Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3425
Revised: September 12, 2024
Accepted: September 23, 2024
Published online: November 27, 2024
Processing time: 122 Days and 23.7 Hours
Current surgical procedures for anorectal abscesses, including incision and drainage alone or combined concurrent fistulotomy, remain controversial pri
To evaluate factors that predict postoperative recurrence of anorectal abscesses and propose a new classification to guide surgical procedures.
In this retrospective study, 525 patients with anorectal abscesses treated by incision and drainage alone, at a tertiary general hospital from August 2012 to July 2022, were included. A new classification for anorectal abscesses based on their propensity to develop into fistulas, considering 18 other potential risk fac
One year post-follow-up, the overall recurrence rate was 39%:81.0% and 23.5% for fistula-prone and non-fistula-prone abscesses, respectively. Univariate χ² analysis showed significant differences in recurrence rates based on anatomical classifications and pus culture results (P < 0.05). Fistula-prone abscess, ≥ 7 days between symptom onset and surgery, chronic diarrhea, preoperative antibiotic use, and local anesthesia were risk factors for re
The choice of surgical procedure for treating anorectal abscesses should follow this new classification. Prompt and thorough incision and drainage can significantly reduce postoperative recurrence.
Core Tip: This retrospective study evaluated the predictive factors for postoperative anorectal abscess recurrence and proposed a new classification to guide surgical procedures, including incision and drainage alone or a combined concurrent fistulotomy. We found that the recurrence rate of fistula-prone and non-fistula-prone abscesses (FPAs) was 81.0% and 23.5%, respectively. Additionally, we demonstrated that FPAs, a duration ≥ 7 days from symptom onset to surgery, chronic diarrhea, and local anesthesia were independent risk factors for postoperative anorectal abscess recurrence. Our findings support using this new classification to guide the choice of surgical procedures for treating anorectal abscesses.
- Citation: Chen SZ, Sun KJ, Gu YF, Zhao HY, Wang D, Shi YF, Shi RJ. Proposal for a new classification of anorectal abscesses based on clinical characteristics and postoperative recurrence. World J Gastrointest Surg 2024; 16(11): 3425-3436
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3425.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3425
Anorectal abscesses are among the most common conditions encountered in the Department of Anorectal Surgery, with an estimated annual incidence of 68000–96000 in the United States[1]. Predominantly, in 90% of cases, anorectal abscesses develop due to cryptoglandular infections and can spread in multiple directions, leading to various types of abscesses[2]. In severe cases, the condition can progress to life-threatening necrotizing fasciitis[3].
Undoubtedly, anorectal abscesses require surgical treatment; however, only a proportion of patients with this con
Currently, anorectal abscesses are described according to their anatomical locations: (1) Ischiorectal; (2) Intersph
Through years of clinical observation, we found that fistula-prone abscesses (FPAs) have an extremely high rate of recurrence or fistula formation after simple I and D, while non-FPAs (NFPAs) exhibit a substantially lower rate of re
This study was approved by the Ethics Committee of Yangzhong People's Hospital (No. KY202317), and the requirement for written informed consent was waived due to its retrospective and anonymous nature.
Clinical data from outpatients and inpatients diagnosed with anorectal abscesses, treated with I and D alone in our hospital (a tertiary general hospital) from August 2012 to July 2022, were collected.
The inclusion criteria were as follows: (1) Patients diagnosed with an anorectal abscess, not limited by sex; (2) Patients with ages ranging from 18–70 years; and (3) Patients who underwent I and D alone at our hospital.
The exclusion criteria were as follows: (1) A non-cryptoglandular anorectal abscesses (caused by specific infection or trauma); (2) History of anal fistula and anorectal surgery; (3) Comorbidities including human immunodeficiency virus, malignant tumors, tuberculosis, inflammatory bowel disease, and necrotizing fasciitis; (4) Missing or incomplete clinical data; and (5) Pregnant patients.
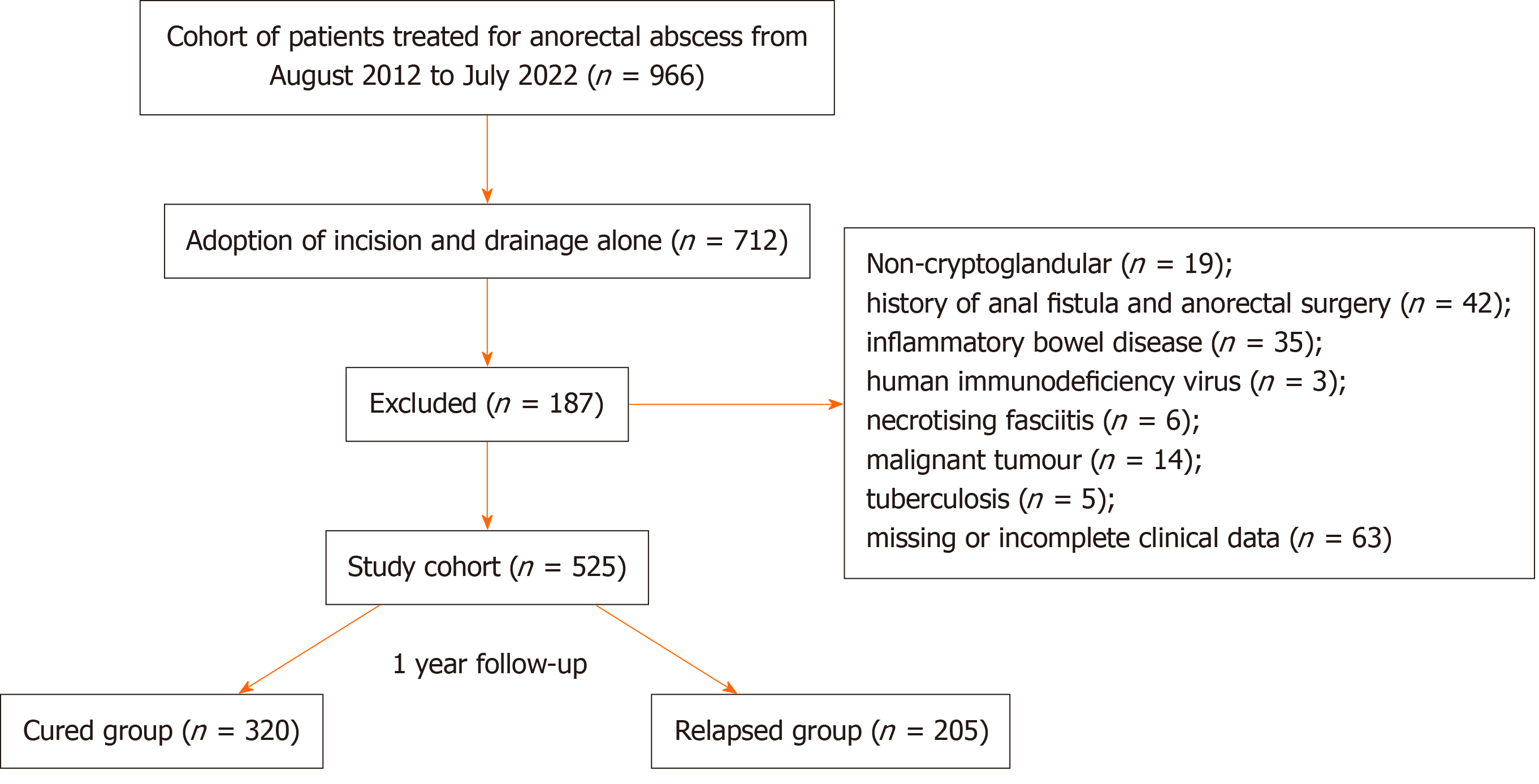
Finally, 525 patients were enrolled in the study (Figure 1).
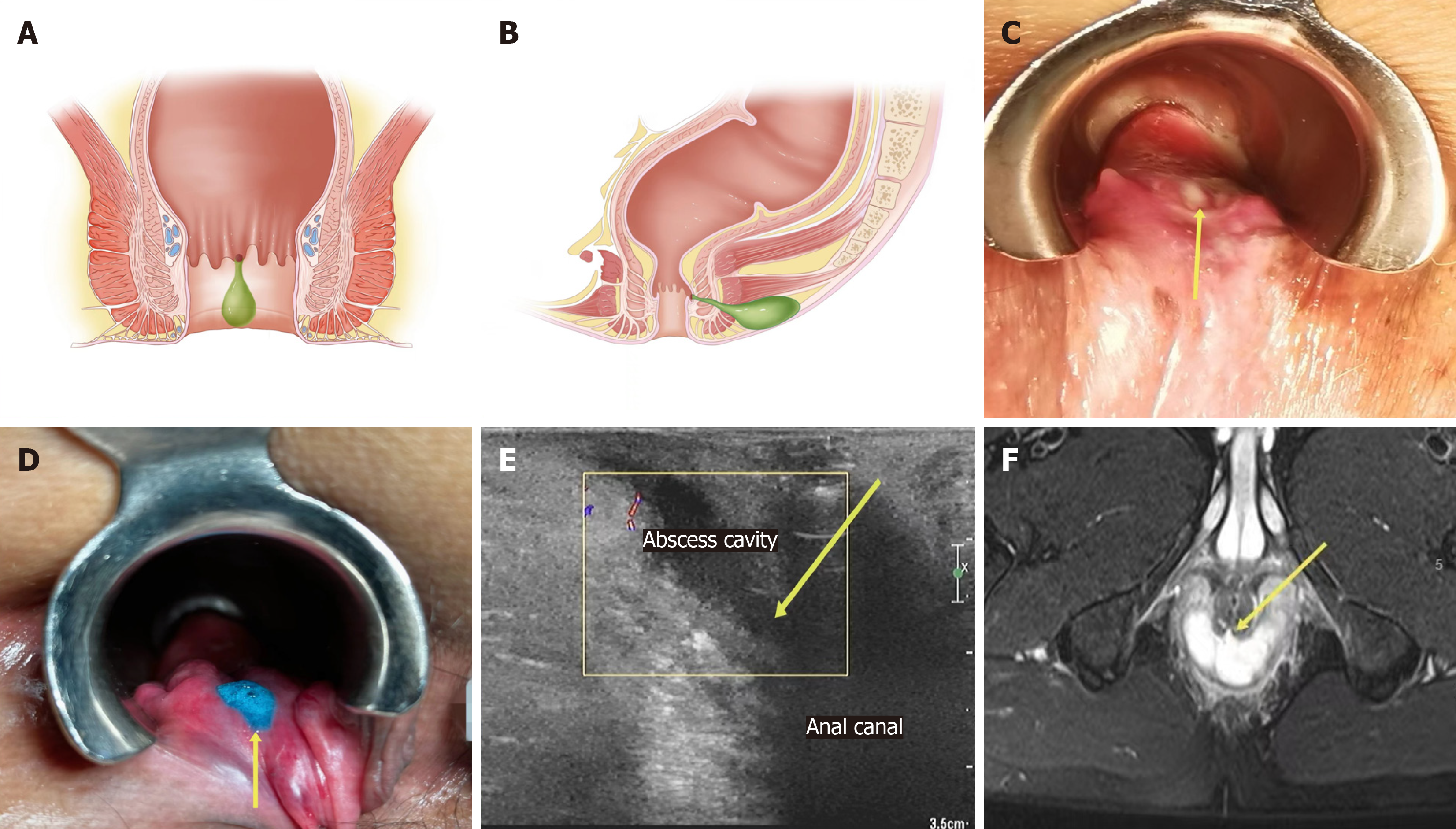
An fistula-prone abscess (FPA) is defined by several criteria: (1) It is characterized by a hard nodule, depression, or defect at the dentate line or sclerotic tissue between the pus cavity and the anal canal, as detected by a finger touch (characteristics of anorectal clinical examination); (2) During surgery, pus oozes from the corresponding area when pressing the pus cavity or liquid overflows when hydrogen peroxide and methylene blue are instilled into the pus cavity (characteristics of surgery); and (3) Ultrasound or magnetic resonance imaging (MRI) reveals an abscess cavity leading to the anal canal, internal sphincter involvement, or discontinuity (characteristics of imaging). If any of the above conditions are satisfied, it is classified as an FPA; otherwise, it is classified as a non-FPA (NFPA) (Figure 2).
All patients completed a routine preoperative examination to rule out contraindications to surgery and underwent ultrasound or MRI. Additionally, their medical histories, including multiple potential risk factors, were obtained. The sites of the anorectal abscesses and anatomical classifications were recorded.
Patients with perianal abscesses were divided into two groups based on the assessment of their conditions and patient preference: I and D was performed under outpatient local anesthesia or inpatient spinal anesthesia. Patients with other anatomical types of anorectal abscesses were hospitalized and received I and D under spinal anesthesia.
All surgeries were performed by the same experienced surgical team of attending or chief surgeons. First, anorectal clinical examinations were conducted, followed by pressing the pus cavity to check for any oozing pus. If this process failed, a small incision was made at the point of maximal bulge to drain the pus, and the pus volume was recorded. Subsequently, hydrogen peroxide was instilled with methylene blue into the pus cavity to observe whether any liquid overflowed. Notably, that there were some differences in the surgical approaches to different anatomical types. For perianal and ischiorectal abscesses, a radial incision was made at the point of maximal bulge. Necrotic tissues was re
The pus was cultured. Antibiotics were administered intravenously for 1–2 days postoperatively. Sitz baths were administered twice a day, and the dressing was changed once a day until the wounds healed.
The patients were instructed to visit the outpatient clinic immediately if they experienced any discomfort, such as perianal swelling and pain or pus discharge, after wound healing. If they remained asymptomatic, a follow-up was scheduled in the outpatient clinic 6 months and 1 year postoperatively, supplemented with telephone interviews. The study's primary endpoint was recurrence, defined as abscess relapse or fistula formation. A team of experienced anorectal surgeons judged recurrence based on a clinical exam and an ultrasound or MRI examination.
The original data were extracted from the electronic medical records and stored in Microsoft Excel; Statistical Package for the Social Sciences 26.0 software was used for statistical analysis. Categorical data were expressed as numbers (n) and percentage (%). Comparisons between groups were performed using the χ² test, and differences were defined as statistically significant at P < 0.05. A multivariate logistic regression analysis included the screened positive variables to explore the relationship between postoperative recurrence of anorectal abscesses and various factors. The same statistical ap
A total of 966 patients with anorectal abscesses were operated on in our hospital from August 2012 to July 2022. Among them, 712 patients underwent I and D alone, and 187 patients met the exclusion criteria. Consequently, 525 patients were enrolled in the study; 464 were male, and 61 were female, with this condition most prevalent among those aged 30–59 years. The most common anatomical classification was perianal (n = 322, 61.3%), followed by intersphincteric abscesses (n = 94,17.9%). Regarding abscess sites, approximately half were posterior (n = 261, 49.7%) compared with anterior abscesses (n = 14, 2.7%). Among the 322 patients with perianal abscesses, 181 underwent outpatient surgeries under local anes
Based on clinical experience and previous literature, 19 factors were included in the study. Given that the number of factors may affect the final results of the analysis, we first conducted a univariate analysis using the χ² test; the results showed that the recurrence rate was significantly different among patients with different anatomic classifications and pus culture results (P < 0.05). FPA, a duration ≥ 7 days from symptom onset to surgery, chronic diarrhea, preoperative antibiotic use, and local anesthesia were risk factors for recurrence. Diabetes mellitus appeared to be a protective factor (P < 0.05). The differences in postoperative recurrence rates of anorectal abscesses between the groups when classified based on sex, age, body mass index (BMI), pus volume, smoking status, drinking and spicy food habits, fever, constipation, insomnia, and abscess site were not significant (P > 0.05). Data are presented in Table 1.
| Variables | Total (n = 525) | Recurrence (n = 205) (39%) | Cured (n = 320) (61%) | χ2 value | P value |
| New classification | |||||
| Fistula-prone abscess | 142 | 115 (81.0) | 27 (19.0) | 143.842 | < 0.001 |
| Non-fistula-prone abscess | 383 | 90 (23.5) | 293 (76.5) | ||
| Gender | |||||
| Male | 464 | 180 (38.8) | 284 (61.2) | 0.109 | 0.742 |
| Female | 61 | 25 (41.0) | 36 (59.0) | ||
| Age (years) | |||||
| < 20 | 7 | 3 (42.9) | 4 (57.1) | ||
| 20-29 | 112 | 41 (36.6) | 71 (63.4) | ||
| 30-39 | 126 | 51 (40.5) | 75 (59.5) | 1.808 | 0.771 |
| 40-49 | 129 | 55 (42.6) | 74 (57.4) | ||
| 50-59 | 120 | 45 (37.5) | 75 (62.5) | ||
| > 60 | 31 | 10 (32.3) | 21 (67.7) | ||
| Body mass index (kg/m2) | |||||
| < 28 | 436 | 168 (38.5) | 268 (61.5) | 0.278 | 0.592 |
| ≥ 28 | 89 | 37 (41.6) | 52 (58.4) | ||
| Time from symptom onset to surgery (days) | |||||
| < 7 | 404 | 117 (29.0) | 287 (71.0) | 74.940 | < 0.001 |
| ≥ 7 | 121 | 88 (72.7) | 33 (27.3) | ||
| Smoking status | |||||
| Smoker | 121 | 46 (38.0) | 75 (62.0) | 0.070 | 0.791 |
| Non smoker | 404 | 159 (39.4) | 245 (60.6) | ||
| Drinking habit | |||||
| Yes | 89 | 39 (43.8) | 50 (56.2) | 1.026 | 0.311 |
| No | 436 | 166 (38.1) | 270 (61.9) | ||
| Spicy food habit | |||||
| Yes | 149 | 55 (36.9) | 94 (63.1) | 0.398 | 0.528 |
| No | 376 | 150 (39.9) | 226 (60.1) | ||
| Diabetes mellitus | |||||
| Yes | 58 | 15 (25.9) | 43 (74.1) | 4.763 | 0.029 |
| No | 467 | 190 (40.7) | 277 (59.3) | ||
| Fever | |||||
| Yes | 122 | 51 (41.8) | 71 (58.2) | 0.507 | 0.476 |
| No | 403 | 154 (38.2) | 249 (61.8) | ||
| Preoperative antibiotic use | |||||
| Yes | 194 | 99 (51.0) | 95 (49.0) | 18.565 | < 0.001 |
| No | 331 | 106 (32.0) | 225 (68.0) | ||
| Constipation | |||||
| Yes | 42 | 16 (38.1) | 26 (61.9) | 0.017 | 0.895 |
| No | 483 | 189 (39.1) | 294 (60.9) | ||
| Chronic diarrhea | |||||
| Yes | 98 | 61 (62.2) | 37 (37.8) | 27.242 | < 0.001 |
| No | 427 | 144 (33.7) | 283 (66.3) | ||
| Insomnia | |||||
| Yes | 84 | 35 (41.7) | 49 (58.3) | 0.288 | 0.591 |
| No | 441 | 170 (38.5) | 271 (61.5) | ||
| Anesthesia method of perianal abscess | |||||
| Local | 181 | 74 (40.9) | 107 (59.1) | 8.305 | 0.004 |
| Spinal | 141 | 36 (25.5) | 105 (74.5) | ||
| Abscess site | |||||
| Anterior | 14 | 4 (28.6) | 10 (71.4) | 0.537 | 0.911 |
| Posterior | 261 | 95 (36.4) | 166 (63.6) | ||
| Left | 92 | 32 (34.8) | 60 (65.2) | ||
| Right | 117 | 44 (37.6) | 73 (62.4) | ||
| Anatomic classification | |||||
| Perianal | 322 | 110 (34.2) | 212 (65.8) | 43.458 | < 0.001 |
| Intersphincteric | 94 | 24 (25.5) | 70 (74.5) | ||
| Ischiorectal | 68 | 41 (60.3) | 27 (39.7) | ||
| Horseshoe | 38 | 28 (73.7) | 10 (26.3) | ||
| Supralevator | 3 | 2 (66.7) | 1 (33.3) | ||
| Pus volume (mL) | |||||
| < 5 | 349 | 131 (37.5) | 218 (62.5) | 1.001 | 0.317 |
| ≥ 5 | 176 | 74 (42.0) | 102 (58.0) | ||
| Pus culture | |||||
| With results | 230 | 99 (43.0) | 131 (57.0) | 17.214 | 0.002 |
| Escherichia coli | 154 | 76 (49.4) | 78 (50.6) | ||
| Klebsiella pneumoniae | 37 | 14 (37.8) | 23 (62.2) | ||
| Staphylococcus aureus | 19 | 1 (5.3) | 18 (94.7) | ||
| Others | 20 | 8 (40.0) | 12 (60.0) | ||
| No growth | 295 | 106 (35.9) | 189 (64.1) |
The eight statistically significant variables mentioned above were included in the multivariate logistic regression analysis as independent variables, with recurrence as the dependent variable (the variable assignment is shown in Table 2). The results, detailed in Table 3, identified the following independent risk factors for postoperative recurrence of anorectal abscesses: FPA [odds ratio (OR) = 7.651, 95%CI: 4.049–14.458, P < 0.001], time ≥ 7 days from symptom onset to surgery (OR = 2.137, 95%CI: 1.090–4.190, P = 0.027), chronic diarrhea (OR = 2.508, 95%CI: 1.216–5.173, P = 0.013), and local anesthesia (OR = 2.308, 95%CI: 1.313–4.059, P = 0.004).
| Variables | Assignment |
| Postoperative prognosis | 1: Recurrence, 0: Cured |
| New classification | 1: Fistula-prone abscess, 0: Non-fistula-prone abscess |
| Time from symptom onset to surgery (days) | 1: ≥ 7, 0: < 7 |
| Diabetes mellitus | 1: Yes, 0 : No |
| Preoperative antibiotic use | 1: Yes, 0: No |
| Chronic diarrhea | 1: Yes, 0: No |
| Anesthesia method of perianal abscess | 1: Local, 0: Spinal |
| Anatomic classification | 1: Perianal, 2: Intersphincteric, 3: Ischiorectal, 4: Horseshoe, 5: Supralevator |
| Pus culture | 1: Escherichia coli, 2: Klebsiella pneumoniae, 3: Staphylococcus aureus, 4: Others, 0: No results |
| Variables | B value | SE | Wald | P value | Odds ratio | 95%CI |
| New classification | 2.035 | 0.325 | 39.271 | 0.000 | 7.651 | 4.049-14.458 |
| Time from symptom onset to surgery (days) | 0.760 | 0.343 | 4.890 | 0.027 | 2.137 | 1.090-4.190 |
| Chronic diarrhea | 0.920 | 0.369 | 6.203 | 0.013 | 2.508 | 1.216-5.173 |
| Anesthesia method of perianal abscess | 0.836 | 0.288 | 8.436 | 0.004 | 2.308 | 1.313-4.059 |
The FPA group could not be analyzed because the sample size was too small to satisfy the conditions for applying statistical methods. Univariate analysis of the risk factors for postoperative recurrence in the NFPA subgroup was performed using the χ² test, and the results showed that the recurrence rate differed significantly among patients with different anatomical classifications (P < 0.05). A BMI ≥ 28, time ≥ 7 days from symptom onset to surgery, chronic diarrhea, and local anesthesia were risk factors for recurrence (P < 0.05). None of the remaining 13 factors significantly influenced the recurrence rate (P > 0.05). Details are presented in Table 4.
| Variables | Total (n = 383) | Recurrence (n = 90) (23.5%) | Cured (n = 293) (76.5%) | χ2 | P value |
| Gender | |||||
| Male | 346 | 86 (24.9) | 260 (75.1) | 3.668 | 0.055 |
| Female | 37 | 4 (10.8) | 33 (89.2) | ||
| Age (years) | |||||
| < 20 | 5 | 1 (20.0) | 4 (80.0) | ||
| 20-29 | 88 | 22 (25.0) | 66 (75.0) | ||
| 30-39 | 95 | 24 (25.3) | 71 (74.7) | 2.580 | 0.764 |
| 40-49 | 88 | 22 (25.0) | 66 (75.0) | ||
| 50-59 | 87 | 19 (21.8) | 68 (78.2) | ||
| > 60 | 20 | 2 (10.0) | 18 (90.0) | ||
| Body mass index (kg/m2) | |||||
| < 28 | 312 | 66 (21.2) | 246 (78.8) | 5.148 | 0.023 |
| ≥ 28 | 71 | 24 (33.8) | 47 (66.2) | ||
| Time from symptom onset to surgery(days) | |||||
| < 7 | 342 | 66 (19.3) | 276 (80.7) | 31.356 | < 0.001 |
| ≥ 7 | 41 | 24 (58.5) | 17 (41.5) | ||
| Smoking status | |||||
| Smoker | 93 | 24 (25.8) | 69 (74.2) | 0.364 | 0.546 |
| Non smoker | 290 | 66 (22.8) | 224 (77.2) | ||
| Drinking habit | |||||
| Yes | 64 | 20 (31.3) | 44 (68.7) | 2.568 | 0.109 |
| No | 319 | 70 (21.9) | 249 (78.1) | ||
| Spicy food habit | |||||
| Yes | 116 | 31 (26.7) | 85 (73.3) | 0.963 | 0.326 |
| No | 267 | 59 (22.1) | 208 (77.9) | ||
| Diabetes mellitus | |||||
| Yes | 46 | 8 (17.4) | 38 (82.6) | 1.085 | 0.298 |
| No | 337 | 82 (24.3) | 255 (75.7) | ||
| Fever | |||||
| Yes | 78 | 15 (19.2) | 63 (80.8) | 0.992 | 0.319 |
| No | 305 | 75 (24.6) | 230 (75.4) | ||
| Preoperative antibiotic use | |||||
| Yes | 119 | 24 (20.2) | 95 (79.8) | 1.065 | 0.302 |
| No | 264 | 66 (25.0) | 198 (75.0) | ||
| Constipation | |||||
| Yes | 31 | 8 (25.8) | 23 (74.2) | 0.100 | 0.752 |
| No | 352 | 82 (23.3) | 270 (76.7) | ||
| Chronic diarrhea | |||||
| Yes | 58 | 27 (46.6) | 31 (53.4) | 20.206 | < 0.001 |
| No | 325 | 63 (19.4) | 262 (80.6) | ||
| Insomnia | |||||
| Yes | 59 | 15 (25.4) | 44 (74.6) | 0.144 | 0.705 |
| No | 324 | 75 (23.1) | 249 (76.9) | ||
| Anesthesia method of perianal abscess | |||||
| Local | 132 | 36 (27.3) | 96 (72.7) | 7.191 | 0.007 |
| Spinal | 107 | 14 (13.1) | 93 (86.9) | ||
| Abscess site | |||||
| Anterior | 12 | 2 (16.7) | 10 (83.3) | 2.062 | 0.560 |
| Posterior | 189 | 38 (20.1) | 151 (79.9) | ||
| Left | 71 | 15 (21.1) | 56 (78.9) | ||
| Right | 92 | 25 (27.2) | 67 (72.8) | ||
| Anatomic classification | |||||
| Perianal | 239 | 50 (20.9) | 189 (79.1) | 40.211 | < 0.001 |
| Intersphincteric | 73 | 5 (6.8) | 68 (93.2) | ||
| Ischiorectal | 52 | 25 (48.1) | 27 (51.9) | ||
| Horseshoe | 18 | 10 (55.6) | 8 (44.4) | ||
| Supralevator | 1 | 0 (0.0) | 1 (100.0) | ||
| Pus volume (mL) | |||||
| < 5 | 253 | 52 (20.6) | 201 (79.4) | 3.597 | 0.058 |
| ≥ 5 | 130 | 38 (29.2) | 92 (70.8) | ||
| Pus culture | |||||
| With results | 158 | 36 (22.8) | 122 (77.2) | 3.760 | 0.452 |
| Escherichia coli | 99 | 26 (26.3) | 73 (73.7) | ||
| Klebsiella pneumoniae | 26 | 5 (19.2) | 21 (80.8) | ||
| Staphylococcus aureus | 17 | 1 (5.9) | 16 (94.1) | ||
| Others | 16 | 4 (25.0) | 12 (75.0) | ||
| No growth | 225 | 54 (24.0) | 171 (76.0) |
The five statistically significant variables mentioned above and pus volume were included in the multivariate logistic regression analysis as independent variables, with recurrence as the dependent variable (the variable assignment is shown in Table 5). The results are shown in Table 6. BMI ≥ 28 (OR = 2.935, 95%CI: 1.203–7.165, P = 0.018), time ≥ 7 days from symptom onset to surgery (OR = 5.978, 95%CI: 2.043–17.489, P = 0.001), chronic diarrhea (OR = 3.417, 95%CI: 1.388–8.412, P = 0.008), and local anesthesia (OR = 3.341, 95%CI: 1.565–7.133, P = 0.002) were identified as independent risk factors for postoperative recurrence of NFPAs.
| Variables | Assignment |
| Postoperative prognosis | 1: Recurrence, 0: Cured |
| Body mass index | 1: ≥ 28, 0: < 28 |
| Time from symptom onset to surgery(days) | 1: ≥ 7, 0: < 7 |
| Pus volume | 1: ≥ 5, 0: < 5 |
| Chronic diarrhea | 1: Yes, 0: No |
| Anesthesia method of perianal abscess | 1: Local, 0: Spinal |
| Anatomic classification | 1: Perianal, 2: Intersphincteric, 3: Ischiorectal, 4: Horseshoe, 5: Supralevator |
| Variables | B value | SE | Wald | P value | Odds ratio | 95%CI |
| Body mass index | 1.077 | 0.455 | 5.593 | 0.018 | 2.935 | 1.203-7.165 |
| Time from symptom onset to surgery (days) | 1.788 | 0.548 | 10.659 | 0.001 | 5.978 | 2.043-17.489 |
| Chronic diarrhea | 1.229 | 0.460 | 7.144 | 0.008 | 3.417 | 1.388-8.412 |
| Anesthesia method of perianal abscess | 1.206 | 0.387 | 9.721 | 0.002 | 3.341 | 1.565-7.133 |
In the current study, 39.0% of patients reported recurrence, nearly consistent with findings from previous studies[13-15]. Among the 525 enrolled patients, 142 were classified and assigned to the FPA group and 383 to NFPA group. The recurrence rate significantly differed between the two groups: 81.0% and 23.5% for the FPA and NFPA groups, respec
The presence of FPAs was identified as a significant risk factor for recurrence, in both the univariate and multivariate analysis (χ2 = 143.842, OR = 7.651). Possible explanations for this result could be related to the three clinical characteristics of FPAs, which all imply disruption of the local barrier and increased permeability. The internal sphincter plays an important role in maintaining this barrier. As inflammation spreads and destroys the integrity of the internal sphincter, it ultimately compromises the local barrier, facilitating the continual migration of the intestinal bacteria to the perianal space. Eventually, a fistula will inevitably persist.This suggests that intraoperative use of a probe to explore a potential fistula tract is inadvisable. The new classification is necessary for guiding the choice of surgical procedure (I and D alone or with concurrent fistulotomy): (1) In the case of NFPAs, a simple I and D is sufficient, thus avoiding unnecessary injuries; and (2) For FPAs, fistulotomy or seton placement can be attempted to prevent recurrence. Notably, 19% of FPAs did not progress to form an anal fistula during the follow-up period. This could be due to a small proportion of FPAs undergoing a reversal of fistulogenic tendency or errors in classification from clinical or imaging assessments, which resulted in the misclassification of patients, who would have belonged to the NFPA group, into the FPA group. This suggests that the accuracy of the classification system required continuous improvement.
Consistent with the previous findings[14], we also found that patients with a time ≥ 7 days from symptom onset to surgery were more likely to relapse than those with a time < 7 days (χ2 = 74.940, OR = 2.137). A substantial proportion of these patients were in the FPA group, suggesting that prolonged inflammation may progressively compromise the local barrier function. Thus, FPA appears to be an intermediate stage between abscess and fistula, indicating that timely I and D is essential to prevent postoperative recurrence.
In contrast to two previous studies[17,18], we found that chronic diarrhea was a predisposing factor for anorectal abscess recurrence (χ2 = 27.242, OR = 2.508). Chronic diarrhea is often associated with intestinal flora dysbiosis[19]. Anorectal abscesses are caused mainly by bacterial infection[20] and are associated with the gut microbiota[21]. Signi
Furthermore, anorectal abscesses drained in the operating room have a lower recurrence rate[23]. Our study yielded similar results; patients who underwent surgery under local anesthesia on an outpatient basis had a recurrence rate more than 2-fold higher than those who received spinal anesthesia on an inpatient basis (χ2 = 8.305, OR = 2.308). This may be because, compared to local anesthesia, spinal anesthesia provides fuller exposure, more thorough drainage, and a lower likelihood of residual inflammation. Based on this finding, we recommend that patients with anorectal abscesses should be hospitalized and operated on under spinal anesthesia.
Notably, the postoperative recurrence rate of intersphincteric abscesses is significantly lower than those of the other classifications, contrary to this study[24]. We performed partial internal sphincterotomy to drain intersphincteric absce
The impact of postoperative antibiotic use on subsequent fistula formation in patients with anorectal abscesses has been studied in a series of studies with disparate results[24,26]. Nonetheless, preoperative antibiotic use has rarely been evaluated previously. Our study showed that this variable was a risk factor for postoperative recurrence (χ2 = 18.565), although it was not significant in multivariate logistic regression analysis. In today’s society, with the use of the internet and social media, many patients self-diagnose and self-medicate. Additionally, it is relatively easy to obtain antibiotics in China. Consequently, patients only seek medical care when their self-treatment is ineffective. It is our view that these patients tended to have a more extended period from symptom onset to seeking medical advice, which may explain the higher rate of postoperative recurrence in patients who had used antibiotics preoperatively. While these antibiotics may be helpful for symptom relief, they may increase the risk of postoperative recurrence. Therefore, future research should investigate how the preoperative use of antibiotics affects the prognosis of patients with anorectal abscesses.
We found that patients with pus culture results of Escherichia coli and Klebsiella pneumoniae were more prone to recurrence than those with Staphylococcus aureus (S. aureus), which supports previous findings[9,27]. Interestingly, 18 of 19 patients with S. aureus positive pus culture were cured, and all these patients had diabetes mellitus; the only one who relapsed did not have diabetes mellitus. Our study had similar observations to this one[13]. Diabetes mellitus may be a contributory factor for the development of anorectal abscesses; however, it seems to be a protective factor for recurrence. For patients with diabetes mellitus, we routinely monitored blood glucose levels and consulted an endocrinologist for glucose control. We analyzed the probable cause of this phenomenon: Patients with anorectal abscesses arising from poor glycemic control were strictly controlled for blood sugar postoperatively. Furthermore, it is also possible that patients with diabetes mellitus that have anorectal abscesses have different pathogenesis compared to other patients. It is essential to examine the mechanism of the effect of diabetes mellitus on patients with anorectal abscesses.
Several previous studies have yielded different results regarding BMI as a potential risk factor for postoperative recurrence of anorectal abscesses[17,23]. In our study, when analyzed in all patients, the recurrence rate was higher in the group with a BMI ≥ 28 than in the group with BMI < 28, although statistical significance was not reached. When BMI was analyzed in the NFPA subgroup; BMI ≥ 28 was a risk factor for relapse in both the univariate and multivariate logistic regression analysis. This may be due to the control of some confounding factors through stratification. This suggests that special attention should be paid to postoperative follow-up of obese patients in the NFPA group.
Furthermore, our study indicated that sex, age, pus volume, smoking status, drinking and spicy food habits, fever, constipation, insomnia, and abscess site were not associated with the postoperative recurrence of anorectal abscesses.
Our study has several strengths, including: (1) The inclusion of a large population of patients with anorectal abscesses over a relatively long period; (2) The relatively comprehensive risk factors analyzed; and (3) To the best of our know
However, this study had some limitations: (1) The retrospective nature of the study makes it difficult to ensure com
A future multicenter prospective randomized clinical trial is essential to compare different surgical procedures for FPAs or NFPAs. Additionally, the pathophysiological, immunological, and microbiological differences between FPAs and NFPAs deserve to be studied, which may yield biomarkers that help predict the postoperative recurrence of ano
The new classification of anorectal abscesses proposed in this study is a reliable predictor of postoperative recurrence, and can be effectively used by anorectal surgeons. It provides specific guidance for selecting surgical procedure: For NFPAs, I and D alone is recommended; for FPAs, concurrent fistulotomy or seton placement can be attempted, and prompt and thorough I and D can significantly reduce postoperative recurrence. Furthermore, attention should to be paid to regulating the intestinal flora in patients with chronic diarrhea and closely monitoring obese patients postoperatively.
| 1. | Abcarian H. Anorectal infection: abscess-fistula. Clin Colon Rectal Surg. 2011;24:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Pearce L, Newton K, Smith SR, Barrow P, Smith J, Hancock L, Kirwan CC, Hill J; North West Research Collaborative. Multicentre observational study of outcomes after drainage of acute perianal abscess. Br J Surg. 2016;103:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Lohsiriwat V. Anorectal emergencies. World J Gastroenterol. 2016;22:5867-5878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (4)] |
| 4. | Ho YH, Tan M, Chui CH, Leong A, Eu KW, Seow-Choen F. Randomized controlled trial of primary fistulotomy with drainage alone for perianal abscesses. Dis Colon Rectum. 1997;40:1435-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ramanujam PS, Prasad ML, Abcarian H, Tan AB. Perianal abscesses and fistulas. A study of 1023 patients. Dis Colon Rectum. 1984;27:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 131] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg. 1997;63:686-689. [PubMed] |
| 7. | Tang CL, Chew SP, Seow-Choen F. Prospective randomized trial of drainage alone vs. drainage and fistulotomy for acute perianal abscesses with proven internal opening. Dis Colon Rectum. 1996;39:1415-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Schouten WR, van Vroonhoven TJ. Treatment of anorectal abscess with or without primary fistulectomy. Results of a prospective randomized trial. Dis Colon Rectum. 1991;34:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Hämäläinen KP, Sainio AP. Incidence of fistulas after drainage of acute anorectal abscesses. Dis Colon Rectum. 1998;41:1357-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Tarasconi A, Perrone G, Davies J, Coimbra R, Moore E, Azzaroli F, Abongwa H, De Simone B, Gallo G, Rossi G, Abu-Zidan F, Agnoletti V, de'Angelis G, de'Angelis N, Ansaloni L, Baiocchi GL, Carcoforo P, Ceresoli M, Chichom-Mefire A, Di Saverio S, Gaiani F, Giuffrida M, Hecker A, Inaba K, Kelly M, Kirkpatrick A, Kluger Y, Leppäniemi A, Litvin A, Ordoñez C, Pattonieri V, Peitzman A, Pikoulis M, Sakakushev B, Sartelli M, Shelat V, Tan E, Testini M, Velmahos G, Wani I, Weber D, Biffl W, Coccolini F, Catena F. Anorectal emergencies: WSES-AAST guidelines. World J Emerg Surg. 2021;16:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Gaertner WB, Burgess PL, Davids JS, Lightner AL, Shogan BD, Sun MY, Steele SR, Paquette IM, Feingold DL; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Anorectal Abscess, Fistula-in-Ano, and Rectovaginal Fistula. Dis Colon Rectum. 2022;65:964-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 12. | Huang X. The "Hands" teaching method for the classification of anorectal abscess. Asian J Surg. 2024;47:1093-1094. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Hamadani A, Haigh PI, Liu IL, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis Colon Rectum. 2009;52:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Yano T, Asano M, Matsuda Y, Kawakami K, Nakai K, Nonaka M. Prognostic factors for recurrence following the initial drainage of an anorectal abscess. Int J Colorectal Dis. 2010;25:1495-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Hasan ZAIY, Mohamed B, AlSayegh R, AlMarzooq R. Incidence of anal fistula after pyogenic perianal abscess drainage in Kingdom of Bahrain. Ann Coloproctol. 2023;39:27-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Holzheimer RG, Siebeck M. Treatment procedures for anal fistulous cryptoglandular abscess--how to get the best results. Eur J Med Res. 2006;11:501-515. [PubMed] |
| 17. | Lu D, Lu L, Cao B, Li Y, Cao Y, Li Z, Wang Z, Lu J. Relationship Between Body Mass Index and Recurrence/Anal Fistula Formation Following Initial Operation for Anorectal Abscess. Med Sci Monit. 2019;25:7942-7950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Ding C, Chen Y, Yan J, Wang K, Tan SS. Risk factors for therapy failure after incision and drainage alone for perianal abscesses in children. Front Pediatr. 2024;12:1342892. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Scaldaferri F, Pizzoferrato M, Pecere S, Forte F, Gasbarrini A. Bacterial flora as a cause or treatment of chronic diarrhea. Gastroenterol Clin North Am. 2012;41:581-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Alabbad J, Abdul Raheem F, Alkhalifa F, Hassan Y, Al-Banoun A, Alfouzan W. Retrospective Clinical and Microbiologic Analysis of Patients with Anorectal Abscess. Surg Infect (Larchmt). 2019;20:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Brook I. The role of anaerobic bacteria in cutaneous and soft tissue abscesses and infected cysts. Anaerobe. 2007;13:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Yin H, Luo B, Wang Q, Hong Z, Chen H, Shen L, Shen B, Hu B. Differences in Gut Microbiota between Healthy Individuals and Patients with Perianal Abscess before and after Surgery. Mediators Inflamm. 2023;2023:1165916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Narayanan S, Althans AR, Reitz KM, Allen LH, Kurukulasuriya C, Larkin TM, Reinert NJ, Cunningham KE, Watson AR, Celebrezze JP, Medich DS, Holder-Murray J. Drainage of anorectal abscesses in the operating room is associated with a decreased risk of abscess recurrence and fistula formation. Am J Surg. 2023;225:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Sözener U, Gedik E, Kessaf Aslar A, Ergun H, Halil Elhan A, Memikoğlu O, Bulent Erkek A, Ayhan Kuzu M. Does adjuvant antibiotic treatment after drainage of anorectal abscess prevent development of anal fistulas? A randomized, placebo-controlled, double-blind, multicenter study. Dis Colon Rectum. 2011;54:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Zhou ZY, Hu B, Liu DC, Peng H, Xie SK, Su D, Ren DL. Clinical Significance of 2 Deep Posterior Perianal Spaces to Complex Cryptoglandular Fistulas. Dis Colon Rectum. 2016;59:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Ghahramani L, Minaie MR, Arasteh P, Hosseini SV, Izadpanah A, Bananzadeh AM, Ahmadbeigi M, Hooshanginejad Z. Antibiotic therapy for prevention of fistula in-ano after incision and drainage of simple perianal abscess: A randomized single blind clinical trial. Surgery. 2017;162:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Toyonaga T, Matsushima M, Tanaka Y, Shimojima Y, Matsumura N, Kannyama H, Nozawa M, Hatakeyama T, Suzuki K, Yanagita K, Tanaka M. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fistula in acute anorectal sepsis. Int J Colorectal Dis. 2007;22:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |