Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3484
Revised: August 27, 2024
Accepted: September 10, 2024
Published online: November 27, 2024
Processing time: 112 Days and 2 Hours
Prolonged postoperative ileus (PPOI) delays the postoperative recovery of gastrointestinal function in patients with gastric cancer (GC), leading to longer hospitalization and higher healthcare expenditure. However, effective monitoring of gastrointestinal recovery in patients with GC remains challenging because of the lack of noninvasive methods.
To explore the risk factors for delayed postoperative bowel function recovery and evaluate bowel sound indicators collected via an intelligent auscultation system to guide clinical practice.
This study included data from 120 patients diagnosed with GC who had undergone surgical treatment and postoperative bowel sound monitoring in the Department of General Surgery II at Shaanxi Provincial People's Hospital between January 2019 and January 2021. Among them, PPOI was reported in 33 cases. The patients were randomly divided into the training and validation cohorts. Significant variables from the training cohort were identified using univariate and multivariable analyses and were included in the model.
The analysis identified six potential variables associated with PPOI among the included participants. The incidence rate of PPOI was 27.5%. Age ≥ 70 years, cTNM stage (I and IV), preoperative hypoproteinemia, recovery time of bowel sounds (RTBS), number of bowel sounds (NBS), and frequency of bowel sounds (FBS) were independent risk factors for PPOI. The Bayesian model demonstrated good performance with internal validation: Training cohort [area under the curve (AUC) = 0.880, accuracy = 0.823, Brier score = 0.139] and validation cohort (AUC = 0.747, accuracy = 0.690, Brier score = 0.215). The model showed a good fit and calibration in the decision curve analysis, indicating a significant net benefit.
PPOI is a common complication following gastrectomy in patients with GC and is associated with age, cTNM stage, preoperative hypoproteinemia, and specific bowel sound-related indices (RTBS, NBS, and FBS). To facilitate early intervention and improve patient outcomes, clinicians should consider these factors, optimize preoperative nutritional status, and implement routine postoperative bowel sound monitoring. This study introduces an accessible machine learning model for predicting PPOI in patients with GC.
Core Tip: Postoperative recovery of gastrointestinal function in patients with gastric cancer (GC) is crucial. However, it is frequently delayed by prolonged postoperative ileus, leading to increased hospital stays and economic burdens. Effective monitoring of postoperative gastrointestinal recovery in patients with GC remains challenging due to the lack of noninvasive methods. This study investigated risk factors for delayed postoperative bowel function recovery and evaluated bowel sound indicators collected via an intelligent auscultation system to guide clinical practice.
- Citation: Shi S, Lu C, Shan L, Yan L, Liang Y, Feng T, Chen Z, Chen X, Wu X, Liu SD, Duan XL, Wang ZZ. Predicting prolonged postoperative ileus in gastric cancer patients based on bowel sounds using intelligent auscultation and machine learning. World J Gastrointest Surg 2024; 16(11): 3484-3498
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3484.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3484
Gastric cancer (GC) is a common malignancy of the digestive tract, often requiring comprehensive surgery-based treatment[1]. Radical GC surgery is extensive and traumatic and impedes the recovery of gastrointestinal function[2]. Postoperative ileus (POI) is a common gastrointestinal dysfunction that occurs following abdominal surgery and is characterized by abdominal distension, weakened or absent bowel sounds, constipation, and intolerance to oral intake[3]. Although POI usually resolves within 3 days, some patients experience persistent or recurrent POI, known as prolonged POI (PPOI)[4]. PPOI is a frequent complication after GC surgery, with a complex and partially understood mechanism. PPOI is influenced by various factors, including surgical trauma, sympathetic nervous system hyperactivity, inflammatory responses, fluid and electrolyte imbalance, and pharmacological effects[5-8]. PPOI contributes to postoperative complications, prolongs hospitalization, and increases healthcare costs[9]. Clinically, PPOI is often diagnosed based on the time of defecation, which is a delayed indicator. Furthermore, computed tomography (CT) and radiographic examinations cannot assess gastrointestinal function in real time and involve radiation exposure[2,10]. To address this, real-time monitoring of bowel sounds was used to effectively assess the gastrointestinal function of patients by intestinal contractions Bowel sound auscultation, a crucial part of abdominal examinations, is a simple and effective method for evaluating gastrointestinal function[11,12]. However, their clinical value can be limited by factors such as weak signals, individual variations, randomness, susceptibility to interference from vocalizations of other organs in the body, and environmental noise[13]. The reliability of bowel sounds is doubtful because of the reliance on stethoscopes for short-term auscultation and subjective interpretation by clinicians, in conjunction with clinical symptoms. Therefore, bowel sound auscultation is inefficient, yields unreliable results, and is not recommended for clinical practice[14,15]. However, technological advancements and the application of intelligent medical equipment in clinical practice have substantially improved bowel sound research. Gu et al[15] investigated the diagnostic accuracy of clinicians using bowel sounds in patients with mechanical bowel obstruction and intestinal paralysis (43 patients in total). They reported an overall diagnostic accuracy of 69.8%, with an accuracy rate of 84.5% specifically for patients with intestinal paralysis. Kaneshiro et al[16] used a non-invasive monitor to predict postoperative bowel paralysis in patients undergoing abdominal surgery. Their study revealed that monitoring bowel sounds 1 hour postoperatively had a sensitivity of 63% and specificity of 72% for predicting bowel paralysis. This highlights the practical value of monitoring bowel sounds to predict postoperative recovery of gastrointestinal function. The continuous auscultation recorder used in this study was clinically tested, which demonstrated a sensitivity of 90.92%, specificity of 94.21%, and an average accuracy of 92.56% for bowel sound recognition[17]. Therefore, a portable continuous bowel sound auscultation recorder was used in this study to record the postoperative bowel sounds of patients with GC in real time until the first postoperative day. The predictive value of clinical and bowel sound-related indices for postoperative PPOI was investigated by analyzing bowel sound-related indices. Establishing a prediction model to predict the probability of postoperative PPOI can enable early intervention and implementation of optimal preventive measures, ultimately benefiting patients by improving outcomes and reducing healthcare expenditure.
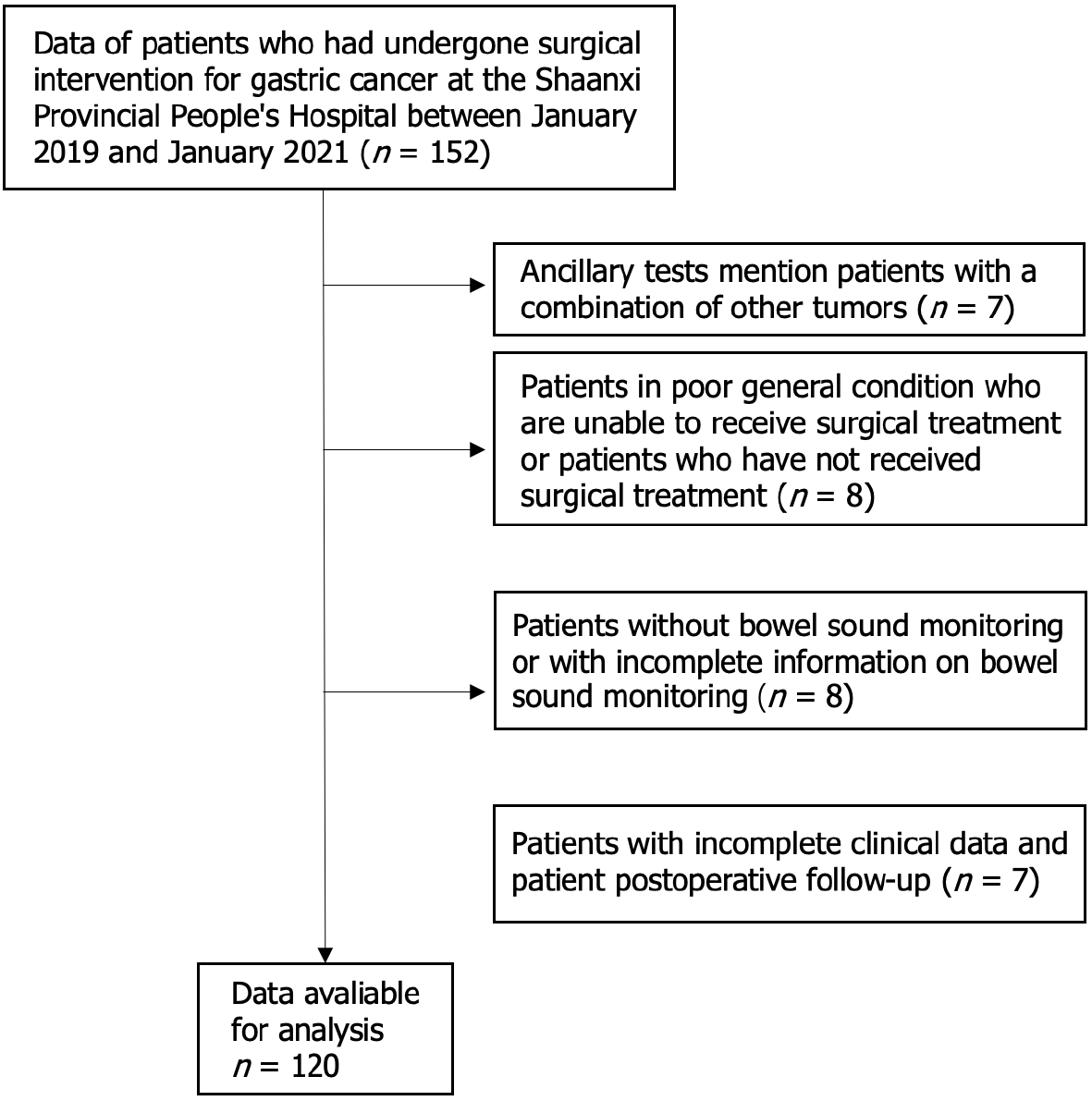
This study included data of 120 patients diagnosed with GC who had undergone surgical treatment in the Department of General Surgery II at Shaanxi Provincial People's Hospital between January 2019 and January 2021. The inclusion criteria were as follows: (1) Patients with confirmed diagnosis of GC through preoperative auxiliary examination; (2) Those with no apparent contraindications to surgery and indications for GC surgery; (3) Those who underwent postoperative bowel sound monitoring with complete monitoring data available; and (4) Those with complete clinical data from postoperative investigation and follow-up. The exclusion criteria were as follows: (1) Patients with auxiliary examinations suggesting non-GC or with other tumors; (2) patients in poor general condition who could not receive surgical treatment or who had not undergone surgical treatment; (3) Patients who did not undergo bowel sound monitoring or had incomplete bowel sound monitoring data; (4) Patients with incomplete clinical and postoperative follow-up data; and (5) Patients with inflammatory bowel disease or other conditions that affect bowel sounds (Figure 1).
The Ethics Committee of Shaanxi Provincial People's Hospital approved the study, and all participants provided written informed consent (approval number: SPPH-LLBG-17-32).
PPOI was diagnosed by two experienced deputy chief physicians at our hospital using the following criteria[3-7]. Patients presenting with two or more of the following conditions 96 hours after surgery: (1) Moderate to severe nausea or vomiting in the past 12 hours; (2) Intolerance to solid food in the past two meals (self-reported food intake < 25%); (3) No bowel movement or defecation in the past 24 hours; (4) Moderate to severe abdominal distension (assessed by percussion); and (5) Imaging confirmation of bowel paralysis (abdominal radiographic or CT findings) within the past 24 hours. Additionally, the presence of two or more of the following findings, including gastric dilatation, presence of a fluid-air plane, and dilatation of small or large bowel loops, were indicative of PPOI.
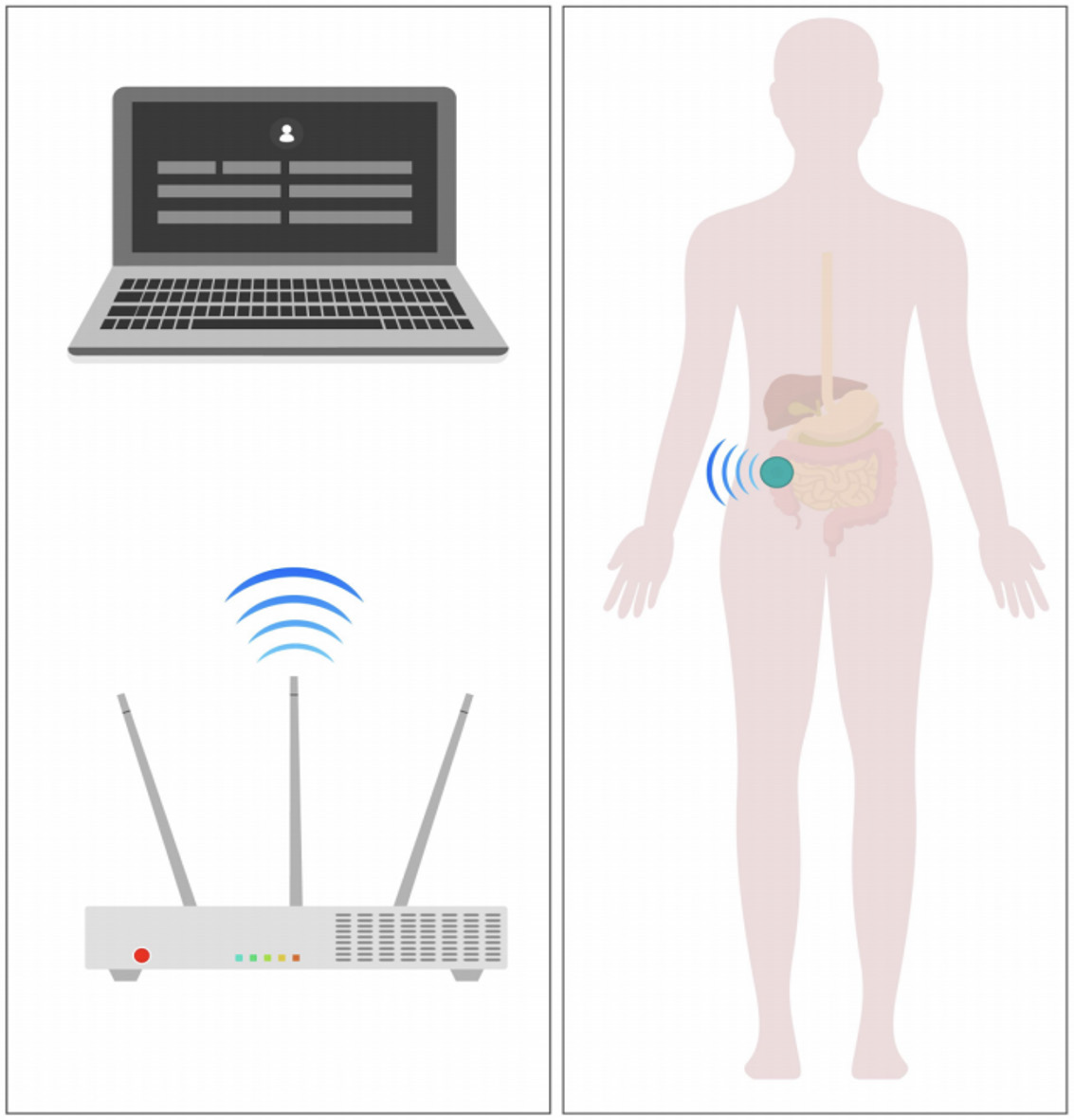
This study used a continuous auscultation recorder (Model: YM-TYJL-01) jointly developed by Tsinghua University and Beijing YiMai Medical Technology Co., Ltd. This device transmits data to the receiver via Bluetooth. The receiver uploads the data to a server, where the accompanying software stores the data and records relevant information. A bowel sound patch was applied to the patient's right lower abdomen for long-term monitoring of bowel sound changes (Figure 2).
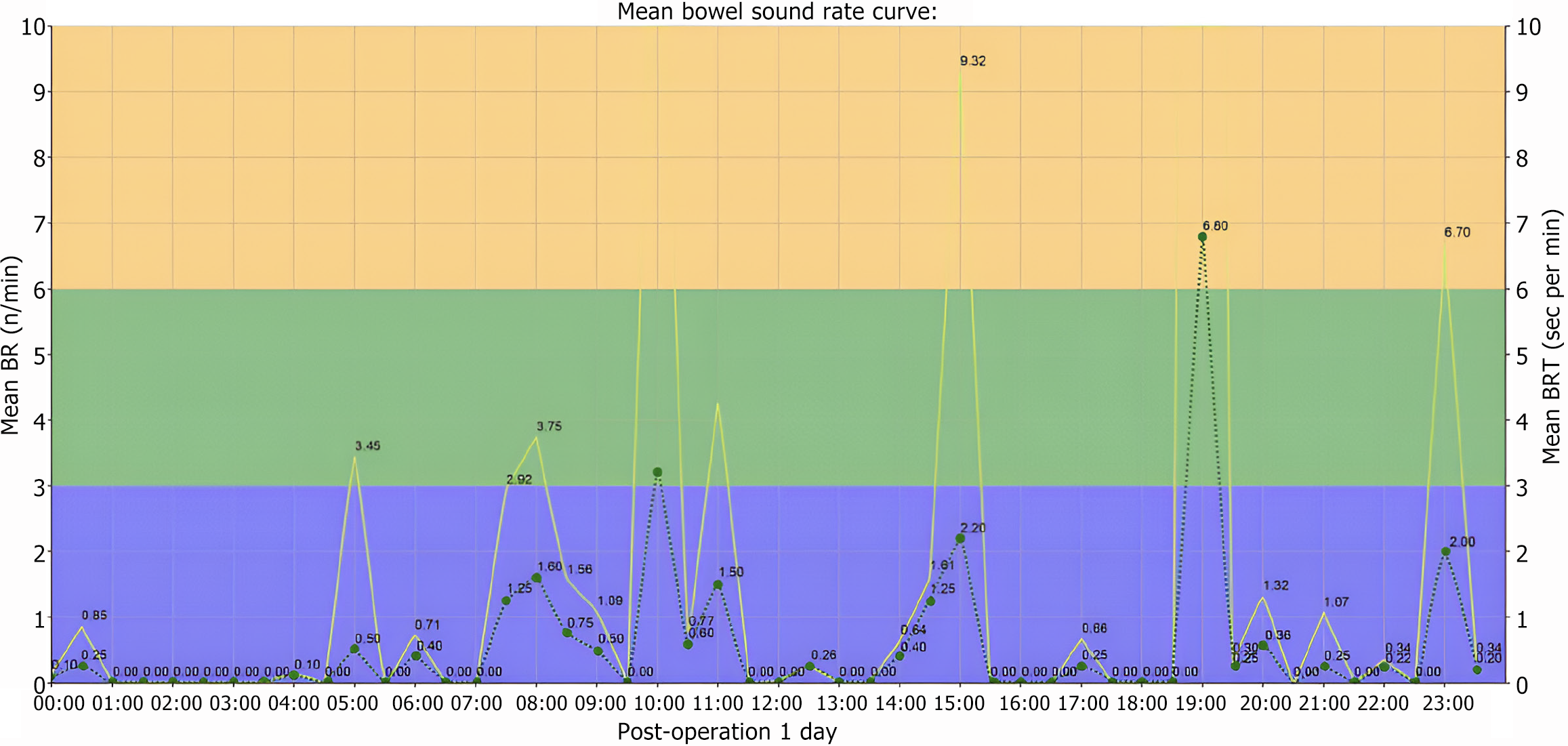
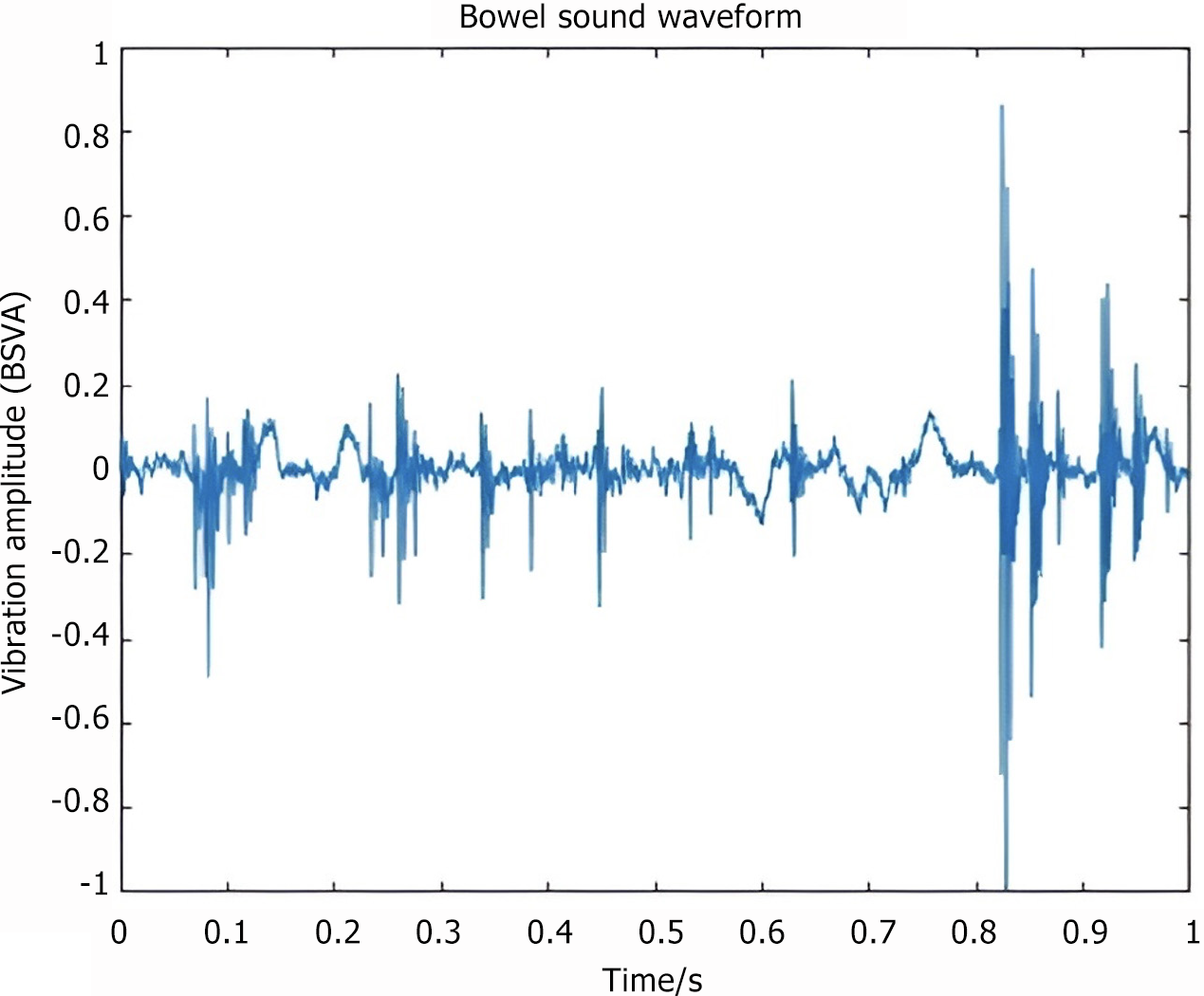
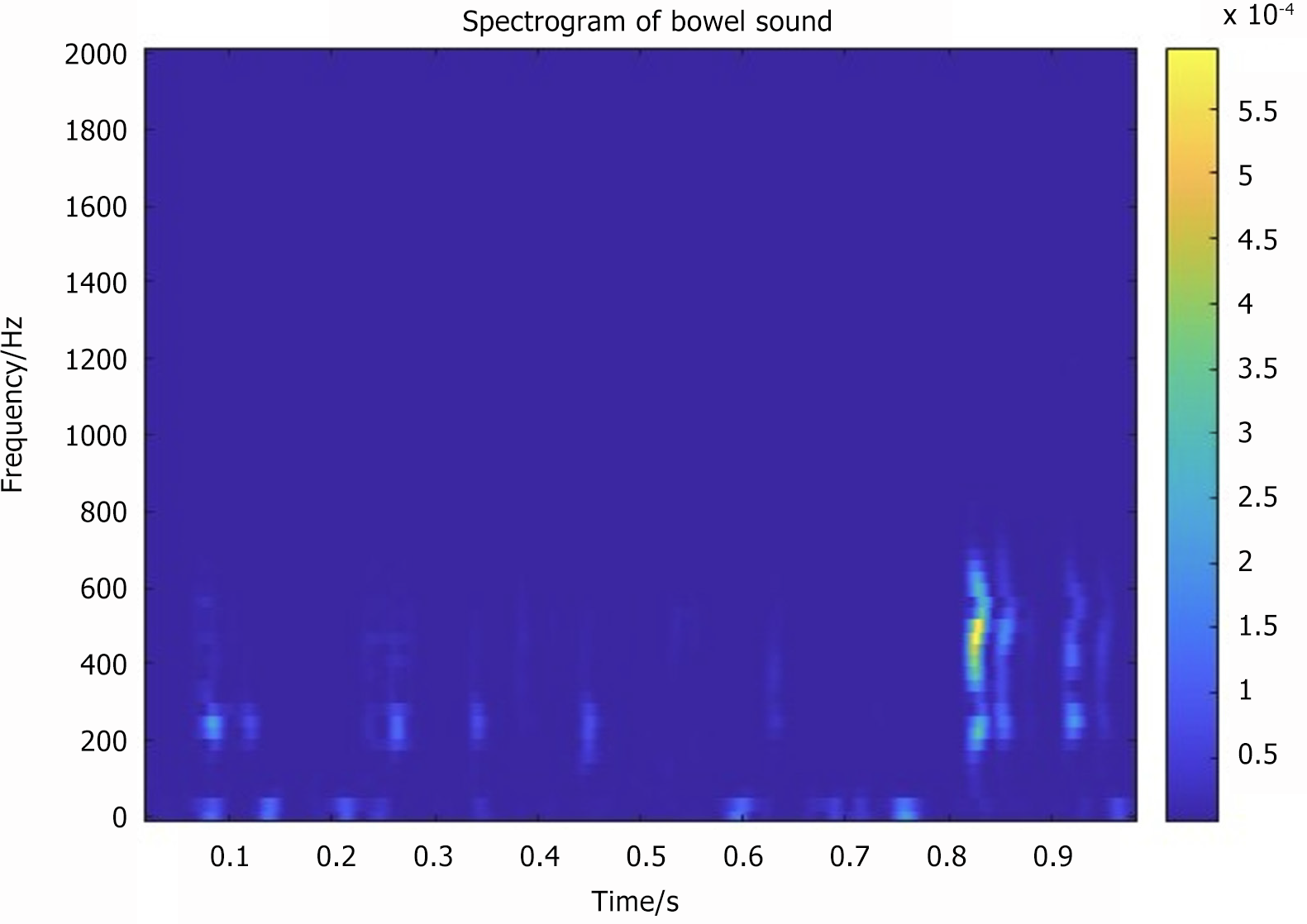
Bowel sound-related indicators included the following: (1) Number of bowel sounds (NBS) and NBSs per minute expressed as counts per minute (CPM) (Figure 3); (2) Bowel sound vibration amplitude (BSVA): The intensity of the bowel sound in the time or frequency domain, measured in decibels (Figure 4); (3) Frequency of bowel sounds (FBS): The significant frequency component of the bowel sound, representing the frequency band where the bowel sound energy is concentrated, measured in hertz (Hz) (Figure 5); and (4) Recovery time of bowel sounds (RTBS): The first postoperative occurrence of bowel sounds is defined as the first instance when the bowel rate exceeds 1 CPM. This time point, subtracted from the end of surgery, was marked as the bowel sound recovery time (Figure 5).
All patients underwent comprehensive preoperative evaluation, which included adequate doctor-patient communication and health education upon admission. Preoperative measures focused on nutritional support and respiratory health, with recommendations for smoking cessation, and respiratory function exercises 2 weeks before surgery. The patients were instructed to fast for 12 hours and abstain from oral intake for 6 hours before surgery; however, mechanical gastroin
Information on baseline demographic characteristics was obtained from the patients’ electronic medical records downloaded from the hospital information system. These characteristics included sex, age, body mass index (BMI), history of smoking and alcohol consumption, previous abdominal surgery, hypertension, diabetes, bowel sound indicators (RTBS, NBS, BSVA, and FBS), presence of a recurrent hernia, preoperative conditions, emergency surgery status, tumor site, tumor size, preoperative obstruction, and cTNM stage. Surgical indicators included the surgical method (laparoscopic or open), excision range (distal gastrectomy, proximal gastrectomy, or total gastrectomy), combined resection, operation time, and duration of anesthesia. Laboratory indicators included baseline and preoperative levels of hemoglobin, potassium, and albumin.
Machine learning model selection: This study employed eight supervised learning algorithms, including decision tree, extreme gradient boosting (XGBoost), logistic regression (LR), neural network, naïve Bayes, K-nearest neighbor (KNN), random forest (RF), and support vector machine (SVM), to develop models for predicting the onset of PPOI in patients with GC. To ensure a robust machine learning model, a random sampling approach was used to select a subset of patients with significant differences in the data between the two groups. Among the total sample of 120 patients, the data were randomly divided into training (n = 84) and internal testing (n = 36) cohorts in a 7:3 ratio. To optimize the training model, a 10-fold cross-validation was applied to the training cohort. Subsequently, the eight established machine-learning models were validated on an independent test cohort to assess their generalization ability, record relevant parameters, and evaluate model performance. Model performance and discriminative properties were assessed using the area under the curve (AUC) and calibration curves. An AUC value > 0.7 indicates a better predictive value, with values closer to 1 indicating superior model performance. The machine learning model with the highest AUC value in the training cohort was selected to develop the final predictive model, which demonstrated good clinical utility as confirmed by decision curve analysis (DCA).
Data analysis was performed using the R software (version 4.3.2; R Core Team, Vienna, Austria). All variables were categorized, and categorical data were compared using the χ2 or Fisher’s exact tests. The patients were randomly assigned to the training (n = 84) or internal validation (n = 36) cohorts in a 7:3 ratio. Univariate logistic analysis was applied to all variables, and significant variables with P < 0.05 were included in the multivariable logistic analysis to identify the independent risk factors for PPOI.
Of the 120 patients who underwent surgical treatment for GC, 33 (27.5%) developed PPOI. Table 1 presents the baseline demographic information, clinical parameters, and bowel sound-related indices of the regular patients and those with postoperative comorbid PPOI. The age distribution of the enrolled patients with GC ranged from 54 to 71 years, and 80 (66.7%) were men. Moreover, 26 patients (21.7%) had a history of abdominal surgery, 22 (18.3%) were diagnosed with hypoproteinemia, 12 (10.0%) with preoperative hypokalemia, and 12 (10.0%) with preoperative intestinal obstruction. Bowel sound-related indicators such as RTBS, NBS, BSVA, and FBS were included in the analysis (Table 1).
| Characteristics | Total (n = 120) | Characteristics | Total (n = 120) |
| Age (years) | 64 (54.25, 71) | Surgical method | |
| Open | 33 (27.5) | ||
| Laparoscope | 87 (72.5) | ||
| Gender | Range of excision | ||
| Male | 80 (66.7) | Distal | 53 (44.2) |
| Female | 40 (33.3) | Proximal | 21 (17.5) |
| Total | 46 (38.3) | ||
| BMI (kg/m2) | 22.79 (20.22, 20.38) | Operation time (min) | 280 (225, 339) |
| Diabetes | Anesthesia duration (min) | 325 (271.25, 390) | |
| Yes | 11 (9.2) | ||
| No | 109 (90.8) | ||
| Anemia | RTBS (h) | 20.4 (15.2, 25.775) | |
| Yes | 44 (36.7) | ||
| No | 76 (63.3) | ||
| Hypoproteinemia | NBS (cpm) | 1.95 (0.89, 3.06) | |
| Yes | 22 (18.3) | ||
| No | 98 (81.7) | ||
| Tumor site | BSVA (dB) | 47.179 (44.75, 49.28) | |
| Cardiac | 21 (17.5) | ||
| Body | 63 (52.5) | ||
| Antrum | 36 (30.0) | ||
| Tumor size | 4 (3.00, 5.00) | FBS (Hz) | 424.32(383.45,485.76) |
| Stage cTNM | |||
| 0 | 17 (14.2) | ||
| I | 20 (16.7) | ||
| II | 32 (26.7) | ||
| III | 41 (34.2) | ||
| IV | 8 (3.3) |
Table 2 indicates that no significant clinical or surgical treatment differences were observed between the training and validation cohorts, demonstrating comparability. As shown in Table 3, a univariate analysis of the 28 variables identified six PPOI-related factors: Age ≥ 70 years, cTNM staging (I and IV), preoperative hypoproteinemia, RTBS ≥ 15, NBS ≥ 2.436, and FBS ≥ 461.56. Variables with P < 0.05 in the univariate analysis were included in the multivariate Cox regression analysis. The results identified age ≥ 70 years [odds ratio (OR) = 5.779; 95% confidence interval (CI): 1.620-23.840; P = 0.009], cTNM stage (I: OR = 19.643, 95%CI: 1.566-646.928, P = 0.042; IV: OR = 98.75, 95%CI: 4.553-4617.345, P = 0.006), preoperative hypoproteinemia (OR = 14.975; 95%CI: 3.438-87.185; P < 0.001), bowel sound-related indices (RTBS ≥ 15: OR = 0.145, 95%CI: 0.033-0.588, P = 0.001; NBS ≥ 2.436: OR = 0.077, 95%CI: 0.012-0.331, P = 0.001; FBS ≥ 461.56: OR = 8.811, 95%CI: 2.351-10.912, P = 0.002) as independent risk factors for PPOI in patients with GC.
| Variable | Overall (N = 120) | Training (N = 84) | Validation (N = 36) | P value |
| Age (years) | 0.291 | |||
| ≥ 70 | 40 (33.3) | 25 (29.8) | 15 (41.7) | |
| < 70 | 80 (66.7) | 59 (70.2) | 21 (58.3) | |
| Gender | 0.291 | |||
| Male | 80 (66.7) | 59 (70.2) | 21 (58.3) | |
| Female | 40 (33.3) | 25 (29.8) | 15 (41.7) | |
| BMI (kg/m2) | 0.768 | |||
| < 18.5 | 16 (13.3) | 10 (16.7) | 6 (8.3) | |
| > 18.8 < 25 | 82 (68.3) | 58 (18.1) | 24 (18.8) | |
| > 25 | 22 (18.4) | 16 (65.3) | 6 (72.9) | |
| Smoking | 0.078 | |||
| Yes | 46 (38.3) | 37 (40.0) | 9 (25) | |
| No | 74 (61.7) | 47 (60.0) | 27 (75) | |
| Drinking | 0.345 | |||
| Yes | 21 (17.5) | 17 (20.2) | 4 (11.1) | |
| No | 99 (82.5) | 67 (79.8) | 32 (88.9) | |
| Hypertension | 0.05 | |||
| Yes | 33 (27.5) | 28 (33.3) | 5 (13.9) | |
| No | 87 (72.5) | 56 (66.7) | 31 (86.1) | |
| Diabetes | 0.581 | |||
| Yes | 11 (9.2) | 9 (10.7) | 2 (5.6) | |
| No | 109 (90.8) | 75 (89.3) | 34 (94.4) | |
| Previous abdominal surgery | 0.885 | |||
| Yes | 26 (21.7) | 19 (22.6) | 7 (19.4) | |
| No | 94 (78.3) | 65 (77.4) | 29 (80.6) | |
| Anemia | 1 | |||
| Yes | 44 (36.7) | 31 (36.9) | 13 (36.1) | |
| No | 76 (63.3) | 53 (63.1) | 23 (63.9) | |
| Hypoproteinemia | 0.135 | |||
| Yes | 22 (18.3) | 12 (14.3) | 10 (16.8) | |
| No | 98 (81.7) | 72 (85.7) | 26 (72.2) | |
| Preoperative potassium | 1 | |||
| Normal | 108 (90.0) | 76 (90.5) | 32 (88.9) | |
| Abnormal | 12 (10.0) | 8 (9.5) | 4 (11.1) | |
| Emergency surgery | 0.444 | |||
| Yes | 3 (2.5) | 1 (1.2) | 2 (5.6) | |
| No | 117 (97.5) | 83 (98.8) | 34 (94.4) | |
| Preoperative bowel preparation | 0.784 | |||
| Yes | 114 (95.0) | 79 (94.0) | 35 (97.2) | |
| No | 6 (5.0) | 5 (6.0) | 1 (2.8) | |
| Tumor size (cm) | 0.693 | |||
| < 5 | 76 (63.3) | 52 (61.9) | 24 (66.7) | |
| ≥ 5 < 10 | 36 (30.0) | 27 (32.1) | 9 (25.0) | |
| ≥ 10 | 8 (6.7) | 5 (6.0) | 3 (8.3) | |
| Tumor site | 0.627 | |||
| Cardiac | 21 (17.5) | 13 (15.5) | 8 (22.2) | |
| Body | 63 (52.5) | 46 (54.8) | 17 (47.2) | |
| Antrum | 36 (30.0) | 25 (29.8) | 11 (30.6) | |
| Stage cTNM | 0.537 | |||
| 0 | 17 (14.2) | 11 (13.1) | 6 (16.7) | |
| I | 20 (16.7) | 13 (15.5) | 7 (19.4) | |
| II | 32 (26.7) | 25 (29.8) | 7 (19.4) | |
| III | 43 (35.8) | 28 (33.3) | 15 (41.7) | |
| IV | 8 (6.7) | 7 (8.3) | 1 (2.8) | |
| Surgical method | 0.858 | |||
| Open | 33 (27.5) | 24 (28.6) | 9 (25) | |
| Laparoscope | 87 (72.5) | 60 (71.4) | 27 (75) | |
| Range_of_excision | 0.427 | |||
| Distal | 21 (17.5) | 17 (20.2) | 4 (11.1) | |
| Proximal | 53 (44.2) | 37 (44.0) | 16 (44.4) | |
| Total | 46 (38.3) | 30 (35.7) | 16 (44.4) | |
| Operation time (min) | 1 | |||
| < 275 | 63 (52.5) | 44 (52.4) | 19 (52.8) | |
| ≥ 275 | 57 (47.5) | 40 (47.6) | 17 (47.2) | |
| Anesthesia time (min) | 0.803 | |||
| < 365 | 43 (35.8) | 29 (34.5) | 14 (38.9) | |
| ≥ 365 | 77 (64.2) | 55 (65.5) | 22 (61.1) | |
| RTBS (h) | 0.144 | |||
| < 15 | 92 (76.7) | 68 (81.0) | 24 (66.7) | |
| ≥ 15 | 28 (23.3) | 16 (19.0) | 12 (33.3) | |
| NBS (cpm) | 1 | |||
| ≥ 2.436 | 48 (40.0) | 34 (40.5) | 14 (38.9) | |
| < 2.436 | 72 (60.0) | 50 (59.5) | 22 (61.1) | |
| BSVA (dB) | 0.964 | |||
| < 44.793 | 88 (73.3) | 61 (72.6) | 27 (75.0) | |
| ≥ 44.793 | 32 (26.7) | 23 (27.4) | 9 (25.0) | |
| FBS (Hz) | 1 | |||
| < 461.560 | 36 (30.0) | 25 (29.8) | 11 (30.6) | |
| ≥ 461.560 | 84 (70.0) | 59 (70.2) | 25 (69.4) |
| Variable | PPOI number (%) | P value | Multivariable OR (95CI) | P value | |
| Age | 0.009 | ||||
| ≥ 70 | 11/25 (44) | < 0.001 | 5.779 | ||
| < 70 | 12/59 (56) | (1.620-23.84) | |||
| Gender | |||||
| Male | 15/59 (25.4) | 0.386 | |||
| Female | 8/25 (32.0) | ||||
| BMI | |||||
| < 18.5 | 4/10 (40.0) | - | |||
| ≥ 18.8 < 25 | 16/58 (27.5) | 0.181 | |||
| ≥ 25 | 3/16 (18.8) | 0.094 | |||
| Smoking | 0.267 | ||||
| Yes | 7/37 (18.9) | ||||
| No | 16/47 (34.0) | ||||
| Drinking | 0.235 | ||||
| Yes | 6/17 (35.3) | ||||
| No | 17/67 (25.3) | ||||
| Hypertension | 0.972 | ||||
| Yes | 7/28 (25.0) | ||||
| No | 16/56 (28.6) | ||||
| Diabetes | 0.182 | ||||
| Yes | 1/9 (11.1) | ||||
| No | 22/75 (29.3) | ||||
| Previous abdominal surgery | 0.36 | ||||
| Yes | 8/19 (42.1) | ||||
| No | 15/65 (23.1) | ||||
| Anemia | 0.702 | ||||
| Yes | 8/31 (25.8) | ||||
| No | 15/53 (28.3) | ||||
| Hypoproteinemia | < 0.001 | 15.0 (3.44-87.19) | < 0.001 | ||
| Yes | 7/28 (25.0) | ||||
| No | 16/56 (28.6) | ||||
| Preoperative potassium | 0.634 | ||||
| Normal | 21/76 (27.6) | ||||
| Abnormal | 2/8 (25.0) | ||||
| Emergency surgery | 0.819 | ||||
| Yes | 0/1 (0.0) | ||||
| No | 23/83 (27.1) | ||||
| Tumor size (cm) | |||||
| < 5 | 12/52 (23.1) | - | |||
| ≥ 5 < 10 | 8/27 (29.6) | 0.535 | |||
| ≥ 10 | 3/5 (60.0) | 0.449 | |||
| Tumor site | |||||
| Cardiac | 3/13 (23.1) | - | |||
| Body | 14/46 (30.4) | 1 | |||
| Antrum | 6/25 (24.0) | 0.767 | |||
| Stage cTNM | |||||
| 0 | 0/11 (0.0) | - | - | ||
| I | 5/13 (38.5) | 0.091 | 19.64 (1.57-646.93) | 0.042 | |
| II | 6/25 (24.0) | 0.096 | 5.46 (0.49-149.62) | 0.216 | |
| III | 8/28 (28.6) | 0.073 | 9.71 (0.99-256.35) | 0.089 | |
| IV | 4/7 (57.1) | 0.026 | 98.8 (4.55-4617.35) | 0.006 | |
| RTBS | 0.012 | 0.15 (0.03-0.59) | 0.001 | ||
| ≥ 15 | 7/16 (43.8) | ||||
| < 15 | 16/68 (23.5) | ||||
| NBS (cpm) | 0.001 | 0.077 (0.01-0.33) | 0.001 | ||
| ≥ 2.436 | 18/64 (28.1) | ||||
| < 2.436 | 5/20 (25.0) | ||||
| BSVA (dB) | 0.2 | ||||
| ≥ 44.793 | 5/23 (21.7) | ||||
| < 44.793 | 18/61 (29.5) | ||||
| FBS (Hz) | 0.007 | 8.81 (2.35-10.91) | 0.002 | ||
| ≥ 461.560 | 12/59 (20.3) | ||||
| < 461.560 | 11/25 (44.0) | ||||
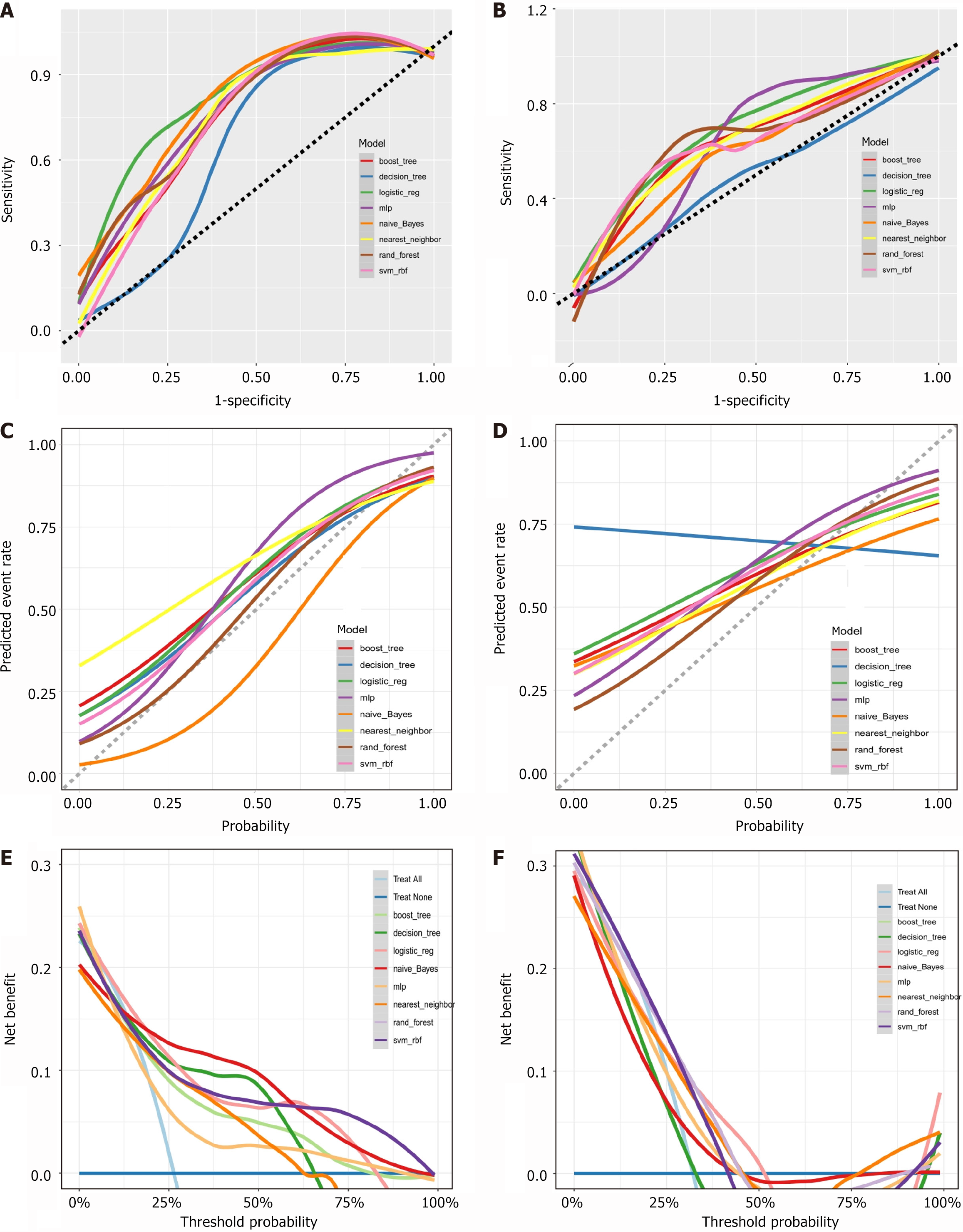
This study evaluated eight machine learning models for predicting PPOI. The naïve Bayes model demonstrated excellent performance in both the training (AUC = 0.880, accuracy = 0.823, Brier score = 0.139) and validation cohorts (AUC = 0.747, accuracy = 0.680, Brier score = 0.236). The RF model exhibited optimal performance with an AUC of 0.833 and accuracy of 0.805 (Brier score = 0.137) in the training cohort and achieved an AUC of 0.751 and accuracy of 0.690 (Brier score = 0.192) in the validation cohort. The LR model achieved an AUC of 0.825 with an accuracy of 0.816 (Brier score = 0.145) in the training cohort and an AUC of 0.685 with an accuracy of 0.685 (Brier score = 0.284) in the validation cohort. The XGBoost model achieved an AUC of 0.811 and an accuracy of 0.796 (Brier score = 0.154) in the training cohort, and an AUC of 0.646 and an accuracy of 0.700 (Brier score = 0.223) in the validation cohort. The SVM model exhibited an AUC of 0.800 and an accuracy of 0.830 (Brier score = 0.135) in the training cohort and an AUC of 0.748 and an accuracy of 0.674 (Brier score = 0.214) in the validation cohort. The KNN model showed an AUC of 0.760 and an accuracy of 0.775 (Brier score = 0.189) in the training cohort and achieved an AUC of 0.670 and an accuracy of 0.700 (Brier score = 0.215) in the validation cohort. The decision tree model yielded an AUC of 0.726 and accuracy of 0.805 (Brier score = 0.161) in the training cohort and showed good performance in the validation cohort (AUC = 0.511, accuracy = 0.627, Brier score = 0.295). The NN model exhibited an AUC of 0.726 and an accuracy of 0.805 (Brier score = 0.161) in the training cohort but lower performance in the validation cohort (AUC = 0.511, accuracy = 0.627, Brier score = 0.295). These indices and model evaluations indicated that the naive Bayesian model, demonstrated consistent performances in both the training and validation cohorts and outperformed all the other models. Consequently, a Bayesian model was established to predict PPOI, which is available online at https://plc-predict.shinyapps.io/PPOI/ (Figure 6).
The DCA curve demonstrated that the naïve Bayes model has substantial clinical utility for diagnosing postoperative PPOI in patients with GC (Figure 6).
Advancements in medical and healthcare standards in China have enhanced the understanding of various malignancies, and comprehensive treatment, including surgical treatment, has emerged as the preferred clinical approach[18]. Consequently, the effectiveness of radical surgical treatments and perioperative recovery have become critical concerns for clinicians and patients. This study employed continuous auscultatory recorders for prolonged postoperative monitoring of patients with GC to explore new avenues for assessing gastrointestinal function and predicting postoperative PPOI to enhance recovery through early intervention.
This study analyzed 29 potential variables, including bowel sound-related indicators, in 120 surgically treated patients with GC to identify independent risk factors for PPOI. Notably, age > 70 years, cTNM staging (I and IV), hypoproteinemia, RTBS < 15, NBS < 2.436, and FBS ≥ 461.560 were identified as significant risk factors. Eight machine learning models were constructed and evaluated using ROC curves, calibration curves, AUC values, accuracy, and Brier scores. The results highlighted the superior performance of the naïve Bayes models in both the training and validation cohorts. DCA highlighted the clinical utility of Bayesian models.
A study on PPOI in intestinal tumors[19] underscored significant differences in PPOI definitions, surgical approaches (open vs laparoscopic), and procedure duration, leading to disparate PPOI incidence rates. In this study, the incidence of PPOI after GC was 27.5%, which is frequently associated with conservative medical management practices that delay early feeding protocols for patients. Existing literature on PPOI risk factors reported diverse findings. For instance, in a meta-analysis, Lee et al[20] reported that abdominal surgery, age, BMI, medical comorbidities, or smoking status had no significant impact on the development of PPOI; however, they observed a male predisposition to intestinal obstruction. Liang et al[8] identified age ≥ 60 years, open surgery, advanced stage (III-IV), and postoperative opioid use as independent risk factors of PPOI in patients with GC. Similarly, Sugawara et al[21] associated PPOI with a history of smoking (OR = 2.31, 95%CI: 1.11-5.17, P = 0.025), colorectal surgery (OR = 2.31, 95%CI: 1.11–5.17, P = 0.004), and open surgical approaches (OR = 3.74, 95%CI: 1.56-11.12, P = 0.002). However, in our study, the results of multifactorial analysis identified age ≥ 70 years, cTNM stage (I and IV), preoperative hypoproteinemia, and specific bowel sound-related indicators (RTBS ≥ 15, NBS ≥ 2.436, and FBS ≥ 461.56) as predictive indicators for postoperative PPOI in patients with GC. This observation underscores the predictive potential of bowel sound-related indices for clinical outcome assessments.
Our study had several notable advantages. First, unlike most previous studies that focused on the incidence of PPOI in patients with colon or rectal cancer, this study provided new evidence on the presence of PPOI in patients with GC. To our knowledge, this is the first study to include indices related to bowel sounds, offering a novel method for clinicians to evaluate gastrointestinal function. Continuous, uninterrupted, and contactless auscultation is crucial for the real-time understanding of patients' gastrointestinal function status. The inclusion of bowel sound indicators in the predictive model demonstrated significant potential for clinical recommendations. Internal validation confirmed the stability of the model, and DCA revealed a positive net benefit. However, this study had certain limitations. The sample size was relatively small, and the single-center, retrospective study design limited the generalizability of our results. Some indicators with significant ORs might have introduced potential bias, likely because of the limited data. Moreover, this study lacked an external validation cohort to validate the model, which will be addressed in future studies.
In summary, PPOI is a common complication following gastrectomy in patients with GC. Age, cTNM stage, preoperative hypoproteinemia, and bowel sound-related indices (RTBS, NBS, and FBS) correlated with PPOI. Clinicians should focus on older patients, correct their nutritional status preoperatively, and routinely monitor bowel sounds postoperatively for early intervention to improve patient outcomes. An easy-to-use clinical model was established for predicting PPOI in patients with GC.
This study was conducted in the Second Surgery Department. We are grateful to all the staff members for their assistance with the experiments.
| 1. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 159] [Reference Citation Analysis (3)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2838] [Article Influence: 567.6] [Reference Citation Analysis (5)] |
| 3. | Traeger L, Koullouros M, Bedrikovetski S, Kroon HM, Thomas ML, Moore JW, Sammour T. Cost of postoperative ileus following colorectal surgery: A cost analysis in the Australian public hospital setting. Colorectal Dis. 2022;24:1416-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Yang JW, Yan SY, Lu Y, Han JG, Pei W, Zhao JJ, Li ZK, Zhou H, Yang NN, Wang LQ, Yang YC, Liu CZ. Electroacupuncture vs Sham Electroacupuncture in the Treatment of Postoperative Ileus After Laparoscopic Surgery for Colorectal Cancer: A Multicenter, Randomized Clinical Trial. JAMA Surg. 2023;158:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Li ZL, Zhao BC, Deng WT, Zhuang PP, Liu WF, Li C, Liu KX. Incidence and risk factors of postoperative ileus after hysterectomy for benign indications. Int J Colorectal Dis. 2020;35:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Wehner S, Vilz TO, Stoffels B, Kalff JC. Immune mediators of postoperative ileus. Langenbecks Arch Surg. 2012;397:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Namba Y, Hirata Y, Mukai S, Okimoto S, Fujisaki S, Takahashi M, Fukuda T, Ohdan H. Clinical indicators for the incidence of postoperative ileus after elective surgery for colorectal cancer. BMC Surg. 2021;21:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Liang WQ, Zhang KC, Cui JX, Xi HQ, Cai AZ, Li JY, Liu YH, Liu J, Zhang W, Wang PP, Wei B, Chen L. Nomogram to predict prolonged postoperative ileus after gastrectomy in gastric cancer. World J Gastroenterol. 2019;25:5838-5849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013;17:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 10. | Rocha DM, Brasil LM, Lamas JM, Luz GVS, Bacelar SS. Evidence of the benefits, advantages and potentialities of the structured radiological report: An integrative review. Artif Intell Med. 2020;102:101770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wang G, Wang M, Liu H, Zhao S, Liu L, Wang W. Changes in bowel sounds of inpatients undergoing general anesthesia. Biomed Eng Online. 2020;19:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Nowak JK, Nowak R, Radzikowski K, Grulkowski I, Walkowiak J. Automated Bowel Sound Analysis: An Overview. Sensors (Basel). 2021;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Namikawa T, Yamaguchi S, Fujisawa K, Ogawa M, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M, Matsuda K, Hanazaki K. Real-time bowel sound analysis using newly developed device in patients undergoing gastric surgery for gastric tumor. JGH Open. 2021;5:454-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Read TE, Brozovich M, Andujar JE, Ricciardi R, Caushaj PF. Bowel Sounds Are Not Associated With Flatus, Bowel Movement, or Tolerance of Oral Intake in Patients After Major Abdominal Surgery. Dis Colon Rectum. 2017;60:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Gu Y, Lim HJ, Moser MA. How useful are bowel sounds in assessing the abdomen? Dig Surg. 2010;27:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kaneshiro M, Kaiser W, Pourmorady J, Fleshner P, Russell M, Zaghiyan K, Lin A, Martinez B, Patel A, Nguyen A, Singh D, Zegarski V, Reid M, Dailey F, Xu J, Robbins K, Spiegel B. Postoperative Gastrointestinal Telemetry with an Acoustic Biosensor Predicts Ileus vs. Uneventful GI Recovery. J Gastrointest Surg. 2016;20:132-9; discussion 139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Zhao K, Jiang H, Wang Z, Chen P, Zhu B, Duan X. Long-Term Bowel Sound Monitoring and Segmentation by Wearable Devices and Convolutional Neural Networks. IEEE Trans Biomed Circuits Syst. 2020;14:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Halama N, Haberkorn U. The Unmet Needs of the Diagnosis, Staging, and Treatment of Gastrointestinal Tumors. Semin Nucl Med. 2020;50:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gong W, Qi X. Association of Frailty with Delayed Recovery of Gastrointestinal Function after Elective Colorectal Cancer Resections. J Invest Surg. 2020;33:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Lee MJ, Vaughan-Shaw P, Vimalachandran D; ACPGBI GI Recovery Group. A systematic review and meta-analysis of baseline risk factors for the development of postoperative ileus in patients undergoing gastrointestinal surgery. Ann R Coll Surg Engl. 2020;102:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sugawara K, Kawaguchi Y, Nomura Y, Suka Y, Kawasaki K, Uemura Y, Koike D, Nagai M, Furuya T, Tanaka N. Perioperative Factors Predicting Prolonged Postoperative Ileus After Major Abdominal Surgery. J Gastrointest Surg. 2018;22:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |