univariate analysis: Parameters that were not normally distributed were expressed in the form of median (25% to 75% interquartile range) and were analyzed by the Mann-Whitney test, while normally distributed parameters were expressed in the form of mean ± standard deviation and were analyzed by Student’s t-test. Categorical variables were analyzed by the χ2 test. The test level α = 0.05.
Multivariate analysis: The least absolute shrinkage and selection operator (LASSO) method was used to select the most useful predictive factors for outcomes of NAC response (P < 0.05). The regression coefficient and odds ratio with 95% confidence intervals were estimated.
Nomogram construction: To predict the response of NAC, a nomogram including significant prognostic factors was constructed based on logistic regression analysis using glm R package (version 4.0.3). The consistency index was calculated. Decision curve analysis and correction curve were drawn to evaluate the predictive efficiency of the nomogram.
Patient information is listed in Table 1. Due to incomplete clinical data, receiving targeted therapy, or pathological results for non-adenocarcinoma, 29 patients were excluded. A total of 230 patients entered the study, consisting of 154 males (67.0%) and 76 females (33.0%). All patients were aged 24-80 years (average, 59.37 ± 10.60). In line with the TRG standard, 95 patients were assigned to the obvious response group (grades 0-1), whereas 135 patients were assigned to the poor response group (grades 2-3), with the obvious response rate being 41.3%. The cases of depth of invasion T2 or T3 were 71, and T4 were 159. There were 83 patients (36.1%) whose tumors were at the esophagogastric junction. In total, 180 patients showed positive lymph node metastasis, accounting for 78.3%.
| Characteristics | Obvious response (grade 0/grade 1), n = 95 (%) | Poor response (grade 2/grade 3), n = 135 (%) | t/χ2 | P value |
| Age | 59.88 ± 10.00 | 59.00 ± 11.03 | -0.62 | 0.535 |
| Sex, n | ||||
| Male | 70 (73.68) | 84 (62.22) | 3.31 | 0.069 |
| Female | 25 (26.32) | 51 (37.78) | ||
| BMI, kg/m2 | 22.90 ± 3.55 | 22.85 ± 2.99 | -0.12 | 0.907 |
| Location | ||||
| Esophagogastric junction | 53 (55.79) | 30 (22.22) | 27.24 | < 0.001 |
| Non-esophagogastric junction | 42 (44.21) | 105 (77.78) | ||
| Tumor size, cm | 5.65 ± 2.51 | 5.97 ± 2.97 | 0.86 | 0.393 |
| Tumor differentiation | ||||
| Well + moderately differentiated | 47 (49.47) | 38 (28.15) | 10.88 | 0.001 |
| Poorly differentiated + Signet ring cell | 48 (50.53) | 97 (71.85) | ||
| cT stage | ||||
| T2 | 6 (6.32) | 4 (2.96) | 9.64 | 0.008 |
| T3 | 34 (35.79) | 27 (20.00) | ||
| T4 | 55 (57.89) | 104 (77.04) | ||
| cN stage | ||||
| N0 | 26 (27.37) | 24 (17.78) | 3.02 | 0.083 |
| N+ | 69 (72.63) | 111 (82.22) | ||
| Blood type | ||||
| Type A | 25 (26.32) | 42 (31.11) | 0.84 | 0.840 |
| Type B | 27 (28.42) | 39 (28.89) | ||
| Type AB | 11 (11.58) | 13 (9.63) | ||
| Type O | 32 (33.68) | 41 (30.37) | ||
| CA724, U/mL | ||||
| ≤ 6.5 | 71 (74.74) | 78 (57.78) | 7.03 | 0.008 |
| > 6.5 | 24 (25.26) | 57 (42.22) | ||
| CEA, ng/mL | ||||
| ≤ 5 | 76 (80.00) | 102 (75.56) | 0.63 | 0.428 |
| > 5 | 19 (20.00) | 33 (24.44) | ||
| CA125, U/mL | ||||
| ≤ 24 | 82 (86.32) | 123 (91.11) | 1.32 | 0.250 |
| > 24 | 13 (13.68) | 12 (8.89) | ||
| CA199, U/mL | ||||
| ≤ 30 | 79 (83.16) | 112 (82.96) | 0.01 | 0.969 |
| > 30 | 16 (16.84) | 23 (17.04) | ||
| Serum albumin, g/L | 41.77 (41.01-42.52) | 41.64 (40.98-42.29) | -0.25 | 0.803 |
| PLT, 109/L | 224.56 ± 95.13 | 214.83 ± 73.90 | -0.87 | 0.384 |
| Lymphocyte, 109/L | 1.57 ± 0.51 | 1.59 ± 0.44 | 0.25 | 0.806 |
| PLR | 153.65 ± 70.73 | 144.45 ± 64.21 | -1.03 | 0.306 |
| Neutrophil cell, 109/L | 3.65 ± 1.43 | 3.57 ± 1.36 | -0.47 | 0.637 |
| Monocyte, 109/L | 0.43 ± 0.16 | 0.41 ± 0.14 | -1.19 | 0.235 |
| NMR | 9.07 ± 3.98 | 9.24 ± 3.50 | 0.346 | 0.730 |
| NLR | 2.29 (2.26-2.73) | 2.41 (2.21-2.62) | -0.48 | 0.629 |
| Smoking history | ||||
| Yes | 36 (37.89) | 50 (37.04) | 0.02 | 0.895 |
| No | 59 (62.11) | 85 (62.96) |
Table 1 displays univariable associations between the clinical parameters and response of NAC. Significant factors (P < 0.05) included tumor location, differentiation, clinical T stage, and CA724. The results showed that tumors in the esophagogastric junction displayed better efficacy than that of non-esophagogastric junction tumors. Greater differentiation level (well/moderate vs poor differentiation), lower T stage (T2/T3 vs T4 stage), and lower CA724 level were associated with a better NAC efficacy.
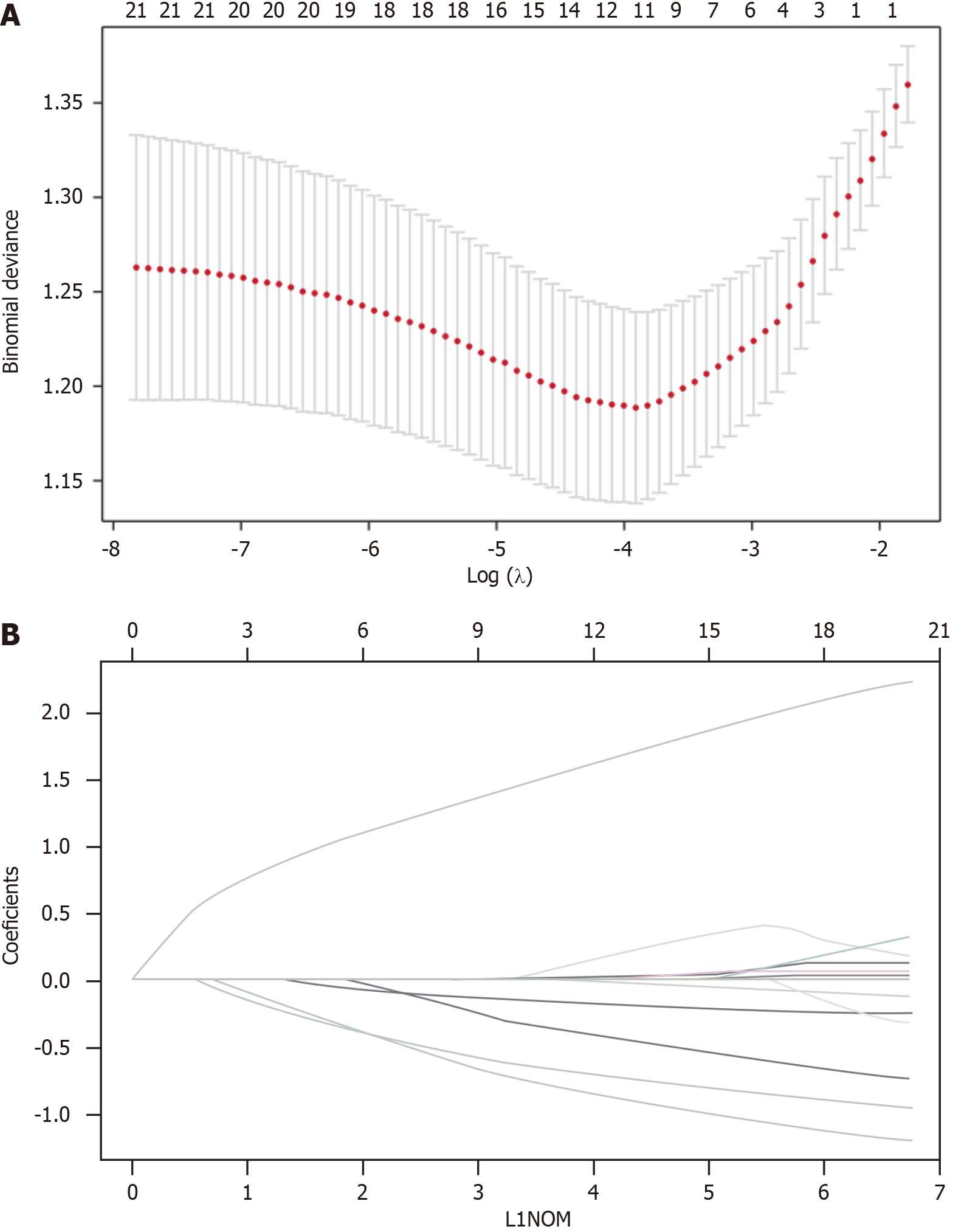
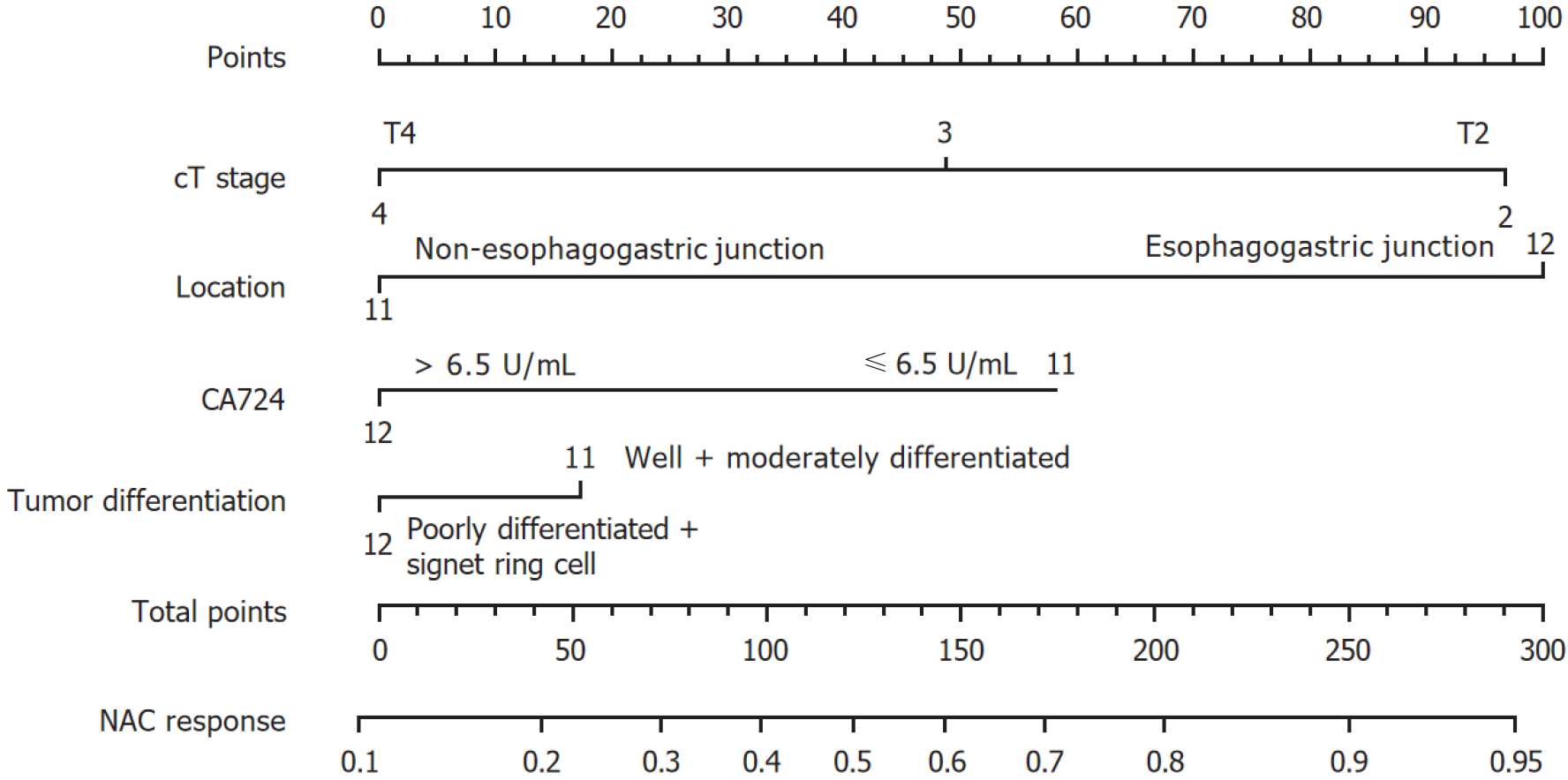
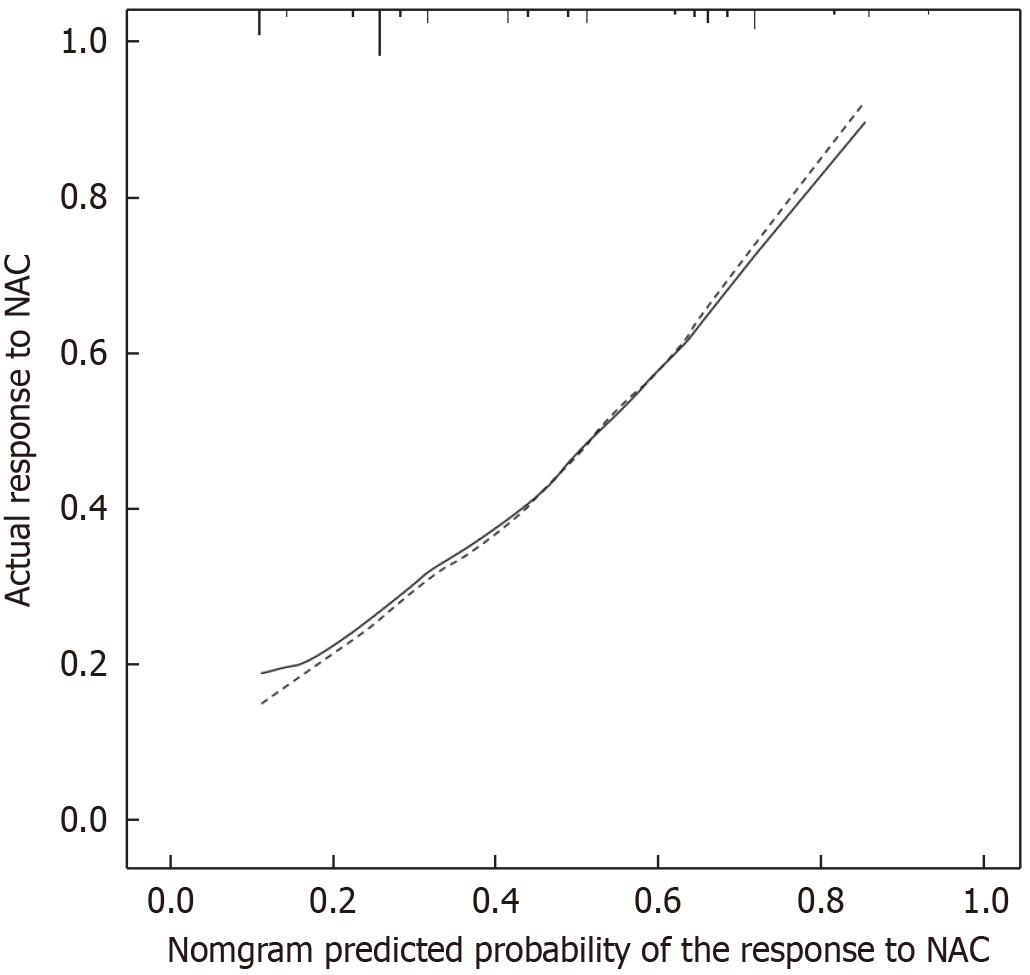
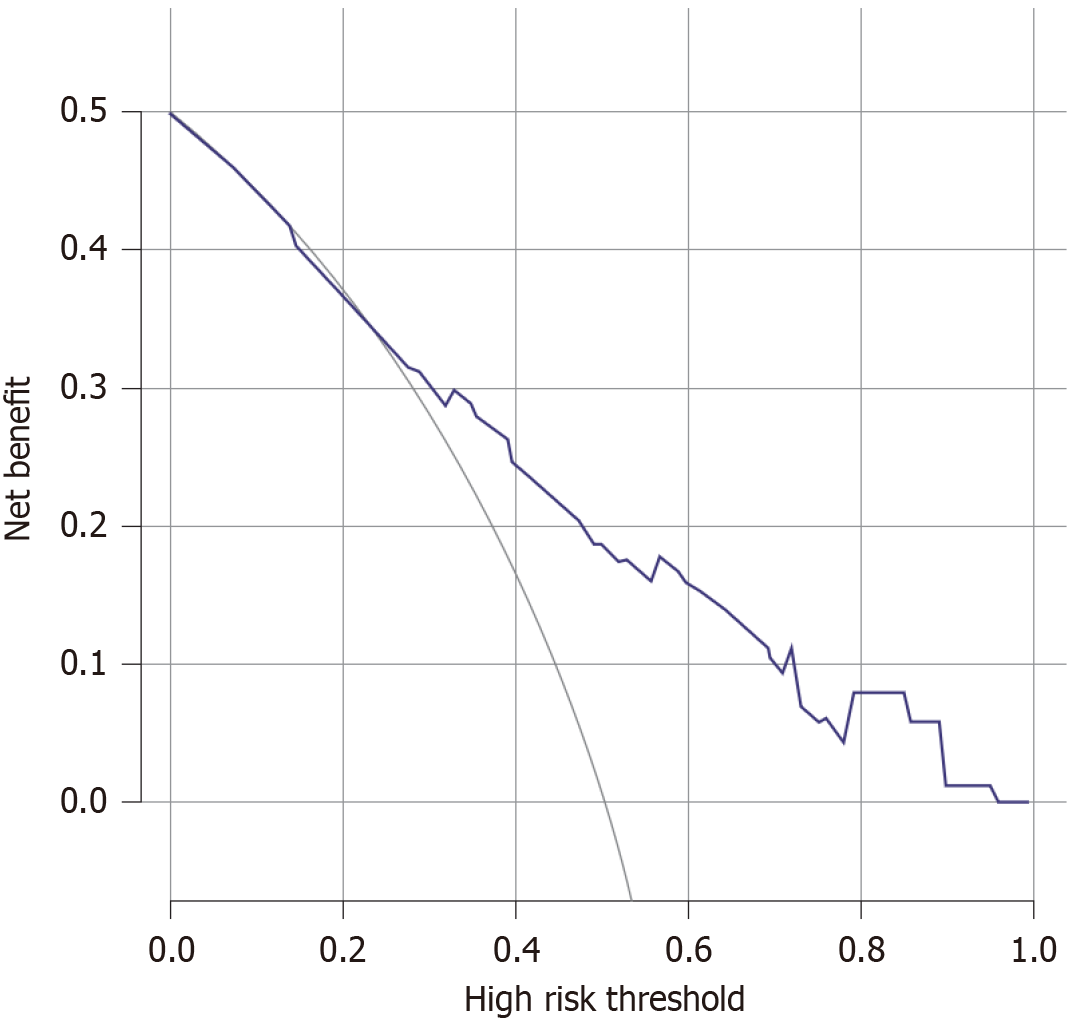
To avoid the multicollinearity problem in regression analysis, the distribution coefficient was analyzed by LASSO regression with an elastic net penalty. The results of the LASSO regression analysis were the same as those of the univariate analysis. Four independent predictors including tumor location, differentiation, clinical T stage, and CA724 were included in the final model, as shown in Figure 1. The model incorporating the above independent predictors was developed and presented as the nomogram (Figure 2). The C-index for the prediction nomogram was 0.806, indicating that the prediction performance of this nomogram has good feasibility. The calibration curve of the NAC nomogram demonstrated a good consistency between prediction and actual observations in the primary cohort (Figure 3). The value of the nomogram and its use in the clinic was evaluated by the decision curve analysis, evaluating the value in terms of clinical application for the NAC nomogram (Figure 4).
Based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0, the overall incidence of NAC adverse events was 85.7%, and the rate of grade 3/4 toxicity was 33.5%. The main side effects were hematological toxicity and gastrointestinal reaction. Anemia (15.7%) was the most common grade 3/4 adverse event (Table 2). In addition, we found that in the gastrointestinal, hematological, and neurological systems, the incidence of adverse reaction in the group with poor response was slightly higher than that in the group with obvious response, even though the differences were not statistically significant (P > 0.05), as shown in Table 3.
| Feature | Grade 1/2, n = 120 (%) | Grade 3/4, n = 77 (%) | Total, n = 197 |
| Anemia | 107 (89.17) | 36 (46.75) | 143 (72.59) |
| Leukopenia | 33 (27.50) | 20 (25.97) | 53 (26.90) |
| Neutropenia | 25 (20.83) | 6 (7.79) | 31 (15.74) |
| Thrombocytopenia | 27 (22.50) | 17 (22.08) | 44 (22.34) |
| Nausea/vomiting | 57 (47.50) | 12 (15.58) | 69 (35.03) |
| Diarrhea | 12 (10.00) | 1 (1.30) | 13 (6.60) |
| Hepatic impairment | 21 (17.50) | 10 (13.00) | 31 (15.74) |
| Hand-foot syndrome | 39 (32.50) | 0 | 39 (19.80) |
| Cardiotoxicity | 1 (0.83) | 0 | 1 (0.51) |
| System | Total, n = 230 (%) | Obvious response, n = 95 (%) | Poor response, n = 135 (%) | χ2 | P value |
| Gastrointestinal | 91 (39.57) | 41 (43.16) | 50 (37.04) | 0.874 | 0.350 |
| Hematological | 169 (73.48) | 71 (74.74) | 98 (72.59) | 0.132 | 0.717 |
| Neurological | 39 (16.96) | 19 (20.00) | 20 (14.81) | 1.065 | 0.302 |
| Cardiac | 1 (0.43) | 1 (1.05) | 0 | 1.385 | 0.239 |
In this study, 51 patients (22.2%) suffered from postoperative complications, and most of them were Clavien-Dindo grade 2 complications. The most common complications were pulmonary infection and pleural effusion (15.2%). One patient died of anastomotic leakage and abdominal hemorrhage. There was no statistical difference in the incidence of each complication between the obvious response group and the poor response group. Detailed information was listed in Tables 4 and 5.
| Complication | Grade 1, n = 2 (%) | Grade 2, n = 43 (%) | Grade 3a, n = 5 (%) | Grade 3b, n = 0 (%) | Grade 4a, n = 0 (%) | Grade 4b, n = 0 (%) | Grade 5, n = 1 (%) |
| Pulmonary infection/pleural effusion | 0 | 31 (72.09) | 4 (80.00) | 0 | 0 | 0 | 0 |
| Incision infection | 2 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| Intraperitoneal hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) |
| Digestive tract hemorrhage | 0 | 2 (4.65) | 0 | 0 | 0 | 0 | 0 |
| Anastomotic leakage | 0 | 0 | 1 (20.00) | 0 | 0 | 0 | 1 (100) |
| Duodenal stump fistula | 0 | 2 (4.65) | 0 | 0 | 0 | 0 | 0 |
| Gastroplegia | 0 | 3 (6.98) | 0 | 0 | 0 | 0 | 0 |
| Intestinal obstruction | 0 | 3 (6.98) | 0 | 0 | 0 | 0 | 0 |
| Peritoneal effusion/abscess formation | 0 | 4 (9.30) | 0 | 0 | 0 | 0 | 1 (100) |
| Lymphatic leakage | 0 | 1 (2.33) | 0 | 0 | 0 | 0 | 0 |
| Urinary tract infection | 0 | 3 (6.98) | 0 | 0 | 0 | 0 | 0 |
| Complication | Obvious-response, n = 95 (%) | Poor response, n = 135 (%) | χ2 | P value |
| Pulmonary infection/pleural effusion | 17 (17.89) | 18 (13.33) | 0.899 | 0.343 |
| Incision infection | 0 | 1 (0.74) | 0.00 | > 0.990 |
| Intraperitoneal hemorrhage | 1 (1.05) | 0 | 0.031 | 0.860 |
| Digestive tract hemorrhage | 2 (2.11) | 0 | 0.945 | 0.331 |
| Anastomotic leakage | 1 (1.05) | 1 (0.74) | 0.000 | > 0.990 |
| Duodenal stump fistula | 0 | 2 (1.48) | 0.221 | 0.638 |
| Gastroplegia | 1 (1.05) | 2 (1.48) | 0.095 | 0.758 |
| Intestinal obstruction | 1 (1.05) | 2 (1.48) | 0.000 | > 0.990 |
| Peritoneal effusion/abscess formation | 1 (1.05) | 4 (2.96) | 0.269 | 0.604 |
| Lymphatic leakage | 0 | 1 (0.74) | 0.000 | > 0.990 |
| Urinary tract infection | 2 (2.11) | 1 (0.74) | 0.095 | 0.758 |
Surgery is the most vital treatment for GC. More than 60% of patients have reached the advanced stage at the time of diagnosis, which leads to a low radical resection rate. Therefore, an efficient method for increasing the radical resection rate is urgently needed in the clinic[23].
Previous studies have indicated that surgery can induce tumor cells to transform into drug-resistant clones and increase the production of tumor growth stimulating factors, which can promote tumor cell proliferation. In the early stage, cell proliferation and DNA replication are active with the small number of tumor cells; at this time, tumor cells are more sensitive to chemotherapeutic drugs[24]. Therefore, giving chemotherapy drugs before tumor resection can not only kill the primary tumor but also inhibit the growth stimulating factors of cancer cells, which is also effective for micrometastases. It indicates that the earlier chemotherapy is administered, the fewer drug-resistant cell lines[12]. This highlights the importance of NAC.
At present, preoperative chemotherapy is receiving increasing attention. The role of NAC is to help surgeons decrease the primary tumor size and stage, eliminate micrometastasis, alleviate tumor related symptoms, improve curative resection rate, and reduce postoperative recurrence rate. However, some patients who are not sensitive to chemotherapy drugs cannot benefit from NAC, causing tumor progression and delaying the time to surgical resection. Studies have shown that approximately 15% of patients receiving preoperative neoadjuvant therapy have the risk of tumor progression[25]. Moreover, patients often suffer from side effects of NAC including cardiotoxicity, hepatotoxicity, and nephrotoxicity, increasing the risk of complications and mortality during surgery. Therefore, it is particularly important to predict the efficacy of NAC. Thus, we performed an exploratory study to identify pretreatment parameters that can predict NAC sensitivity, aiming to provide the basis for individualized treatment of GC patients. For patients with promising responsiveness to NAC, NAC should be considered. Otherwise, surgery or other comprehensive treatment should be performed as soon as possible.
Our data showed that the obvious response rate of NAC for advanced GC was 41.3%, which further indicated that only a portion of patients can benefit from NAC, thereby emphasizing the importance of predicting the responses to NAC. According to the results of the univariate and multivariate analysis, we found that tumor location, differentiation, depth of invasion, and CA724 were significant influencing factors for predicting the response of NAC. Using the four factors, we constructed a nomogram to predict the NAC response before performing gastrectomy with lymph node dissection.
A German retrospective cohort study including 410 patients indicated that a tumor in the upper two-thirds of the stomach tended to have a better response to NAC[26]. Another study performed by Li et al[27] also showed a similar finding. This was consistent with our result that the obvious response rate of NAC in patients with tumors located in the esophagogastric junction (63.86%) was higher than that in patients with tumors elsewhere (28.57%). The difference was statistically significant (P < 0.05).
Many studies have shown that serum tumor markers were associated with diagnosis, prognosis, and the therapeutic effect of preoperative or postoperative chemotherapy in GC[28,29]. Another study had indicated that CA724 was an independent factor for efficacy of NAC in GC[30]. Consistently, this work suggested that an increased CA724 level was related to the poor NAC response. Nonetheless, as reported in another study, CA724 only achieved a 45.0% sensitivity[31]. Additionally, CA724 was related to environmental factors and Helicobacter pylori infection[32,33]. Based on the above findings, a bias might exist in evaluating the patient condition according to CA724 alone, and many studies are needed to solve this problem.
Patients with a well-differentiated tumor had better survival than those with poor differentiation in GC[34,35], and previous studies suggested that differentiation is a vital predictor of pathological response[36,37], conforming to our study. However, in contrast to a previous study[38], our results showed that patients with a lower T stage (T2, T3) had a better response to NAC than advanced T stage (T4). The reason is that NAC regimens bring relatively serious toxicity and side effects in patients, damaging hematological, digestive, and nervous systems[10]. In this study, the overall incidence of NAC adverse reactions was 85.7%, and the rate of grade 3/4 toxicity was 33.5%. Therefore, it is important to select the optimal treatment options for different patients. We suggest that for these patients who are not sensitive to NAC, one solution is to apply other regimens of NAC, such as fluorouracil, leucovorin, oxaliplatin, docetaxel, resulting in superior OS compared with cisplatin and capecitabine[39]. The other is to implement surgery as soon as possible to avoid the time interval of chemotherapy.
Recent articles have concentrated on the relationship of the tumor with serum inflammatory factors, suggesting that lymphocytes, neutrophils, and platelets within the tumor microenvironment are associated with tumor metastasis and progression because inflammatory chemokines and cytokines are produced[40-45]. Typically, the increased neutrophil/platelet proportion and the decreased lymphocyte proportion suggests a damaged immune response and strong inflammatory response, thereby promoting cancer cell proliferation, distant organ metastasis, lymph node metastasis, and invasion. However, our study suggests that inflammatory factors such as platelets, neutrophils, and lymphocytes are not independent predictors of chemosensitivity.
Although a nomogram predicting the response of NAC had been established with a C-index of 0.767[10], our study achieved a C-index of 0.806, indicating a better performance for prediction than a previously reported study. LASSO analysis was used to find significant clinical factors in this study, while other similar articles mostly used logistic regression analysis. All patients were treated with XELOX, and thus the results are more reliable. Meanwhile, we also discussed the adverse reactions and postoperative complications of NAC, which further demonstrate the importance of predicting response to NAC.
However, this study has the following limitations. The results may be biased due to the retrospective design. In addition, because most patients enrolled in this study were in the most recent 2 years, there were insufficient survival events to analyze the impact of the predictor and chemosensitivity on OS rate. Therefore, high-quality studies with a larger cohort of patients are warranted to address this issue.
To conclude, four risk factors significantly related to response of NAC included tumor location, differentiation, clinical T stage, and CA724. The established nomogram exhibited a favorable prediction performance in predicting NAC response, which can be applied in identifying the best therapeutic strategies in advanced GC patients by gastrointestinal surgeons.
Neoadjuvant chemotherapy (NAC) has an unclear therapeutic effect on advanced gastric cancer (GC).
This work focused on identifying factors related to chemosensitivity to NAC treatment to be able to offer the best treatments for GC patients receiving NAC.
To find factors associated with chemosensitivity to NAC treatment and to provide the optimal therapeutic strategies for GC patients receiving NAC.
Predicting factors were identified by least absolute shrinkage and selection operator logistic regression. Additionally, a nomogram model was employed to predict the response to NAC.
We enrolled 230 patients, consisting of 154 males (67.0%) and 76 females (33.0%). These patients were aged 24-80 years (average, 59.37 ± 10.60). According to the TRG standard, 95 patients were assigned into the obvious response group (grades 0-1) and 135 into the poor response group (grades 2-3), yielding an obvious response rate of 41.3%. As revealed by the least absolute shrinkage and selection operator regression, tumor location (P < 0.001), histological differentiation (P = 0.001), clinical T stage (P = 0.008), and carbohydrate antigen 724 (P = 0.008) were significant risk factors for NAC efficacy. The C-index of the prediction nomogram was 0.806. According to calibration curve analysis, the predicted value was highly consistent with real measurement. Moreover, decision curve analysis revealed the high application value of this nomogram clinically.
Our nomogram combining tumor location, histological differentiation, clinical T stage, and carbohydrate antigen 724 showed a high performance in predicting NAC response, which can be applied in identifying the best therapeutic strategies for advanced GC patients by gastrointestinal surgeons.
Candidate predictive factors were identified by the least absolute shrinkage and selection operator logistic regression. The response to NAC was predicted by a nomogram model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagaya M, Japan; Nakano H, Japan S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zheng XM
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64634] [Article Influence: 16158.5] [Reference Citation Analysis (176)] |
| 2. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 728] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 3. | De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, Nennecke A, Siesling S, Berrino F, Capocaccia R; EUROCARE-5 Working Group. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 1374] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 4. | Yang JJ, Wang XY, Ma R, Chen MH, Zhang GX, Li X. Prediction of lymph node metastasis in early gastric signet-ring cell carcinoma: A real-world retrospective cohort study. World J Gastroenterol. 2023;29:3807-3824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 5. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 678] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 6. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3422] [Article Influence: 488.9] [Reference Citation Analysis (1)] |
| 7. | Wang Y, Zhang J, Guo S, Meng XY, Zheng ZC, Zhao Y. Indications of neoadjuvant chemotherapy for locally advanced Gastric Cancer patients based on pre-treatment clinicalpathological and laboratory parameters. J Cancer. 2020;11:6000-6008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1337] [Article Influence: 334.3] [Reference Citation Analysis (2)] |
| 10. | Chen YH, Xiao J, Chen XJ, Wang HS, Liu D, Xiang J, Peng JS. Nomogram for predicting pathological complete response to neoadjuvant chemotherapy in patients with advanced gastric cancer. World J Gastroenterol. 2020;26:2427-2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Reddavid R, Sofia S, Chiaro P, Colli F, Trapani R, Esposito L, Solej M, Degiuli M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018;24:274-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Achilli P, De Martini P, Ceresoli M, Mari GM, Costanzi A, Maggioni D, Pugliese R, Ferrari G. Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study. J Gastrointest Oncol. 2017;8:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, Pauligk C, Luley K, Bichev D, Schumacher G, Homann N. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 2013;24:2068-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Lai J, Pan Z, Chen P, Ye G, Chen K, Su F. Development and validation of a nomogram incorporating axillary lymph node ratio to predict survival in node-positive breast cancer patients after neoadjuvant chemotherapy. Jpn J Clin Oncol. 2019;49:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Lai J, Wang H, Peng J, Chen P, Pan Z. Establishment and external validation of a prognostic model for predicting disease-free survival and risk stratification in breast cancer patients treated with neoadjuvant chemotherapy. Cancer Manag Res. 2018;10:2347-2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kim CH, Yeom SS, Lee SY, Kim HR, Kim YJ, Lee KH, Lee JH. Prognostic Impact of Perineural Invasion in Rectal Cancer After Neoadjuvant Chemoradiotherapy. World J Surg. 2019;43:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Tan W, Yang M, Yang H, Zhou F, Shen W. Predicting the response to neoadjuvant therapy for early-stage breast cancer: tumor-, blood-, and imaging-related biomarkers. Cancer Manag Res. 2018;10:4333-4347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Zhu YL, Sun YK, Xue XM, Yue JY, Yang L, Xue LY. Unnecessity of lymph node regression evaluation for predicting gastric adenocarcinoma outcome after neoadjuvant chemotherapy. World J Gastrointest Oncol. 2019;11:48-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Fang M, Tian J, Dong D. Non-invasively predicting response to neoadjuvant chemotherapy in gastric cancer via deep learning radiomics. EClinicalMedicine. 2022;46:101380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518-526. [PubMed] |
| 22. | Yan W, Zhu L, Wang J. Effects of Clavien-Dindo Classification on Long-Term Survival of Patients With Advanced Gastric Cancer After Radical Resection: A Propensity Score-matched Study. Am Surg. 2023;31348231191230. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Liu X, Cai H, Sheng W, Huang H, Long Z, Wang Y. microRNAs expression profile related with response to preoperative radiochemotherapy in patients with locally advanced gastric cancer. BMC Cancer. 2018;18:1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Crookes P, Leichman CG, Leichman L, Tan M, Laine L, Stain S, Baranda J, Casagrande Y, Groshen S, Silberman H. Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy: a final report. Cancer. 1997;79:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Zhou J, Shen J, Seifer BJ, Jiang S, Wang J, Xiong H, Xie L, Wang L, Sui X. Approaches and genetic determinants in predicting response to neoadjuvant chemotherapy in locally advanced gastric cancer. Oncotarget. 2017;8:30477-30494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Lorenzen S, Blank S, Lordick F, Siewert JR, Ott K. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol. 2012;19:2119-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L, Zhang XP, Li JY, Hu Y, Shen L, Ji JF. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 29. | Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 230] [Cited by in RCA: 321] [Article Influence: 45.9] [Reference Citation Analysis (7)] |
| 30. | Tong Y, Zhao Y, Shan Z, Zhang J. CA724 predicts overall survival in locally advanced gastric cancer patients with neoadjuvant chemotherapy. BMC Cancer. 2021;21:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Ning S, Wei W, Li J, Hou B, Zhong J, Xie Y, Liu H, Mo X, Chen J, Zhang L. Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels in gastric and colorectal cancer patients. J Cancer. 2018;9:494-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Hu PJ, Chen MY, Wu MS, Lin YC, Shih PH, Lai CH, Lin HJ. Clinical Evaluation of CA72-4 for Screening Gastric Cancer in A Healthy Population: A Multicenter Retrospective Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Jing J, Ge M, Yang Z, Li P. Spatial distribution characteristics of tumor marker CA724 reference values in China. Cancer Med. 2019;8:4465-4474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Lazăr D, Tăban S, Sporea I, Dema A, Cornianu M, Lazăr E, Goldiş A, Vernic C. Gastric cancer: correlation between clinicopathological factors and survival of patients (III). Rom J Morphol Embryol. 2009;50:369-379. [PubMed] |
| 35. | Kim SM, Min BH, Ahn JH, Jung SH, An JY, Choi MG, Sohn TS, Bae JM, Kim S, Lee H, Lee JH, Kim YW, Ryu KW, Kim JJ. Nomogram to predict lymph node metastasis in patients with early gastric cancer: a useful clinical tool to reduce gastrectomy after endoscopic resection. Endoscopy. 2020;52:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Wang LB, Teng RY, Jiang ZN, Hu WX, Dong MJ, Yuan XM, Chen WJ, Jin M, Shen JG. Clinicopathologic variables predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Xu W, Ma Q, Wang L, He C, Lu S, Ni Z, Hua Z, Zhu Z, Yang Z, Zheng Y, Feng R, Yan C, Li C, Yao X, Chen M, Liu W, Yan M. Prediction Model of Tumor Regression Grade for Advanced Gastric Cancer After Preoperative Chemotherapy. Front Oncol. 2021;11:607640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Reynolds JV, Preston SR, O'Neill B, Lowery MA, Baeksgaard L, Crosby T, Cunningham M, Cuffe S, Griffiths GO, Parker I, Risumlund SL, Roy R, Falk S, Hanna GB, Bartlett FR, Alvarez-Iglesias A, Achiam MP, Nilsson M, Piessen G, Ravi N, O'Toole D, Johnston C, McDermott RS, Turkington RC, Wahed S, Sothi S, Ford H, Wadley MS, Power D; Neo-AEGIS Investigators and Trial Group. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): an open-label, randomised, phase 3 trial. Lancet Gastroenterol Hepatol. 2023;8:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 40. | Kuol N, Stojanovska L, Apostolopoulos V, Nurgali K. Crosstalk between cancer and the neuro-immune system. J Neuroimmunol. 2018;315:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Huong PT, Nguyen LT, Nguyen XB, Lee SK, Bach DH. The Role of Platelets in the Tumor-Microenvironment and the Drug Resistance of Cancer Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 42. | Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, Zhang Q, Lavalle C, McKeown T, Marshall AH, Ni H. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 43. | Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell. 2018;33:965-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 447] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 44. | Felix K, Gaida MM. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. Int J Biol Sci. 2016;12:302-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 45. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |