Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3437
Revised: August 19, 2024
Accepted: August 23, 2024
Published online: November 27, 2024
Processing time: 234 Days and 11.7 Hours
With the widespread use of hemocoagulase in patients with gastrointestinal bleeding, clinicians have become increasingly concerned about coagulation dis
To determine risk factors for hemocoagulase-associated hypofibrinogenemia in patients with gastrointestinal bleeding.
We performed a retrospective analysis of the medical documentation of hospitalized patients treated with hemocoagulase for gastrointestinal bleeding. Hypofibrinogenemia was defined as a decrease in plasma fibrinogen concentration to less than 2.0 g/L. The included patients were divided into two groups: acquired hypofibrinogenemia group and non-hypofibrinogenemia group. We used logistic regression analysis to identify potential risk factors and established risk assess
There were 36 patients in the acquired hypofibrinogenemia group and 73 patients in the non-hypofibrinogenemia group. The hypofibrinogenemia group showed higher rates of intensive care unit admissions (P = 0.021), more female patients (P = 0.005), higher in-hospital mortality (P = 0.027), larger hemocoagulase doses (P = 0.026), more Packed Red Cells transfusions (P = 0.024), and lower baseline fibrinogen levels (P < 0.000). Binary logistic regression was employed to examine the risk factors associated with acquired hypofibrinogenemia. The analysis revealed that baseline fibrinogen [odds ratio (OR) 0.252, 95%CI: 0.137-0.464, P < 0.000], total hemocoagulase doses (OR 1.074, 95%CI: 1.015-1.137, P = 0.014), and female gender (OR 2.856, 95%CI: 1.015–8.037, P = 0.047) were statistically significant risk factors.
Higher doses of total hemocoagulase, female gender, and a lower baseline fibrinogen level were risk factors for hemocoagulase-associated hypofibrinogenemia in patients with gastrointestinal bleeding.
Core Tip: In order to identify risk factors for hemocoagulase-associated hypofibrinogenemia, a retrospective study analyzing data from 109 patients with gastrointestinal bleeding was conducted. We found that higher doses of total hemocoagulase, female gender, and a lower baseline fibrinogen level were risk factors for hemocoagulase-associated hypofibrinogenemia in patients with gastrointestinal bleeding.
- Citation: Zou F, Wu MT, Wang YY. Risk factors for hemocoagulase-associated hypofibrinogenemia in patients with gastrointestinal bleeding. World J Gastrointest Surg 2024; 16(11): 3437-3444
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3437.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3437
Gastrointestinal bleeding (GIB) encompasses various instances of bleeding within the gastrointestinal tract. Manifestations of GIB, particularly when characterized by a rapid blood loss, may include vomiting red blood, vomiting black blood, bloody stool, or black stool[1,2]. Additional symptoms may involve abdominal discomfort, respiratory distress, a pallid complexion, or episodes of syncope. It is noteworthy that individuals experiencing minimal blood loss may remain asymptomatic[1,2].
GIB is commonly classified into three types: Upper GIB (UGIB), lower GIB (LGIB), and middle GIB (MGIB)[1,2]. Causes of UGIB include peptic ulcer disease, esophageal varices resulting from liver cirrhosis and cancer, among other factors[2]. Causes of LGIB involve hemorrhoids, cancer, and inflammatory bowel disease, among other contributing factors[2]. Causes of MGIB comprise inflammatory bowel disease, Meckel’s diverticulum, and small bowel neoplasms[2,3]. The primary interventions for GIB include resuscitation, intravenous fluids, use of proton pump inhibitors, and blood transfusions. Endoscopy of the upper or lower gastrointestinal tract is typically recommended within 24 hours, facilitating both diagnosis and treatment[4].
Hemocoagulase, a hemostatic preparation originating from snake venom, is widely used for bleeding disorders in China. Currently, several hemocoagulase products have received approval from China's National Medical Products Administration[5-7]. These products consist of Slounase (based on viper venom), Hemocoagulase for injection (based on Agkistrodon halys pallas venom), Haemocoagulase Agkistrodon for injection (based on Agkistrodon acutus venom), and Hemocoagulase Bothrops Atrox for injection (based on Bothrops atrox venom)[5-7]. These hemocoagulase agents can effectively treat GIB, traumatic bleeding, surgical hemorrhage, and other bleeding disorders[8,9]. Their proven efficacy lies in diminishing bleeding duration and transfusion needs[5,8,9].
Hemostasis by hemocoagulase is achieved through two primary mechanisms: Enzymatic cleavage of fibrinogen's α-subunit into α-peptide and fibrin, followed by polymerization into insoluble fibrin polymers and fibers facilitated by factor XIII. Additionally, hemocoagulase suppresses factor XIII release, thereby reducing the likelihood of thrombus formation[10,11].
In treating GIB, hemocoagulase is an effective agent, and can be administered both intravenously and locally via endoscopy[5,8-10]. Nevertheless, it is important to emphasize that the utilization of hemocoagulase has the potential to induce acquired hypofibrinogenemia. This, in turn, may contribute to a serious exacerbation of bleeding manifestations. Although there are documented cases of this complication, the risk factors for hypofibrinogenemia induced by hemocoagulase administration are not well understood[9,12,13]. Our research aimed to address this knowledge gap by exploring potential risk factors associated with hemocoagulase-induced hypofibrinogenemia in GIB patients. We hope that this study will provide meaningful insights to healthcare practitioners.
This retrospective investigation, carried out at the First Affiliated Hospital of Chongqing Medical and Pharmaceutical College, was a single-center observational cohort study. The study received approval from the hospital's Medical Ethics Committee, adhering to the ethical principles described in the Declaration of Helsinki. Due to the study's retrospective nature, the necessity to obtain informed consent was exempted[14].
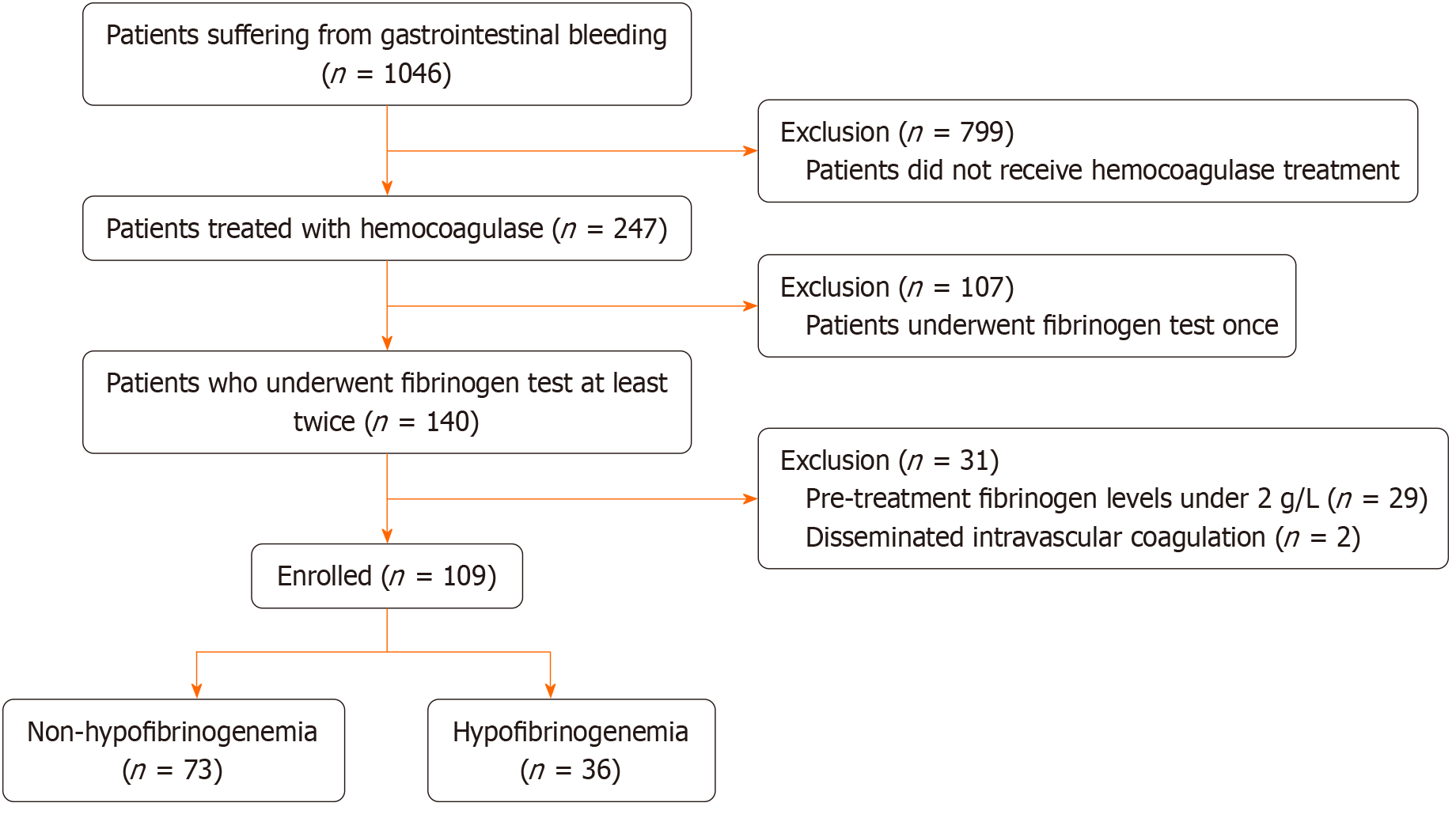
We identified 1046 in-patients suffering from GIB between July 2020 and July 2023. Of these patients, 937 were excluded according to the following criteria: (1) Lack of hemocoagulase treatment; (2) Absence of coagulation status monitoring despite being treated with hemocoagulase; (3) Pre-treatment fibrinogen levels less than 2 g/L; and (4) Diagnosed with disseminated intravascular coagulation. To determine the risk factors associated with hemocoagulase-induced hypofibrinogenemia, we conducted a case-control study, analyzing data from patients who experienced GIB and received hemocoagulase therapy. Acquired hypofibrinogenemia was our principal outcome. Using this outcome as a criterion, we partitioned patients into two groups: Those exhibiting hypofibrinogenemia and those not presenting this condition (Figure 1). We then investigated the correlation between hemocoagulase-induced hypofibrinogenemia and pertinent variables such as patient demographics, causes of bleeding, and laboratory tests, among others.
We extracted patients, clinical data from the digital medical record system. Subsequently, we analyzed the data comprising demographics (age, sex, body mass index), cumulative hemocoagulase dosage, duration of hemocoagulase use, GIB etiological factors, admission to the intensive care unit (ICU), concomitant medications, site of bleeding, in-patient mortality, transfusions and laboratory tests.
The fibrinogen assay used in this study had a reference range of 2-4 g/L. Consistent with the laboratory's established parameters, we defined hypofibrinogenemia as any plasma fibrinogen concentration lower than 2 g/L. This disorder was subsequently categorized into mild (ranging from 1 to 2 g/L), moderate (ranging from 0.5 to 1 g/L), and severe (lower than 0.5 g/L) phases[15]. Additionally, we defined severe blood loss as hemorrhage leading to hemorrhagic shock or any extent of blood loss causing unstable vital signs[1,2].
We utilized the hemocoagulase agent known as Hemocoagulase for Injection (Avanc Pharma, Jinzhou, Liaoning, China). Each Klobusitzky unit (KU) of this hemocoagulase was administered intravenously after diluting in 10 mL of 0.9% saline solution. The hemocoagulase could also be injected intramuscularly after diluting in 2-5 mL of the same saline solution. The administration frequency varied from one to five times daily depending on the individual patient's health status.
We employed SPSS version 23.0 for data analysis, and a P value less than 0.05 was considered statistically significant. Missing data were replaced using the series mean. Continuous variables, which were not normally distributed, were delineated as medians along with interquartile ranges; categorical variables were represented as rates (in percentages). We used the χ2 test or Fisher’s exact test to compare categorical variables; the Mann-Whitney U test was used to evaluate continuous variables that were not normally distributed. Variables that displayed significant differences between the hypofibrinogenemia group and non-hypofibrinogenemia group were incorporated into a multivariate logistic regression model to determine possible risk factors for hemocoagulase-associated hypofibrinogenemia. To predict hypofibrinogenemia, the receiver operating characteristic (ROC) curve was utilized, and parameters (the optimal cut-off, sensitivity, and specificity) within the ROC curve were determined using the Youden index.
A total of 109 patients were included in the study and divided into two groups. The acquired hypofibrinogenemia group, also known as the case group, comprised 36 patients, while the non-hypofibrinogenemia group, also referred to as the control group, consisted of 73 patients. Following the administration of hemocoagulase, we noted a range in hypofibrinogenemia severity: 22 patients (20.2%) presented mild conditions, 13 patients (11.9%) demonstrated moderate levels, and severe hypofibrinogenemia was observed in one patient, accounting for 0.9%.
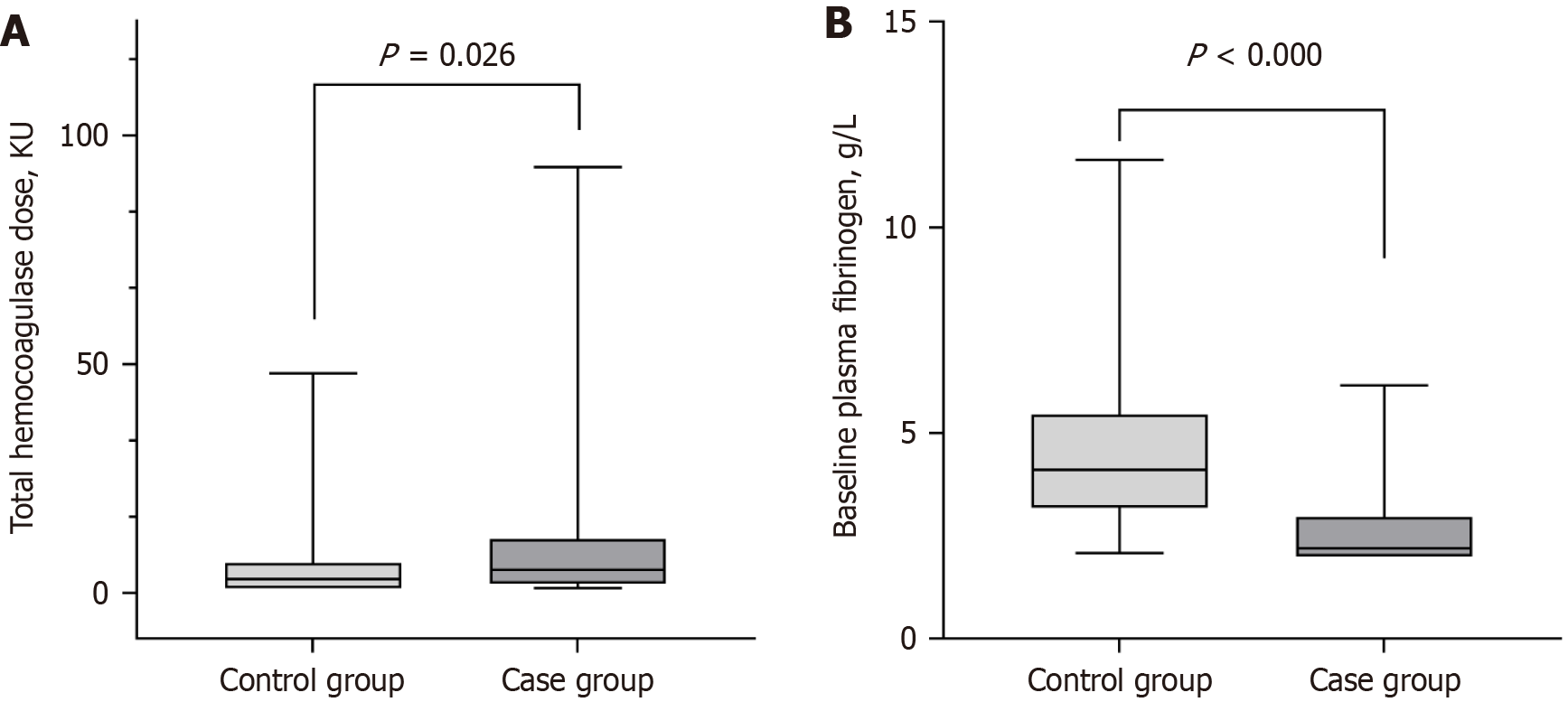
Table 1 summarizes the baseline demographics and clinical characteristics in the two groups. No significant differences were found between the two groups in terms of age and body mass index. Similarly, there were no significant differences in bleeding sites, duration of treatment, and administration of concomitant drugs (Table 1). However, admissions to the ICU were notably higher in the hypofibrinogenemia group compared to the non-hypofibrinogenemia group (5.5% vs 22.2%; P = 0.021; Table 1). The hypofibrinogenemia group also had a higher percentage of female patients (32.9% vs 61.1%; P = 0.005), along with an increased incidence of severe blood loss (9.6% vs 41.7%; P < 0.000) and in-hospital mortality (19.2% vs 38.9%; P = 0.027; Table 1). Median total hemocoagulase doses were significantly higher in the hypofibrinogenemia group (3.0 vs 5.0 KU; P = 0.026; Table 1 and Figure 2A). Bleeding from esophagogastric varices was more prevalent in the hypofibrinogenemia group (4.1% vs 27.8%; P = 0.001; Table 1). Packed red cells (PRC) transfusion rate was significantly higher in the hypofibrinogenemia group (23.3% vs 44.4%; P = 0.024; Table 1). Laboratory tests showed that the hypofibrinogenemia group had lower baseline fibrinogen levels (4.11 vs 2.20 g/L; P < 0.000; Figure 2B), lower fibrinogen levels after treatment (3.83 vs 1.23 g/L; P < 0.000), lower platelet count (PLT) (202 vs 122.5 × 109/L; P = 0.001), and higher total bilirubin (TBil) (11.00 vs 15.87 μmol/L; P = 0.003; Table 1). No significant differences were observed in other laboratory tests between the two groups (Table 1).
| Variables | Non-hypofibrinogenemia (n = 73) | Hypofibrinogenemia (n = 36) | P value |
| Age (years) | 76.0 (66.5-82.0) | 73.0 (57.3-81.0) | 0.29 |
| Sex, female | 24 (32.9) | 22 (61.1) | 0.005a |
| Body mass index (kg/m2) | 20.3 (19.1-21.8) | 20.3 (19.2-20.3) | 0.183 |
| Total hemocoagulase doses (KU) | 3.0 (1-6.5) | 5.0 (2.0-11.8) | 0.026a |
| Treatment duration (days) | 2 (1-5) | 3 (1-6.8) | 0.1 |
| ICU admission | 4 (5.5) | 8 (22.2) | 0.021a |
| Total deaths | 14 (19.2) | 14 (38.9) | 0.027a |
| Severe blood loss | 7 (9.6) | 15 (41.7) | 0.000a |
| Bleeding location | |||
| Upper gastrointestinal | 25 (34.2) | 17 (47.2) | 0.190 |
| Small bowel | 1 (1.4) | 1 (2.8) | 1.0 |
| Lower gastrointestinal | 5 (6.8) | 1 (2.8) | 0.667 |
| Unknown site of bleeding | 42 (57.5) | 17 (47.2) | 0.310 |
| Cause of bleeding | |||
| Esophagogastric varices | 3 (4.1) | 10 (27.8) | 0.001a |
| Malignant neoplasm | 21 (28.8) | 8 (22.2) | 0.467 |
| Peptic ulcer | 10 (13.7) | 2 (5.6) | 0.341 |
| Ischemic bowel disease | 3 (4.1) | 0 (0) | 0.549 |
| Severe infection | 22 (30.1) | 8 (22.2) | 0.384 |
| Unexplained GIB | 14 (19.2) | 8 (20.2) | 0.710 |
| Concomitant drugs | |||
| Cephalosporin | 12 (16.4) | 7 (19.4) | 0.697 |
| Carbopenems | 10 (13.7) | 3 (8.3) | 0.618 |
| CSS | 48 (65.8) | 27 (75.0) | 0.327 |
| Vitamin K1 | 5 (6.8) | 3 (8.3) | 1.0 |
| Tranexamic acid | 4 (5.5) | 3 (8.3) | 0.876 |
| Transfusion | |||
| PRC | 17 (23.3) | 16 (44.4) | 0.024a |
| FFP | 6 (8.2) | 7 (19.4) | 0.166 |
| Laboratory tests | |||
| Baseline FIB (g/L) | 4.11 (3.18-5.44) | 2.20 (2.00-2.96) | 0.000a |
| Post-hemocoagulase FIB (g/L) | 3.83 (2.81-5.16) | 1.23 (0.67-1.62) | 0.000a |
| PLT (× 109/L) | 202 (137.5-277.5) | 122.5 (79-216.75) | 0.001a |
| WBC (× 109/L) | 9.08 (7.11-12.38) | 8.00 (5.79-11.51) | 0.285 |
| ALT (U/L) | 19.00 (14.20-37.78) | 20.13 (12.50-34.85) | 0.867 |
| AST (U/L) | 28.04 (19.90-39.89) | 27.89 (20.18-54.28) | 0.556 |
| TBil (μmol/L) | 11.00 (8.32-20.08) | 15.87 (11.51-39.84) | 0.003a |
In the multivariate analysis, we included the following variables which showed significance at P < 0.05 during univariate analysis: Total hemocoagulase doses, esophagogastric varices, baseline fibrinogen, PLT, sex, and TBil. Moreover, we incorporated treatment duration into the multivariate analysis, despite its P value exceeding 0.05 in the univariate analysis, as a previous study on hemoptysis showed that this variable was associated with acquired hypofibrinogenemia[16].
The multivariate analysis with logistic regression suggested that baseline fibrinogen [odds ratio (OR) 0.252, 95%CI: 0.137-0.464, P < 0.000], total hemocoagulase doses (OR 1.074, 95%CI: 1.015-1.137, P = 0.014), and female gender (OR 2.856, 95%CI: 1.015-8.037, P = 0.047) were risk factors for hypofibrinogenemia induced by hemocoagulase (Table 2). To assess the predictive capacity of both the total doses of hemocoagulase and baseline fibrinogen level for hemocoagulase-associated hypofibrinogenemia, the ROC curve was employed. The area under the receiver operating characteristic curve was 0.630 for total hemocoagulase doses (95%CI: 0.520-0.740, P = 0.028), and was 0.850 for the baseline fibrinogen level (95%CI: 0.769-0.931, P < 0.000; Table 3). The optimal cutoff point, sensitivity, and specificity on the ROC curve were determined by selecting the threshold corresponding to the largest Youden index. For total hemocoagulase doses, the cutoff value was 3.5 KU, with a sensitivity of 63.9% and a specificity of 57.5%. For baseline fibrinogen level, the cutoff value was 3.012 g/L, with a sensitivity of 77.8% and a specificity of 80.8% (Table 3).
To our knowledge, this is the first study to determine the risk factors for hemocoagulase-related hypofibrinogenemia in patients with GIB. Hemocoagulase is a complex of thrombin-like enzymes originating from snake venom. The extensive examination of hemocoagulase spans multiple decades, with the earliest known form, Reptilase (batroxobin), emerging in the 1950s. To date, multiple hemocoagulase products have been commercialized globally, with widespread use in bleeding disorders[6,17].
In the terminal phase of the blood coagulation process, fibrin formation is crucial; hemocoagulase accelerates the conversion of fibrinogen into fibrin monomers, thereby playing a pro-hemostatic role similar to human thrombin. At present, hemocoagulase is extensively used to mitigate traumatic hemorrhage, respiratory system bleeding, and gastrointestinal hemorrhage. Additionally, it plays a preventative role in bleeding complications predominantly stemming from invasive operations. Nevertheless, according to post-marketing studies, hemocoagulase may trigger bleeding associated with hypofibrinogenemia. The current literature on hemocoagulase-related hypofibrinogenemia consists mainly of case reports, with a notable absence of case-control studies[13,18-20].
Our research showed that in patients with GIB, the administration of hemocoagulase may cause acquired hypofibrinogenemia, which was noted in 33% of cases. Before the use of hemocoagulase, fibrinogen levels in all patients were within the normal range. However, following the administration of hemocoagulase, hypofibrinogenemia was noted in 36 patients. In addition, we observed that patients who developed hypofibrinogenemia exhibited more severe blood loss, a higher rate of PRC transfusions, and an increased mortality rate compared to those without hypofibrinogenemia. This suggests that the development of hypofibrinogenemia following hemocoagulase use may result in heightened transfusion needs, and an elevated risk of bleeding and death. In previous studies, a case report detailed the propensity for bleeding in a patient with hemoptysis, caused by hypofibrinogenemia related to long-term hemocoagulase therapy[20]. Moreover, Zhou[13] identified seven patients with hypofibrinogenemia after colonic polypectomy, which was performed following the administration of hemocoagulase; three of these patients had lower gastrointestinal hemorrhage; Notably, no decrease in fibrinogen levels was observed in the 13 patients who did not receive hemocoagulase[13]. The findings of these studies were generally consistent with those of ours. In addition, as hemocoagulase has the potential to induce hypofibrinogenemia, coagulation monitoring and prompt treatment adjustment are necessary for patients. However, coagulation monitoring for all patients is impractical as this would significantly increase medical costs. Therefore, it is important to identify patients at high risk of developing hypofibrinogenemia. In view of this, our study specifically focused on the risk factors associated with the development of hypofibrinogenemia following the administration of hemocoagulase in GIB patients. We identified several risk factors, including total hemocoagulase doses (≥ 3.5 KU), female gender, and a lower baseline fibrinogen level (≤ 3.012 g/L). We believe that these risk factors can help identify patients at high risk of developing hypofibrinogenemia and that patients with GIB who have any of these risk factors require strict coagulation monitoring and prompt treatment adjustment.
Although our research provides meaningful insights, it did have some limitations. First, the single-institution retrospective nature of our study resulted in a limited number of participants. This may constrain the generalizability of our results. Second, there might be factors that could potentially influence the precision of our study. For example, there was uncertainty surrounding the timing and frequency of coagulation function tests. Lastly, due to insufficient laboratory test data, a significant number of GIB patients were not included (for instance, a variety of milder cases were left out as post-treatment coagulation function assessments were not available). This could potentially lead to selection bias.
In patients with GIB, the occurrence of acquired hypofibrinogenemia following treatment with hemocoagulase is associated with a higher likelihood of severe bleeding, increased likelihood of admission to the ICU, and a higher mortality rate compared to those who do not develop acquired hypofibrinogenemia. Higher doses of total hemocoagulase, female gender, and a lower baseline fibrinogen level are risk factors for hemocoagulase-associated hypofibrinogenemia in patients with GIB. We recommend that GIB patients with any of these risk factors receive coagulation monitoring and prompt treatment adjustments during hemocoagulase administration.
| 1. | Kim BS, Li BT, Engel A, Samra JS, Clarke S, Norton ID, Li AE. Diagnosis of gastrointestinal bleeding: A practical guide for clinicians. World J Gastrointest Pathophysiol. 2014;5:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (11)] |
| 2. | Triantafyllou K, Gkolfakis P, Gralnek IM, Oakland K, Manes G, Radaelli F, Awadie H, Camus Duboc M, Christodoulou D, Fedorov E, Guy RJ, Hollenbach M, Ibrahim M, Neeman Z, Regge D, Rodriguez de Santiago E, Tham TC, Thelin-Schmidt P, van Hooft JE. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:850-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | Pennazio M, Cortegoso Valdivia P, Triantafyllou K, Gralnek IM. Diagnosis and management of small-bowel bleeding. Best Pract Res Clin Gastroenterol. 2023;64-65:101844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 4. | Rodrigues A, Carrilho A, Almeida N, Baldaia C, Alves Â, Gomes M, Gonçalves L, Nunes AR, Pereira CL, Silva MJ, Aguiar J, Orfão R, Duarte P, Marinho RT. Interventional Algorithm in Gastrointestinal Bleeding-An Expert Consensus Multimodal Approach Based on a Multidisciplinary Team. Clin Appl Thromb Hemost. 2020;26:1076029620931943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Lu S, Han M, Song Y, Liu C. Hemocoagulase agkistrodon can prevent bleeding and induce hypofibrinogenemia in hepatic disease cases . Int J Clin Pharmacol Ther. 2020;58:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Castro HC, Zingali RB, Albuquerque MG, Pujol-Luz M, Rodrigues CR. Snake venom thrombin-like enzymes: from reptilase to now. Cell Mol Life Sci. 2004;61:843-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | National Medical Products Administration. Online Drug Search in China. Aug 6, 2021. [cited 13 August 2024]. Available from: https://www.nmpa.gov.cn/datasearch/home-index.html#category=yp. |
| 8. | Qiu M, Zhang X, Cai H, Xu Z, Lin H. The impact of hemocoagulase for improvement of coagulation and reduction of bleeding in fracture-related hip hemiarthroplasty geriatric patients: A prospective, single-blinded, randomized, controlled study. Injury. 2017;48:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Zhang H. The Effects of Hemocoagulase on Coagulation Factors in an Elderly Patient with Upper Gastrointestinal Hemorrhage: A Case Report. Curr Drug Saf. 2019;14:230-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Wei JM, Zhu MW, Zhang ZT, Jia ZG, He XD, Wan YL, Wang S, Xiu DR, Tang Y, Li J, Xu JY, Heng QS. A multicenter, phase III trial of hemocoagulase Agkistrodon: hemostasis, coagulation, and safety in patients undergoing abdominal surgery. Chin Med J (Engl). 2010;123:589-593. [PubMed] |
| 11. | Xian R, Wang C, Gong L, Hang B, Wang W, Zhang X, Du H, Wang F, Shi F. A Species-Specific Strategy for the Identification of Hemocoagulase Agkistrodon halys pallas Based on LC-MS/MS-MRM. Front Mol Biosci. 2022;9:831293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Qi X, Wang J, Yu X, De Stefano V, Li H, Wu C, Zeng Q, Zhang Y, Ren L, Lin H, Deng J, Guo X. Hemocoagulase might not control but worsen gastrointestinal bleeding in an elderly patient with type II respiratory failure. Transl Gastroenterol Hepatol. 2017;2:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Zhou HB. Hypofibrinogenemia Caused by Hemocoagulase After Colon Polyps Excision. Am J Case Rep. 2017;18:291-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 18329] [Article Influence: 1527.4] [Reference Citation Analysis (0)] |
| 15. | Brunclikova M, Simurda T, Zolkova J, Sterankova M, Skornova I, Dobrotova M, Kolkova Z, Loderer D, Grendar M, Hudecek J, Stasko J, Kubisz P. Heterogeneity of Genotype-Phenotype in Congenital Hypofibrinogenemia-A Review of Case Reports Associated with Bleeding and Thrombosis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Lee JK, Yoon CS, Na YO, Park HK, Oh HJ, Kho BG, Park HY, Kim TO, Shin HJ, Kwon YS, Kim YI, Lim SC. Risk factors and clinical outcomes associated with acquired hypofibrinogenemia in patients administered hemocoagulase batroxobin for hemoptysis. J Thorac Dis. 2023;15:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | BRUCK H, SALEM G. [Reptilase, a hemostatic for prophylaxis and therapy in surgical operations]. Wien Klin Wochenschr. 1954;66:395-397. [PubMed] |
| 18. | Wang L, Wang C, Zhang D, Wang W, Wang F. Effectiveness and safeties of hemocoagulase and tranexamic acid to reduce perioperative blood loss in intertrochanteric fracture PFNA fixation. Acta Orthop Belg. 2023;89:645-650. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Linglong X, Dijiong W. Prolonged Hemocoagulase Agkistrodon Halys Pallas Administration Induces Hypofibrinogenemia in Patients with Hematological Disorders: A Clinical Analysis of 11 Patients. Indian J Hematol Blood Transfus. 2018;34:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kim TO, Kim MS, Kho BG, Park HY, Kwon YS, Kim YI, Lim SC, Shin HJ. Paradoxical pulmonary hemorrhage associated with hemocoagulase batroxobin in a patient with hemoptysis: A CARE-compliant case report. Medicine (Baltimore). 2021;100:e24040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |